- Insulin replacement therapy is considered the only effective and feasible treatment for type 1 diabetes mellitus (T1DM) as only insulin is capable of reversing the metabolic disturbances and restoring a near-normal quality of life in patients with T1DM.
- Despite rigorous measures and major advances in health care provided for patients with T1DM, increased morbidity and mortality are still common from complications, which commonly develop within 10–12 years after clinical onset.
- Advances in the understanding of the natural history of T1DM and increased abilities to predict the disease have made it possible to design and implement prevention and intervention clinical trials.
- Clinical trials are aimed at: (a) preventing the initiation of islet autoimmunity (primary prevention); (b) reducing autoimmune (β-cell killing and progression to clinical diabetes (secondary prevention); or (c) suppressing or modulating the immune response in order to halt β- cell killing and enhance β-cell regeneration (tertiary prevention or intervention).
- Several trials were implemented or are currently ongoing with dietary manipulation, parenteral or oral insulin or immune-suppressing or immune-modulating agents with the aim of preventing the disease or retarding its progression.
- The search for safe, effective and feasible drugs to prevent or cure T1DM is still ongoing. So far, immune modulation with alum-formulated GAD65 has been shown to be the most promising intervention to reduce the loss of (β-cells. Anti-CD3 monocloncal autoantibodies also showed some benefits in patients with newly diagnosed T1DM.
Introduction
The pathophysiologic mechanisms in type 1 diabetes mellitus (T1DM) are proposed to progress over several stages, in which autoimmunity is triggered in genetically predisposed individuals and in which β-cell killing by cellular immunity is activated, leading to insulin deficiency [1]. The autoimmune insult, which leads to selective killing of β-cells, may take months to years to develop. Throughout this autoimmune prodrome several genetic, autoimmune and biochemical markers may predict the disease prior to clinical onset. Once the clinical disease is established, the patient will be dependent on exogenous insulin and will require strict control to sustain euglycemia and minimize complications. Shortly after clinical onset of diabetes and initiation of insulin treatment, the remaining β-cells often increase their capacity to produce insulin, thereby decreasing the need for insulin treatment. This period of low insulin requirement, known as “partial remission” or the “honeymoon” period, may last for months or even years and is advantageous for the patient because it simplifies treatment and improves metabolic control, leading to less risk of long-term complications [2]. The “honeymoon” period seems to be longer and appears more frequently in older children and adults than in children diagnosed at a younger age [3].
Several trials seeking to prevent or delay the clinical onset of T1DM in genetically predisposed individuals as well as attempting to spare or even improve residual β-cell function in recently diagnosed patients are planned, implemented or currently ongoing.
Short history of type 1 diabetes prevention trials
Several approaches to modulate the immune system have been tested to prolong the partial remission in newly diagnosed patients. In the 1980s, plasmapheresis was performed in patients newly diagnosed with T1DM. A tendency of improved residual β-cell function and metabolic control was observed [4]. Later, several attempts with immune-modulating drugs were carried out. In order to suppress the immune response against the β-cells, small doses of cyclosporine were administered in a pilot study to autoantibody-positive first-degree relatives (FDR) of patients with T1DM with decreased first-phase insulin release (FPIR). The drug increased the FPIR, suggesting that cyclosporine may delay the onset of T1DM in glucose-intolerant siblings [5]. Cyclosporine was further tested in the Canadian–European Randomized Control Trial [6] as well as in series of controlled or uncontrolled smaller studies [5,7]. It was found that cyclosporine temporarily reduced insulin requirement and enhanced endogenous β-cell function in patients with newly diagnosed T1DM [6]. Although cyclosporine had some effect on preserving β-cell function, further clinical trials were abandoned because of severe kidney lesions [6,8]. Other immunosuppressive drugs such as azathioprine were also tested, either alone [9] or with prednisolone [10]. These immunosuppression trials had verified the immunopathogenic basis of T1DM, indicated by improved indicators of β-cell function in some trials; however, the outcomes were hard to maintain because of concerns about serious side effects [5,7].
Concerns about safety and sustainability of therapeutic effects directed attention towards the search for other preventive agents, with the focus on initiating preventive trials based on the stages of disease progression; however, our understanding of the natural history of T1DM made it possible to identify highly predictive markers (genetic, immunologic and metabolic) and recognize putative etiologic factors and possible intervention methods.
Several studies tested non-antigen-specific agents as possible preventive agents. The Bacille Calmette–Guérin (BCG) vaccine was found to prevent T1DM in experimental animals such as the non-obese diabetic (NOD) mice [11] and the bio-breeding (BB) rat [12]; however, similar results were not achieved in a group of human pilot studies [13,14]. The histamine antagonist, ketotifen [15], used orally in a pilot study in islet cell antibody (ICA) positive children with low FPIR showed no significant effect. Nicotinamide (vitamin B3) protected β-cells in rodent studies [16] and so the effect of nicotinamide was tested in the European Nicotinamide Diabetes Intervention Trial (ENDIT) [17]. Nicotinamide was administered orally to ICA-positive FDR of patients with T1DM but the drug did not prevent progression to diabetes. High doses of nicotinamide were tested in other efficacy studies such as the DENIS [18] but this study also failed to show beneficial effects among high-risk children. Additionally, a group of pilot studies tested the combination of nicotinamide with cyclosporine [19], vitamin E [20] or intensive insulin regimen [21], none of which showed a preventive effect. These studies showed that nicotinamide could not protect against the loss of β-cells in high-risk subjects and overall no significant beneficial effects were detected.
Insulin, as an antigen-specific agent, has been tested through different routes, parenteral [22], oral [23] or intranasal [24,25] in genetically predisposed individuals, FDR and patients with T1DM in several trials. These trials showed no effect on disease progression or the rate of β-cell loss; however, subanalysis from the oral arm of the Diabetes Prevention Trial-1 (DPT-1) revealed a statistically significant delay in onset of T1DM in subjects with high titer insulin autoantibodies (IAA). A repeat study of the oral insulin trial has therefore been initiated.
Several prevention and intervention trials with long follow-up durations, some even including a combination of therapies, are now ongoing or planned. These trials use different approaches, with goals of preventing the triggering of autoimmunity (primary prevention), halting β-cell destruction (secondary prevention) or preserving remaining β-cells and enhancing their regeneration (tertiary prevention). This chapter presents an evidence-based review of the main ongoing drug clinical trials in subjects at-risk for or in those with newly diagnosed T1DM.
Prediction
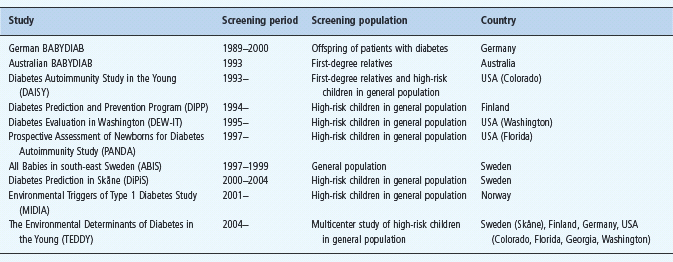
Since the late 1970s, several studies have indicated that it is possible to predict T1DM among FDR through testing for human leukocyte antigen (HLA) class II genes and IAA. Several such studies utilized HLA genotyping, islet autoantibody analyses and biochemical indicators, as predictive markers are currently ongoing (Table 59.1). These prospective screening studies, may allow us to define the environmental factors that may trigger islet autoimmunity or accelerate the development of T1DM in subjects with islet autoimmunity. These studies should make it possible to follow the disease process from initiation to the clinical onset of T1DM to help us understand the course of events during β-cell autoimmunity. At the same time, our knowledge about patterns of disease markers during subclinical disease and the predictive value of islet cell autoantibodies should be improved.
Table 59.1 Some of the recent or ongoing screening studies for T1DM in first-degree relatives or high-risk children in the general population.
Predicting a disease at an early stage of pathologic insult is a prerequisite for attempts to prevent disease progression and initiating interventions to ameliorate symptoms and reduce complications. T1DM fulfils the criteria for predictable disease:
Combining these markers provides highly predictive tools that may help to identify individuals at risk and initiate prevention or intervention at an appropriate point in time.
Currently, exogenous insulin injection is the only therapeutic measure that is able to correct hyperglycemia and enables patients with T1DM to live an almost normal life. Nevertheless, complications are common and may develop within 10–12 years after onset even with adequate metabolic control [30,31]. Prediction of T1DM may provide a window of opportunity to initiate prevention programs to reduce or ideally to prevent β-cell destruction. Identifying susceptible individuals prior to the clinical onset may allow an early and symptom-free diagnosis of diabetes and prevent an otherwise acute T1DM. This is crucially important to prevent the life-threatening condition of diabetic ketoacidosis (DKA) [32], which is commonly seen in children <4 years of age and associated with high morbidity and mortality.
Genetic markers
Much of our knowledge about prediction of T1DM has been generated from studies on FDR of patients with T1DM. These studies have revealed that T1DM can be predicted in siblings of patients with diabetes with analysis of HLA genotypes, number of islet autoantibodies and the titer of IA-2Ab [33]. HLA-identical siblings are at greater risk of developing autoantibodies and T1DM than are non-identical siblings.
Almost 50% of T1DM risk among FDR of Caucasian patients with T1DM is attributed to HLA [34]. One or both of the high-risk class II DQ8 and DQ2 haplotypes were found in more than 90% of patients diagnosed before the age of 30. Additionally, nearly 50% of siblings who carry high-risk HLA genes develop islet autoimmunity by the age of 3 years [35]. By contrast, only 15% of children developing T1DM have FDR with the disease. Therefore, screening of the general population is necessary in order to find a majority of children who are susceptible to T1DM. The diagnostic sensitivity of large-scale genetic screening is low because only 10% of subjects with disease-associated HLA genes develop the disease [36]. Nevertheless, several studies such as the BABYDIAB [37], DAISY [38], DiPiS and TEDDY [39], involving genetically susceptible individuals, continue to improve our understanding of the etiopathologic mechanisms of T1DM. Furthermore, the frequencies of DR-DQ alleles within the high-risk haplotypes DR3-DQ2 (DRB1*03-DQA1*0501-B1*0201) and DR4-DQ8 (DRB1*04-DQA1*0301-B1*0302) vary among different populations [40]. Indeed, T1DM in Asians is associated with HLA haplotypes other than those in Caucasians, indicating that screening strategies may need to be tailored to the population at hand [41]. Longitudinal studies will identify the relationship between HLA variants and the contribution of environmental factors to risk of T1DM. Other non-HLA genetic markers such as INS-VNTR, PTPN22, IL2RA and many others have also been identified [42]. The role of these non-HLA genetic factors in the pathogenesis of islet autoimmunity needs to be clarified, but the relative risk of these genetic factors is shown to be less than that of the HLA genes [42]. HLA and some of the non-HLA genes may be associated with the presence of islet autoantibodies and progression to persistent autoimmunity [43–47]. Combining genetic and autoimmune markers does not improve diagnostic sensitivity of autoantibody testing although the overall positive predictive value may increase.
Autoimmune markers: islet cell autoantibodies
The appearance of diabetes-associated autoantibodies is the first measurable sign of the autoimmune process that eventually leads to T1DM. In 1974, autoantibodies to pancreatic cells were described for the first time [48,49], providing evidence for T1DM being an autoimmune disease. These autoantibodies were named islet cell autoantibodies (ICA) and were detected by immunofluorescence in patients with T1DM. Later, ICA were detected in one patient a year before T1DM onset and in one twin without diabetes who later developed the disease [48], providing the first evidence of an ongoing autoimmune process before the clinical onset of T1DM. ICA are not specific for β-cells and probably represents several different specific autoantigens.
The first autoantigen demonstrated was a Mr64K protein immunoprecipitated by sera from patients with T1DM [50], which was later demonstrated to have glutamic acid decarboxylase (GAD) activity [51], but found not to be a previously recognized isoform of glutamic acid decarboxylase (GAD65) [52]. GAD65 converts glutamic acid to γ-aminobutyric acid (GABA), an inhibitory transmitter in the CNS. GAD65 is primarily present in specific neurons in addition to the pancreatic islet β-cells. Autoantibodies to GAD65 (GAD65Ab) are present in 55–60% of children with T1DM at onset of the disease.
In 1983 autoantibodies to insulin (IAA) were found to be present in patients with diabetes before insulin treatment [53]. This finding suggested that IAA had a role in the autoimmune process of diabetes. IAA are not detected by the immunofluorescence test for ICA. IAA are present in about 35–40% of children with T1DM at onset of the disease.
A third autoantigen, also found to contribute to the ICA reaction, is the insulinoma-associated protein 2 (IA-2), identified in 1996 [54]. IA-2 is a non-functional member of the protein tyrosine phosphatase family. It is localized to the membrane of the secretory vesicles in endocrine and neuronal cells [55]. IA-2 is expressed in both islet α and β-cells, but the function is still unknown because it lacks enzymatic activity. Autoantibodies to IA-2 (IA-2Ab) are directed to the intracellular protein domains [55]. IA-2Ab are present in about 70% of children with T1DM at onset of the disease.
Most recently, autoantibodies to a fourth islet autoantigen, zinc transporter 8 (ZnT8Ab), were reported in patients recently diagnosed with T1DM [56]. This autoantigen is highly β-cell specific. ZnT8 is a protein located in the membrane of the secretory vesicles of the β-cells acting as a zinc ion transporter. Recently, ZnT8Ab have been found in 60–65% of patients with T1DM, compared to <2% in controls [56]. ZnT8Ab are independent markers of islet cell autoimmunity and were detected in otherwise IAA-negative individuals. Furthermore, ZnT8Ab were found to appear and precede disease onset in prospectively studied FDR of patients with T1DM and high-risk individuals from the general population.
Some patients are positive for ICA, but negative for both GAD65Ab and IA-2Ab, suggesting that there are additional, as yet undiscovered, antigens covered by ICA. Some of those patients may have ZnT8Ab. More than 90% of patients with T1DM have previously been reported to have ICA, GAD65Ab, IA-2Ab or IAA at diagnosis. The corresponding number for the general population is about 1% [57]. With the addition of ZnT8Ab to the combined analysis of GAD65Ab, IA-2Ab and IAA, up to 95% of all individuals developing T1DM are positive for at least one autoantibody.
In addition, several other proteins have been proposed as candidate autoantigens (see Chapter 9). Further studies are needed to establish the possible importance of these additional autoantigens for T1DM risk. It is possible that all diabetes-associated autoantibodies have not yet been discovered.
Individuals with clinical T1DM and absence of autoantibodies at diagnosis may still have had an ongoing autoimmune process leading to β-cell destruction. It has been found that 25% of children with T1DM with negative autoantibodies at diagnosis of the disease had autoantibodies present in their cord blood [58].
In genetically predisposed children, detecting islet autoantibodies as early as 3 months of age was associated with high sero-conversion rates, where IAA and GAD65Ab often preceded ICA and IA-2Ab appeared last [59,60]. These autoantibodies seem to appear sequentially, with the appearance of additional autoantibodies usually taking place within a year after the detection of the first one [36,61]. While a single autoantibody may be harmless and often represents non-progressive β-cell autoimmunity, the appearance of multiple autoantibodies most often reflects a progressive process [62–66]. The number of detectable autoantibodies is related to the risk of T1DM, both in FDR and in the general population. In studies of family members of patients with T1DM, 60–100% of individuals with three or more autoantibodies develop clinical T1DM over the next 5–6 years and population-based studies indicate that the risk is similar in the general population [67–69]. The autoantibody pattern differs among age groups. It has been suggested that GAD65Ab is a marker of general non-specific autoimmunity, while IA-2Ab and IAA are more specific markers for β-cell death [70]. The recently discovered ZnT8Ab has been reported to appear generally after 3 years of age and thereafter to increase in frequency with age up to adolescence [56].
Titers of the islet autoantibodies need to be taken into consideration for prediction. Higher titers may better predict risk of persistent autoimmunity and T1DM [33,62,64,66], especially in combination with a high-risk HLA genotype [64,71]. Persistent detection of high titer autoantibodies may mirror the intensity of the autoimmune reaction and the rate of progression to clinical diabetes [64].
In studies of the general population, GAD65Ab and IA-2A have been shown to be of equal predictive value as they are in FDR siblings, but for each separate autoantibody a higher cumulative risk was observed among the siblings. Double autoantibody positivity confers a similar cumulative risk among siblings and the general population [69], with additional predictive information provided by the level of the autoantibodies [64]. In one study of school children, the positive predictive value of multiple autoantibodies in the general population was 25–75%, with a sensitivity of 58–100%, not taking HLA genotypes into account [67].
Biochemical (metabolic) markers
Children progressing to T1DM have been shown to have several autoantibodies, high titers of ICA, IA-2Ab and GAD65Ab and decreased FPIR in response to an intravenous glucose tolerance test (IVGTT). A combination of islet autoantibodies and FPIR might therefore potentially predict diabetes [62]. In one study, siblings of children with diabetes were also examined at the time when the index case was diagnosed. Islet autoantibodies alone predicted T1DM in 36%, but when FPIR was taken into account the rate of prediction increased to 56%, with a median observation time of 3.6 years. A young age, a strong humoral response and reduced FPIR seemed to characterize individuals with a progressive process [62]. Additionally, reduced FPIR levels (<50 mU/L) among autoantibody-positive subjects (mainly ICA or IAA) may predict T1DM among FDR by up to 92% over 10 years [29]. C-peptide may predict β-cell functional loss near diagnosis because C-peptide levels may diminish significantly 6 months prior to onset [72]. Therefore, combining these two tests may detect metabolic disturbances and predict the disease prior to clinical onset, especially when monitored in parallel with autoimmune markers. Children developing diabetes in the DPT-1 study had a gradually deteriorating glucose tolerance with declining 2-hour C-peptide levels after an oral glucose tolerance test (OGTT) [73]. These data were seen over a period of at least 2 years before onset of disease, despite the fact that fasting C-peptide levels remained stable [73]. Moreover, among ICA-positive subjects recruited in the DPT-1 trial, an alternative OGTT index (using area-under-the-curve glucose, 60 and 90-minute glucose, instead of 2-hour glucose) was shown to have better predictive criteria than the standard OGTT, especially when combined with C-peptide measurement from the alternative OGTT [74].
Body mass index (BMI) has also been reported to give additional information in T1DM prediction [75]. It has also been reported that a normal but rising HbA1c, a measure that is proportional to the average blood glucose in the previous 120 days, predicts clinical onset of diabetes in autoantibody-positive children [76].
Other markers
The search for additional biomarkers to predict T1DM is ongoing. Cytokines, chemokines and adhesion molecules are proposed as important inflammatory mediators in the pathogenesis of T1DM (for review see Purohit and She [77]). Antigen-specific T-lymphocytes are also proposed markers for T1DM prediction, but standardized tests to detect these markers have yet to be developed.
Prevention and intervention
Several clinical trials aiming to prevent T1DM have been initiated during the past two decades; some have been completed and others are still ongoing. The general aim of prevention is either to prevent islet autoimmunity from happening or retard the autoimmune destruction of β-cells, or alternatively to preserve remaining β-cell secretory capability before clinical diagnosis. The aim of intervention is to interfere with the natural history of T1DM after the clinical diagnosis. The idea of intervention is to stop or halt the killing of β-cells and preserve β-cell secretory function in patients who have already developed clinical diabetes. Therefore, scientists continue to test new drugs with attempts to halt one or both of these stages. The rationale behind reported prevention and intervention has often been obtained from studies on laboratory animals such as the NOD mouse and BB rat [78–80]. Significant differences exist, however, between these animals and humans; therefore a successful intervention in these animals may not have the exact same effects in humans. Moreover, factors such as the duration of the intervention, the stage of enrolment and drug dosage and safety may influence the outcome. These factors, in addition to the complexity of the etiopathogenesis of T1DM, may explain why little success has been achieved so far in prevention or intervention of T1DM.
All three levels of disease prevention (primary, secondary and tertiary) may be applicable to T1DM.
Primary prevention trials
Primary prevention trials aim to prevent the initiation of autoimmune responses among infants with genetic susceptibility (high-risk HLA genes) or history of T1DM among FDR. Several attempts to prevent T1DM in relatives of patients with the disease have been performed over the years. Such trials are often implemented for longer follow-up durations, especially during the main window of autoimmunity triggering (from birth to 6 years) [81]. Primary prevention trials tend to test the effects of safe, mainly non-antigen-specific agents in relation to environmental risk determinants, of which nutritional factors are important. Results from observational studies encouraged trials that use dietary manipulation to prevent T1DM (Table 59.2).
Non-antigen specific primary prevention trials
Gluten-free diet
A gluten-free diet was given to islet autoantibody-positive children without any significant preventive effect neither on the risk of T1DM nor on the titers of T1DM-associated autoantibodies [82].
Cow’s milk
There are several suggestions that cow’s milk may be associated with higher risk of T1DM [83]. Dietary manipulation using hydrolyzed casein milk formula provided evidence of preventive effect on the risk of T1DM [84,85]. The TRIGR trial is an international effort involving 17 countries to verify if hydrolyzed casein milk formula can reduce T1DM risk among infants at risk (genetically predisposed infants with history of T1DM among FDR), if used instead of cow’s milk. Following a period of 6–8 months’ breastfeeding, infants are randomized into either receiving hydrolyzed casein-based or conventional cow’s milk formulas. This trial is still ongoing and the results of autoantibody analyses are expected in 2012 and the results of T1DM in 2016 [86].
Vitamin D
Supplementary vitamin D has been suggested to protect against T1DM [87], possibly through the effects on the T lymphocytes and through suppression of cytokine production [88]. Lower concentrations of vitamin D have been reported in blood from children with T1DM, and lower vitamin D levels during pregnancy have been suggested to increase the risk for the child to develop T1DM [88,89]. Vitamin D (cholecalciferol) is currently tested in an ongoing randomized feasibility pilot study in Manitoba, Canada, to test if high doses of vitamin D (2000 IU/day) prevent T1DM among genetically susceptible infants ≤4 weeks of age [90].
Nutritional Intervention to Prevent Diabetes study
Supplementation with cod liver oil, an important source of vitamin D and omega-3 fatty acids, during the first year of life led to reduced risk of T1DM in Norwegian children, but no risk reduction was found with other kinds of vitamin D supplementation, suggesting that omega-3 fatty acids were responsible for the effect [91]. In a recent study it was reported that the intake of omega-3 and omega-6 fatty acids were inversely associated with the development of IAA, GAD65Ab and IA-2Ab in children with genetic risk of T1DM [92].
The Nutritional Intervention to Prevent Diabetes (NIP-Diabetes) is a small pilot study to test a proposed preventive effect of oral docosahexanoic acid (DHA) against islet autoimmunity (trial identifier NCT00333554) [93]. Participants included in this trial were infants ≤5 months of age and expected babies of pregnant mothers in their third trimester (>24 weeks) who were genetically predisposed (DR3 or 4) and had family history of T1DM among FDR. DHA is taken in late pregnancy and early infancy and all those infants are followed up for markers of autoimmunity and T1DM (for review see Staeva-Vieira et al. [94]).
Antigen-specific primary prevention trials
Pre-POINT Trial
The primary intervention with oral and/or nasal insulin for prevention of T1DM in infants at high genetic risk to develop diabetes (Pre-POINT) is a trial to identify the dose and the type of insulin that can prevent progression to T1DM. Pre-POINT is an ongoing trial, which has two arms: oral and intranasal. This international effort recruits genetically predisposed FDR infants aged 18 months to 7 years with no islet autoimmunity. In this trial, oral insulin dose is almost 10 times higher than that used in the DPT-1 trial (http://www.diabetes-point.org/nav2uk.html).
Secondary prevention trials
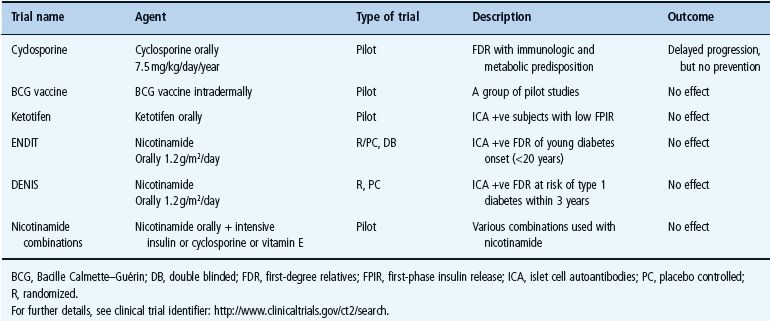
Secondary prevention trials are mainly intended for genetically predisposed children in whom autoimmunity has already developed and also for young adults with multiple islet autoantibodies. These trials aim to reduce β-cell killing by autoimmunity and prevent progression to clinical diabetes [95]. Secondary prevention trials may be divided into two groups: non-antigen-specific (Table 59.3) and antigen-specific trials (Table 59.4).
Table 59.3 Non-antigen-specific secondary prevention trials in T1DM. Adapted from Staeva-Vieira et al. [94].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree