Fever and Shock Syndromes
General concepts
Definitions
Despite its frequency, there is no generally accepted definition of fever. A practical definition is a temperature above 38°C (100.4°F) by mouth or above 38.4°C (101°F) by rectum. Lower temperatures have been proposed as a definition of fever but are not practical and contribute to excessive concern, especially because it is not abnormal for a child to have a rectal temperature between 100°F and 101°F in the afternoon or after exercise.1
Fever is not equivalent to hyperthermia. Fever is an adaptive response that is well regulated by the body and is not dangerous in and of itself (although the cause of the fever may be quite serious). Temperatures due to fever almost never exceed 41°C (105.8°F). In contrast, hyperthermia is an elevated body temperature caused by a dysregulation of the normal mechanisms and can be very dangerous, with temperatures exceeding the body’s set point. Examples include heat stroke (in which body temperature is elevated by external means) and malignant hyperthermia (caused by markedly increased heat production via uncoupling of oxidative phosphorylation).
Normal body temperature shows a diurnal variation, being lowest before awakening and highest in the late afternoon or evening. Fever curves usually follow this diurnal pattern also.
Conversion between Centigrade and Fahrenheit degrees can be made using the following formulas:
(C × 9/5) + 32 = F
(F – 32) × 5/9 = C
Mechanisms
Body temperature is a dynamic balance between heat production and heat loss. In the case of infections, fever is probably produced both by vasoconstriction and by increased heat production. These functions are controlled by the thermoregulatory center in the hypothalamus, which responds to stimulation by pyrogens. Experimental studies have increased the understanding of the exogenous pyrogens of bacteria and the endogenous pyrogens produced by leukocytes. Currently recognized endogenous pyrogens include interleukin-1, TNF-α, interleukin-6, and interferon.2
Elevated core body temperature is the cardinal symptom of fever, but multiple other processes are involved. Fever is a well-regulated and complex physiologic response, involving generation of a host of cytokines and acute phase reactants and activation of numerous physiological, endocrinologic, and immunologic mechanisms.2
The increase in body temperature is modulated by the up-regulation of the thermostatic set point in the hypothalamus. The primary thermoregulatory mechanism used to maintain an elevated temperature is the redirection of blood flow from cutaneous to deep vascular beds, which minimizes heat loss through the skin.3
Dangers and Benefits
Convulsions can occur in children who have fever during relatively minor illnesses, as discussed in the section on febrile convulsions in Chapter 9. Body temperatures in excess of 42°C have multiple harmful effects. However, body temperature caused by the fever mechanism almost never reaches this level, unless there is failure of the thermoregulatory mechanims.4
Body temperatures above 42°C are nearly always the result of hyperthermia, not fever. Temperatures this high have numerous damaging cellular effects—most deaths from hyperthermia are due to cardiac arrhythmias.3
Fever has been postulated to have several effects that might be expected to be useful in the control of infectious diseases: white cell mobility is increased, some viruses and bacteria are killed, natural killer cell activity is increased, killing of bacteria by antibiotics
is enhanced, and the effects of interferon are augmented. Despite these theoretical benefits, there is no clear evidence that fever has a measurable favorable influence on the course of any infectious disease in humans.
is enhanced, and the effects of interferon are augmented. Despite these theoretical benefits, there is no clear evidence that fever has a measurable favorable influence on the course of any infectious disease in humans.
Temperature Measurement
Axillary temperature measurement is slow, has poor sensitivity in detecting fever, and is not recommended for use in infants and children in outpatient settings.5 In rapidly breathing patients, even when there is no obvious mouth breathing, oral temperatures may be erroneously low.6 The so-called tempadots (papers that are placed on the child’s forehead) and temperature-taking pacifiers are inaccurate and should not be used. Tympanic membrane thermometers (“ear thermometers”) have the advantage of being rapid, noninvasive, and painless; however, their accuracy has been questioned in several studies. Mothers’ estimation of high fever in young children (< 2 years old) and their estimation that a child has no significant fever are very accurate most of the time, without the use of a thermometer.7 However, the determination of so-called tactile fever should not be relied on in infants in the first 2 months of life.8
Medical lore holds that children with severe central nervous system (CNS) abnormalities have decreased temperature regulation and exaggerated fever responses, and this is supported by some studies.9,10
Corticosteroids, like antipyretics, can obscure the presence of fever. Sometimes, high fever occurs in children receiving high doses of corticosteroids, and other times, fever is obscured by steroids, especially in tuberculosis.11
Symptomatic Treatment
In each child with a fever, the physician should first ask whether any symptomatic treatment is really necessary. The phrase fever phobia has been used to describe undue worry by parents about fever.12,13 A recent study showed that fever phobia persists despite efforts at education. About 25% of parents became worried enough about their child’s fever that they measured his/her temperature five or more times a day, and an equal percentage of parents slept in the same room as the child with fever.14 Fear of fever has also been documented to exist among medical professionals.15
Guidelines in counseling parents have been recommended for use for children over 6 months of age.1,13 These include retaking the temperature after the child rests for one-half hour, defining 105°F as a high fever, teaching parents that normal body temperature regulation will keep the fever from going “out of control,” and using antipyretic therapy only when fever makes the child uncomfortable.13 Sponging has been recommended only if the temperatures are over 40°C (104°F) and then only if the temperature has not improved within 1 hour of giving an antipyretic. The child is not to be awakened for temperature taking or antipyretic administration because sleep is more important. The parents are taught that temperature taking or “breaking the fever” is not a substitute for more important observations, such as watching for dyspnea, change in consciousness, or pain.1,13
In general, excessive clothing or blankets should be removed to a point of comfort. Hydration with oral fluids is usually advised but should not be forced.
Sponging with tepid (but not cold) water is a comfortable and effective method that is traditional for high fever. One study showed that over the first 30 minutes sponging was more effective than either acetaminophen or ibuprofen, but by 60 minutes it was inferior to both of these agents.16
Sometimes hospitalized patients with high fevers are placed on cooling blankets to decrease their body temperature. A study of febrile adults in an intensive care unit found that the use of cooling blankets plus acetaminophen was no more effective in reducing fever than the use of acetaminophen alone. In addition, the use of cooling blankets was associated with wide fluctuation in temperatures and with rebound hypothermia.17
Unlike antipyretics, external cooling acts not by reducing the elevated set point but by overwhelming metabolically expensive effector mechanisms that have been evoked by the elevated set-point.18
For patients with hyperthermia, external cooling may be lifesaving. However, for patients with fever, the use of cooling blankets is nonsensical, as it causes forced peripheral vasoconstriction at a time when the body is attempting to dissipate heat by vasodilatation.
Antipyretics
Acetaminophen is similar to aspirin as an antipyretic and is less toxic to animals. Acetaminophen
alone is comparable in efficacy to tepid water sponging and when used together, there is some additive effect.19 The pediatric community has amassed a considerable amount of clinical experience using acetaminophen since aspirin (the old standard of care) was dropped as a pediatric antipyretic because of its association with Reye’s syndrome.
alone is comparable in efficacy to tepid water sponging and when used together, there is some additive effect.19 The pediatric community has amassed a considerable amount of clinical experience using acetaminophen since aspirin (the old standard of care) was dropped as a pediatric antipyretic because of its association with Reye’s syndrome.
Over the past decade, ibuprofen has been used with increasing frequency. Early reports about ibuprofen appeared to show that it reduced fever more rapidly and kept the fever down for a longer period of time than acetaminophen.20,21 However, a careful reading of those studies shows that the dosages of acetaminophen used were below the standard dose, which may have biased them toward finding ibuprofen to be superior. Furthermore, more recent studies have failed to find a difference, even when using a suboptimal dose of acetaminophen: no difference in time to lowest temperature, extent of temperature decrease, rate of temperature decrease, or duration of fever was found in groups taking 10 mg/kg of either preparation.22
Finally, although many pediatricians advise parents to alternate acetaminophen and ibuprofen, presumably to give a more frequent dose of antipyretics, there are no data supporting either the safety or the utility of this approach.23 Until such a study has been performed, there is no rationale for the alternating antipyretic approach. Advising such a course of action is unwise; furthermore, it implies that absolute fever control is medically necessary, which it is not.
Complications of Fever Treatment
Sometimes, the treatment of fever produces more problems than the fever. Complications of treatment include antipyretic overdosage, particularly aspirin poisoning (salicylism), which was more common in the past. Acetaminophen can produce delayed liver toxicity or the rare complications of dermatitis, hypoglycemia, agranulocytosis, thrombocytopenia, and methemoglobinemia. Overdosage may produce prolongation of the prothrombin time, vomiting, hepatic failure, and death after 2-7 days. The toxicity of aspirin and acetaminophen have been reviewed in detail.24
Children administered ibuprofen have a higher rate of gastrointestinal bleeding than those treated with acetaminophen. Renal side effects have been reported, too, especially in children with preexisting renal problems or intravascular volume contraction.25 There has been some concern that the use of ibuprofen for antipyresis in children with varicella may increase the risk of necrotizing fasciitis caused by Group A streptococcal superinfection. The evidence in favor of this association is one case-control study and one retrospective cohort study. The case-control study showed an odds ratio of 11.5 for ibuprofen use in children with necrotizing fasciitis versus the control group;26 in many of these cases, ibuprofen use was initiated after the child had developed symptoms of secondary infection. This fact is used by many to say that perhaps ibuprofen use was simply a marker for more severe infection. However, studies in a rabbit model suggest that ibuprofen is capable of preventing neutrophil adhesion, suppressing azurophil granule secretion, and decreasing the production of superoxide,27 all of which are mechanisms by which ibuprofen potentially could increase the risk of progression of an existing superinfection. The cohort study showed that if ibuprofen was dispensed to a child within the 30 days prior to the onset of varicella, the rate of bacterial skin superinfection was 3.1 times higher; if only superinfections severe enough to warrant hospitalization were included, that ratio rose to 5.1.28 Problems with this study include that there is no evidence the children actually took the medication and that the confidence intervals of both odds ratios are wide, and the P values nonsignificant. Nevertheless, the suggestion of a possible association, coupled with the severity of the disease in question and the ready availability of an alternative antipyretic agent would seem to argue against the use of ibuprofen in children with varicella.
Poisoning can occur from inhalation of alcohol used to sponge the skin and may produce hypoglycemia and coma.29,30
In hospitalized patients who are being treated with an antibiotic, the use of antipyretics for moderate fever can interfere with the interpretation of the effectiveness of the antibiotic. However, if the infecting organism is known and the therapy known to be appropriate, then use of antipyretics for discomfort is appropriate.
Classification of Fever Patterns
Interpretation of fever patterns is difficult for many reasons: children are given antipyretics, which may
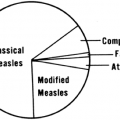
alter the pattern; or consecutive temperatures may be taken by differing routes. Nevertheless, if a pattern of fever can be discerned, this information may provide additional diagnostic clues to the clinician. The classical fever pattern descriptions, along with the syndromes with which they have been associated, are shown in Table 10-1.2
alter the pattern; or consecutive temperatures may be taken by differing routes. Nevertheless, if a pattern of fever can be discerned, this information may provide additional diagnostic clues to the clinician. The classical fever pattern descriptions, along with the syndromes with which they have been associated, are shown in Table 10-1.2
Classification of Fever Syndromes
It is helpful to have a classification of fever syndromes for use in the problem-oriented approach. The classification in Table 10-2 was developed by Dechovitz and Moffet in 1968 by analyzing the records of 155 children hospitalized for fever and is the basis for subsequent individual sections in this chapter.31
Prolonged unexplained fever is defined as a documented fever higher than 38.4°C (101°F) occurring daily for more than 10 days. This pattern is often called “fever of unknown origin (FUO).”
Fever without localizing signs is defined as a fever of less than or equal to 10 days’ duration, with no signs of the source of the infection on physical examination and normal urine. Newborn infants younger than 2 months old are excluded from this category, and children from 2 months to 2 years of age are considered a special subgroup because of an increased risk for occult bacteremia. At the time this classification was devised, many physicians were using the diagnosis of FUO wrongly to describe children with fever of brief duration without localizing signs. Of course, not every child with fever has a urinalysis and/or urine culture done in practice, but it is useful to retain this requirement in the definition to remind the clinician that the possibility of a urinary infection has not been excluded.
TABLE 10-1. SOME FEVER PATTERNS AND ASSOCIATED CAUSES | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Fever with Nonspecific signs is defined as a fever of less than or equal to 10 days’ duration and some abnormal physical findings that direct investigation to a specific area.
Fever complicating a chronic disease is defined as fever in a patient with a disease known to have a predilection for a particular febrile complication. Many chronic diseases have a particular expected febrile complication, such as subacute bacterial endocarditis or brain abscess, which must be considered in fever with congenital heart disease.
Fever in an immunocompromised host should be considered a separate category because the diagnostic considerations must be broadened to include opportunistic infections and because the urgency of providing specific therapy is usually greater (see Chapters 20, 22, and 23).
TABLE 10-2. CLASSIFICATION OF FEVER SYNDROMES | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Recurrent fever is defined as separate episodes of definite high fever.
Periodic fever is defined as recurrent episodes of definite fever that occur at regular or predictable intervals.
Influenza-like illness is defined as fever with prominent respiratory symptoms of cough and sore throat without remarkable respiratory signs such as dyspnea or rales and usually including myalgia. This syndrome is discussed in Chapter 7. “Viral syndrome” is an inadequate working diagnosis, as it lacks the specificity of the previous diagnostic phrases.
Pseudofever is the term used when parents bring a child to medical attention for temperatures that are above their concept of “normal” but do not meet the definition of fever given earlier; that is, temperature greater than 37°C (98.6° F) but less than 38°C (100.4° F) oral or 38.4°C (101°F) rectal. Because this is not true fever at all or is just diurnal or exercise temperature variation, the clinician should try to avoid reiterating the parent’s use of the word fever. It is helpful to explain to the parents that this is a normal variation of temperature, especially when the child’s appearance and examination are normal, as discussed later.
Fever without Localizing Signs
Definition
Fever without localizing signs is a tentative or working diagnosis and is best defined as:
Documented fever (rectal temperature of 38.4°C [101°F] or higher)
Brief duration (less than or equal to 10 days and usually only a few days)
No localizing signs sufficient to account for the fever
Normal urinalysis including microscopic examination and negative urine culture.
A urine dipstick examination that includes testing for leukocyte esterase and bacterial products such as nitrites might be substituted for a microscopic study. However, if a clean-catch urine has been obtained, the additional time involved in the more thorough study is usually minimal. It is not rare to discover important urinary tract disease when a proper urinalysis is done after a number of febrile illnesses attributed to respiratory infections (see Chapter 14).
The preliminary diagnosis of fever without localizing signs should be reserved for patients who do not appear seriously ill. Suspected septicemia should be the preliminary diagnosis if the patient is seriously ill or hypotensive.
Fever without localizing signs (FWLS) also should be defined to exclude the newborn and young infant in the first 2 months of life (Table 10-2). Neonatal fever is a better preliminary descriptive diagnosis and raises the question of sepsis, discussed in Chapter 19.
Fever without localizing signs as a preliminary diagnosis helps to avoid using more exact, but less certain, diagnoses. Other descriptive diagnoses with a similar meaning include “fever only,” “undifferentiated febrile illness,” “undiagnosed fever,” and “fever, not seriously ill-appearing.” All of these preliminary diagnoses are acceptable, but the term fever without localizing signs is being used more widely and is readily understood.
Inadequate Definitions
Although “flu syndrome” has been used to describe this pattern, it is not accurate, because influenza-like illnesses characteristically have prominent respiratory symptoms, especially cough and sore throat, as described in Chapter 7. “Viremia” has also been used to describe this syndrome, but this term is neither useful nor accurate, because documentation of a virus in the blood is rarely possible using currently available methods.
“Upper respiratory infection” (URI) is also an inappropriate diagnosis, because these patients do not have sufficient upper respiratory signs or symptoms to account for the magnitude of the fever. URI is too vague a diagnosis even when respiratory symptoms are present.
Clinical Course
The preliminary diagnosis of fever without localizing signs may be changed to another diagnosis as the course of the illness evolves. There are several courses the illness may take:
Development of new signs. When these signs occur, the physician may make a diagnosis of a specific localized infection, a viral exanthem such as roseola, or a working diagnosis of fever with nonspecific signs, indicating an area for investigation. Fever with nonspecific signs such as splenomegaly is discussed in a subsequent section of this chapter.
Persistence of fever. When fever persists for more than 10 days, the working diagnosis of prolonged unexplained fever or fever of unknown origin (FUO) is applicable, as described in a later section.
Complete, uneventful recovery. When the patient recovers uneventfully from the illness, the retrospective diagnosis can be undifferentiated febrile illness or self-limited febrile illness, as discussed later. In one study of 102 children with fever without localizing signs, about 70% had an uneventful recovery, whereas about 30% developed signs of a specific infectious disease.31
“Self-limited febrile illness” is a retrospective descriptive diagnosis used for a fever persisting for several days from which the patient recovers without antibiotic therapy and without any localized infection, rash, or other signs.32 A urinary tract infection must be reliably excluded to make this diagnosis. If antibiotic therapy is used, the term self-limited is not appropriate. This syndrome is usually presumed to be a viral illness and has also been called “3-day fever,” “acute undifferentiated febrile illness,” “systemic infection,” and “febrile illness of short duration.”
Causes
Common Viruses
Coxsackieviruses and echoviruses are probably the most common causes of self-limited febrile illnesses in the United States.33 Parainfluenza viruses also appear to be a common cause of this syndrome.34 Adenovirus and influenza virus sometimes cause
this syndrome (especially in young infants) but more frequently result in sufficient respiratory symptoms to be classified as an influenza-like illness.35
this syndrome (especially in young infants) but more frequently result in sufficient respiratory symptoms to be classified as an influenza-like illness.35
Bacteria
Many bacterial infections can be self-limited without antibiotic therapy (ear infections, streptococcal pharyngitis). Even bacteremias such as pneumococcemia, discussed later in this section, can be self-limited.
Uncommon
In endemic areas of the United States, arboviruses are an occasional cause of self-limited febrile illnesses during the season of the year that humans can be exposed to the arthropod vector. Such viruses include La Crosse encephalitis virus (California encephalitis virus) and equine encephalitis viruses.
Ehrlichiosis
There are two forms of ehrlichiosis: human monocytic ehrlichiosis (HME) and human granulocytic ehrlichiosis (HGE). HME is much more common. It is caused by a rickettsial organism known as Ehrlichia chaffeensis that can cause fever in humans after a tick bite.39,40 Formerly recognized only in dogs, the human disease was originally described as resembling Rocky Mountain spotted fever (RMSF) without a rash, and therefore was sometimes referred to as Rocky Mountain spotless fever. This name, for several reasons, should not be used. First, the same name is being applied to patients who become infected with Rickettsia rickettsiae and develop symptoms of RMSF but without a rash. Second, some patients do develop a rash during the course of ehrlichiosis. In fact, rash seems to be more common in children than it is in adults and was found in two-thirds of childhood cases of HME in the largest series published to date.41
Fever and myalgia are seen in most patients, and about a fourth have headache, vomiting, and diarrhea.41 Laboratory findings that suggest the diagnosis include thrombocytopenia, lymphopenia or leukopenia, anemia, and hyponatremia. Liver enzymes are commonly mildly elevated. This diagnosis needs to be seriously considered in patients who present during “tick season” with fever, myalgia, and headache, with or without rash, who have suppression of one or more cell lines on complete blood count. Doxycycline is the drug of choice in all age groups.42 The illness can be quite severe and even life-threatening.43 Some patients with long-term cognitive or subtle neurologic abnormalities have been identified,41 but most make a complete recovery with appropriate therapy. The diagnosis is established by demonstrating a fourfold rise in antibody titers to E. chaffeensis, or by an acute titer over 1:64 in a patient with a clinically compatible illness. Polymerase chain reaction (PCR) is very sensitive but is not widely available.43a Morulae (cytoplasmic vacuoles containing the organisms) are usually not demonstrable. Therapy must be started before the diagnosis is confirmed. HGE, caused by Anaplasma (formerly, Ehrlichia) phagocytophila,43b clinically resembles HME but granulocytopenia, rather than lymphopenia, is observed. Because this pathogen is carried by the Ixodes species of tick, its distribution resembles that of Lyme disease.43c
Unknown
Unknown or unidentified viruses may be a cause of this syndrome, but most children do not have any virus recovered when studied using available techniques. Other infectious agents such as Epstein-Barr virus (EBV) or cytomegalovirus (CMV) are often not detected, because no specific diagnostic studies are done when the child does not seem very sick and is afebrile within a few days.
Development of Focal Infections
Pharyngitis
Frequently in young children, infections are manifested only by fever, with considerable delay in localization of the infection. Frontal headaches and abdominal pain may develop and provide a clue to the diagnosis. It is wise to perform a throat culture for Group A streptococci in most young children with fever without localizing signs in the first day or two of the fever (see Table 2-4). However, even school-age children sometimes do not develop signs of exudative pharyngitis until a day after the onset of fever (see Fig. 2-4).32 Because of such delays in localization of infection, the physician
should not omit follow-up physical examination in a patient with continued fever on the assumption that the patient has a benign, self-limiting viral illness.
should not omit follow-up physical examination in a patient with continued fever on the assumption that the patient has a benign, self-limiting viral illness.
Pneumonia
Unrecognized pneumonia is one of the most frequent focal causes of fever without localizing signs. Abdominal pain (probably really pleuritic or referred from the diaphragm) and vomiting may mislead the clinician toward the abdomen.
Other
Although the majority of infections observed will not be serious, a few patients will be found to have such serious illnesses as osteomyelitis, septic arthritis, or meningitis. One value of the preliminary diagnosis of fever without localizing signs is the emphasis on the need to repeat the physical exam ination looking for localizing signs of infection.
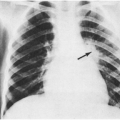
In a 1968 study of hospitalized children, the earliest indication of a localized infection was usually the development of abnormalities on physical examination.31 In fact, about 20% of the 105 patients hospitalized because of undiagnosed fever had evidence of a specific localized infection on the first physical examination after admission. Presumably, they had developed these signs of a specific infection after the physical examination that led to the hospitalization. After throat culture and urinalysis were negative, further laboratory studies rarely provided the first clue to the final diagnosis. A notable exception was the chest x-ray, which revealed six unsuspected cases of pneumonia, usually lobar or segmental, which presumably were pneumococcal.
In children, the most frequent infections that are likely to be recognized by new localized signs are exanthems (especially roseola syndrome, presumed enteroviral rashes, or occasionally rickettsial disease); exudative tonsillitis, otitis media, or stomatitis; meningitis (purulent or aseptic); pneumonia, parotitis or cervical adenitis; and arthritis or osteomyelitis. These localized infections are discussed further in other chapters.
Laboratory Approach
Exposures
In a child older than 2 years, the history and physical examination usually allow the clinician to decide if a laboratory workup is needed. If other family members or school contacts have been having self-limited febrile illnesses, this is an important clue to the probability of a viral etiology, and it is likely that no further laboratory studies are required.
No laboratory test should be done routinely in a child with fever without localizing signs. Sometimes, minor symptoms might suggest some value to a particular test, especially if the general appearance or magnitude of the fever (above 39.5°C [103°F]) is suspicious.
Throat Culture
This may reveal Group A streptococci if sore throat is present early in the illness and the fever is above 38.8°C (102°F), as discussed earlier.
Urinalysis and Culture
This has already been mentioned in the definition of fever without localizing signs. Usually, fever secondary to a urinary infection is accompanied by urinary symptoms, but these may not be recognized in a younger child. The yield for finding a positive urine culture is low (2%) in febrile children,44 but urinary tract infections are important to diagnose and treat.
White Blood Cell Count and Differential
This study may be useful in a teenager with fever and fatigue to detect atypical lymphocytosis in the typhoidal presentation of infectious mononucleosis. It also may be useful for the sicker-appearing child with a higher fever, although leukocytosis is nonspecific and may only stimulate a more careful reexamination or possibly other simple specific studies.
Chest Roentgenogram
In the younger, sicker-appearing child, especially one with leukocytosis and some cough, an unrecognized bacterial pneumonia may be present without many clinical signs. Reexamination after the diagnosis may reveal some slight splinting or tachypnea (beyond that attributable to the fever), but often it does not. A chest roentgenogram was the most useful study that detected a focal infection unsuspected by physical examination in one large study of febrile children.31 This is a lesson frequently relearned even by experienced clinicians. It is reasonable to include a chest x-ray in the evaluation of the child with FWLS, even in the absence of respiratory signs
or symptoms if the fever has been present for three or more days.
or symptoms if the fever has been present for three or more days.
Lumbar Puncture
If there is any clinical suspicion of a CNS infection in febrile infants or young children, a lumbar puncture is indicated. In children beyond infancy, more definite signs are usually present with CNS infections. With increased experience, the physician becomes more skillful at recognizing these findings. However, in situations in which parents are unreliable about observing the young child or seeking care promptly, lumbar puncture should be done for less-obvious medical indications.
Management
In the child over 2 years of age with fever without localizing signs, clinical decisions concerning hospitalization and therapy can be based on the clinical findings, ability of parents to judge changes, and convenience and reliability of follow-up contacts. Usually, the child can be followed by telephone if done carefully, with return visits as necessary for reexamination and without antibiotic therapy if there is no specific likely explanation for the fever. New symptoms or signs may be noted on follow-up, the illness may resolve without a diagnosis, or hospitalization may be needed for further study.
Instructions to Parents
The parents can be told that a bacterial infection is unlikely but that the child should be reexamined if significant new symptoms develop, such as difficulty in arousing the child, difficult breathing, changes in sleep patterns, or pain or tenderness in any area. Parents can make the other clinical observations (such as playfulness) described later. They should be told to note the general appearance and alertness of the child and to call the physician if the child appears worse by the preceding criteria or if a rash is noted. The availability of the physician for follow-up should be emphasized. A return visit in 24 hours should be required for some patients, such as those who have very high fever (>39.5°C). For other children, the parents should be instructed to bring the child back for another examination if the fever is still present in 3 to 4 days. Fluids should be encouraged; solids are unnecessary but permissible. Symptomatic treatment of fever may be indicated, as described earlier in this chapter.
High Fever in Neonates, Infants, and Toddlers
Historical Factors
Beginning in the early 1970s, there was an increasing appreciation of the occurrence of unrecognized (“occult” or “outpatient”) bacteremia in young children (younger than 2 years and younger than 3 months became established as the major age breakpoints). Within recent years, the cutoff age for hospital admission for suspected sepsis in babies with fever has been changed to 2 months in some centers, and to 4–6 weeks in others. Prospective studies have looked at many clinical, laboratory, and epidemiologic variables, so that more accurate information is now available about one of the most difficult clinical diagnostic problems: high fever in young children.
It is not surprising that age breakpoints have become so helpful in assessing the probabilities for various infections, as pediatrics is a field that makes progressively finer age distinctions at younger ages.
Age Differences
Infants younger than 2 months are susceptible to more diseases and are often treated similarly to newborns, but they also may have many of the same diseases as older infants. Early in this period, there is less social eye contact and less social smiling, and as a consequence, the general appearance is harder to evaluate.45 Additionally, babies are unable to communicate, which makes interpretation and diagnosis of their illnesses somewhat trickier. Finally, young infants often develop similar, nonspecific signs and symptoms in response to a wide variety of illnesses; this makes distinguishing the cause of the fever difficult.
Most physicians have routinely hospitalized infants less than 2 months of age because of fever, regardless of the results of preliminary laboratory studies. This policy is discussed later. In one series of 169 children aged 3 months or younger, serious infections were found in 8 (36%) of 22 infants with a temperature of 40°C (104°F) or higher and in 15 (10%) of 147 infants with fever of 37.5°C to 39.9°C (100°F to 103.9°F) who were brought to an emergency room.46
In another series of infants less than 6 months of age, the frequency of serious illness was higher in those with very high temperatures.47 This general correlation of magnitude of fever with seriousness
of illness has been found in many studies, but additional clinical and laboratory findings can be used to develop more predictive accuracy than the fever alone. An exception to this rule is a temperature 41.1°C (106°F) or more, which is frequently associated with serious illness regardless of other findings.48 In general, hospitalization can be individualized rather than routine for those older than 1 month old, depending on the findings and the ability and accessibility of the parents (Table 10-3).
of illness has been found in many studies, but additional clinical and laboratory findings can be used to develop more predictive accuracy than the fever alone. An exception to this rule is a temperature 41.1°C (106°F) or more, which is frequently associated with serious illness regardless of other findings.48 In general, hospitalization can be individualized rather than routine for those older than 1 month old, depending on the findings and the ability and accessibility of the parents (Table 10-3).
Causes
Viral Infections
In a study of 182 young infants with fever, enteroviruses (coxsackieviruses and echovirus) were the most frequent cause,49 as is the case in older infants and preschool children.50 During influenza virus outbreaks, high fever and lethargy secondary to influenza virus infections can mimic septicemia in young infants.51
TABLE 10-3. MODIFIED ROCHESTER CRITERIA AND MODIFIED PHILADELPHIA CRITERIA FOR IDENTIFICATION OF FEBRILE INFANTS 28–90 DAYS WHO ARE AT LOW RISK FOR SERIOUS BACTERIAL INFECTION | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
In a study of 258 febrile children in Finland, 66% of whom were less than 2 years of age, fever from respiratory virus infection was just as likely to be as high and to last as long as in serious bacterial infections, with 37% of the children having fever lasting 5 days or longer.52 In another study of very young infants with fever, nonpurulent meningitis (presumably viral) was the most frequent cause found, but nearly 70% of infants had no cause found and presumably had self-limited viral infections.53
Focal Bacterial Infections
In one study, urinary tract infections were a frequent cause of fever in young infants, particularly
in uncircumcised boys.49 Salmonellosis, with or without diarrhea, was also observed in this study. Other studies have indicated other focal infections such as bone and joint infections.
in uncircumcised boys.49 Salmonellosis, with or without diarrhea, was also observed in this study. Other studies have indicated other focal infections such as bone and joint infections.
Occult Bacteremia
Occult bacteremia is a condition in which a young child is febrile, physical examination reveals no obvious source for the fever, and the child is judged well enough to be managed as an outpatient, but blood culture obtained at the time of evaluation yields a pathogenic bacterium. This condition is most commonly caused by the pneumococcus, but cases of unsuspected bacteremia with meningococcus, Haemophilus influenzae type b, nontyphi Salmonella species, and other bacteria also occur. The incidence of occult bacteremia secondary to Hib has decreased because of the widespread use of conjugated Hib vaccine. The prognosis of occult bacteremia has, therefore, changed; this is discussed in more detail later. Occult bacteremia is an important cause of fever in infants up to about 2 years of age.54,55,56 The child typically has high fever without localizing signs, although a few patients have upper respiratory findings and a few have had a seizure. Historically, the incidence of occult bacteremia in children between the ages of 6 and 24 months who present with FWLS has been estimated to be approximately 5%; a more recent, large study found a lower incidence of 1.9%.57 To a certain extent, the prevalence of occult bacteremia in a study of children with FWLS depends on the stringency of the inclusion criteria used in the study. Bass et al. found that about 16% of children in their study had occult bacteremia; their study enrolled only children with fever greater than 39.5°C and a white blood cell (WBC) count greater than 15,000/μL, or children with fever greater than 40.0°C.58 However, the overall incidence of occult bacteremia continues to decline based on the widespread use of conjugated vaccines against both Hib and Streptococcus pneumoniae.
Marked leukocytosis (greater than 25,000/μL) is often found with pneumococcal bacteremia. In one series of 111 infants and children with pneumococcal bacteremia, 41 (37%) had a WBC count higher than 25,000/μL.54 There were fifteen patients with no clearly defined source of the bacteremia, and seven of these had an initial WBC count higher than 24,000/μL. In another series of twelve patients with unexpected pneumococcal bacteremia, all had a white cell count higher than 20,000/μL.55 In another report of twenty-two patients with occult pneumococcal bacteremia, nine had WBC counts higher than 20,000/μL.56 Although very high WBC counts are frequently seen with pneumococcal bacteremia, this finding is not universal; thus one is not able to rule out bacteremia based on a lower WBC count.
Febrile convulsions occasionally occur in pneumococcal bacteremia.54,55,56 Hyponatremia, petechiae, and vomiting were also observed in some of these patients.54,55,56
The prognosis of occult bacteremia is changing; in general it is better than might be expected given the presence of bacteria in the blood stream. Older, retrospective studies warned the physician of a 5–10% risk of bacterial meningitis, a 10% risk of localized bacterial infection, and another 30% risk of continued bacteremia and fever.59 One study that helped to prompt the widespread use of intramuscular ceftriaxone in the outpatient setting estimated that the risk of meningitis in occult bacteremia was 9.8% in patients given no antibiotic therapy, 8.2% in patients treated with oral antibiotics, and 0.3% in those given parenteral antibiotic treatment.60 This study was a Bayesian meta-analysis of published studies available at the time. Of importance is that all cases of bacterial meningitis that resulted from untreated or insufficiently treated occult bacteremia were caused by Hib. Because Hib is no longer the second most common cause of occult bacteremia, the outcome of pneumococcal bacteremia has become the statistic of most interest. The course of unrecognized pneumococcal bacteremia is varied, but it is better than that of occult bacteremia due to Hib or to the meningococcus. Hib is about 12 times more likely than S. pneumoniae to cause meningitis in patients who present with occult bacteremia.61
Two recent large studies paint a clearer picture of risks in the current environment. The first was a prospective evaluation of children between 3 and 36 months of age with fever of 39°C or higher who had no obvious focus of infection and were discharged home from the emergency department. This study focused primarily on determining the prevalence of occult bacteremia in this population. Blood cultures were obtained from 9,465 children who fit this description; (1.6%) children had blood cultures that were positive for pathogenic organisms.62 The second was a retrospective study of FWLS in children from 2–24 months of age in a
pediatric emergency department. This study found bacteremia in 111 (1.8%) of 5,901 children; 83% were caused by the pneumococcus, and 96% of those underwent resolution of their bacteremia without therapy.57 Salmonella was the second most common isolate, accounting for 5.4% of occult bacteremia, followed by Group A streptococci. No cases of either Hib or meningococcal occult bacteremia were seen.
pediatric emergency department. This study found bacteremia in 111 (1.8%) of 5,901 children; 83% were caused by the pneumococcus, and 96% of those underwent resolution of their bacteremia without therapy.57 Salmonella was the second most common isolate, accounting for 5.4% of occult bacteremia, followed by Group A streptococci. No cases of either Hib or meningococcal occult bacteremia were seen.
Bacteremia caused by the meningococcus, Salmonella, Staphylococcus aureus, and even gram-negative enteric bacteria are uncommon causes of fever in infants.63,64,65 Hib bacteremia has become very rare. The child typically becomes sicker within hours or develops localizing signs. The clinical manifestations of septicemia with these bacteria are discussed later in the section on septicemia and bacteremia.
Clinical Evaluation
The separation of febrile infants with early serious infections from those with benign self-limited illnesses is one of the most difficult decisions in the care of children. Every experienced pediatrician has known the feeling of not recognizing an early serious infection.
Prospective studies have been done, especially by McCarthy and colleagues, to help give guidelines for both clinical and laboratory evaluation.66,67,68,69,70 Unfortunately, rapid and unexpected change is typical of illness in young children. No laboratory tests or clinical observations detect all of the children who progress to a serious illness, so that follow-up clinical observations by the physician and parents are essential.
What observations help the parent or physician detect a worsening course? In studies directed at this question, the child’s playfulness and eye contact were helpful guides.69,70 Other useful criteria were the degree of alertness and of consolability, essential observations needed to determine potential severity. Other definable components include the infant’s use of its eyes to observe people, spontaneous arm and leg movement, appropriate smiling or crying, playing, sucking, reaching, and vocalizing. Generally, patients as a group can be divided into those who are at high risk and those who are at low risk of bacteremia or invasive disease. However, this ability does not extend to the individual patient level; in other words, some children initially designated as being at low risk for serious infection prove to develop serious infection, and some children initially thought to be at high risk prove to have trivial self-limiting illnesses.
Duration of Fever
In young children at risk for occult bacteremia, the duration of fever correlates inversely with the presence of bacteremia. A prospective study of 6,619 children with FWLS showed that a greater proportion of those with fever for less than one day had positive blood cultures than those with fever for greater than one day.71 The incidence in those with fever for less than 2 days, in turn, was higher than for those with fever for longer than 2 days. Presumably, patients with occult bacteremia become ill quite suddenly and seek medical attention more quickly than do patients without bacteremia.
Afebrile Bacteremia
In a review of 182 children with bacteremia during an 18-month period, 24 (13%) were afebrile at the time of evaluation in the emergency room.72 However, half had received recent antipyretics because of a history of fever. Of the five afebrile children who also had no recent history of fever, localizing signs of meningitis, pneumonia, or orbital cellulitis were present in four, and the other appeared clinically “toxic.” This study serves to remind clinicians what they already know: Absence of fever can accompany clinical findings of significant infection.
Response to Acetaminophen
Improvement in clinical appearance and reduction of fever after an appropriate dose of acetaminophen does not exclude the possibility of severe illness.73 A prospective trial of 154 children with FWLS, 19 of whom had positive blood cultures, showed that the response to acetaminophen did not differ between the groups; furthermore, the appearance of the child when afebrile (often cited as a differentiating factor by practicing physicians) was shown to be identical.74 Only those with meningitis remained ill appearing when fever was reduced. Perhaps the clinician should be more wary when a child remains ill appearing after fever reduction, but response to acetaminophen or ibuprofen can be misleading and thus should not be used as a diagnostic test.
Laboratory Approach
White Blood Cell Count and Differential
Most prospective studies have confirmed the value of the WBC count that was observed in retrospective
reviews of “outpatient bacteremia” and occult pneumococcemia in the 1970s.53,75,76,77,78,79,80 It appears to be the simplest and most reliable of the laboratory tests, is applicable to office practice, and can be used together with clinical and social factors either to help decrease the concern for serious disease or to stimulate further laboratory studies.
reviews of “outpatient bacteremia” and occult pneumococcemia in the 1970s.53,75,76,77,78,79,80 It appears to be the simplest and most reliable of the laboratory tests, is applicable to office practice, and can be used together with clinical and social factors either to help decrease the concern for serious disease or to stimulate further laboratory studies.
A marked leukocytosis (greater than 25,000/μL) with a predominance of neutrophils is often taken as presumptive evidence for a bacterial infection, although this criterion has not been adequately studied in a prospective fashion, because the final etiologic diagnosis is often unknown. The most widely used screening values (in conjunction with high fever) are a count of 15,000/μL or more and total segmented neutrophils of 10,000/μL or more.
Bass et al.’s careful, multicenter, prospective study of children with high fever clearly showed an increased risk of occult bacteremia with increasing peripheral WBC count. Bacteremia was documented in none of 99 children with a WBC count less than 10,000/μL; in 21 (8%) of 265 children with a WBC count from 10,000/μL to less than 20,000/μL; in 30 (24%) of 127 children with a WBC count from 20,000/μL to less than 30,000/μL; and in 9 (43%) of 21 children with a WBC count more than 30,000/μL.58
Vacuolization and Toxic Granulation
C-Reactive Protein
Chest Roentgenogram
The principles discussed earlier for the child over 2 years of age with fever without localizing signs also apply to the infant with fever. In the older infant, unsuspected pneumonia may be discovered by a chest x-ray to be the cause of high fever and marked leukocytosis. Outpatient management may be practical for some older infants.
Lumbar Puncture
Meningitis is likely to be subtler in terms of clinical findings as the age of the infant decreases. A lumbar puncture may also reveal nonpurulent meningitis, giving an explanation for the fever that can be managed as described in Chapter 9. Lumbar puncture should not be withheld for fear of producing meningitis in a bacteremic child.86
Urinalysis and Urine Culture
A collaborative study to determine the frequency of urinary infection in febrile infants indicated a rate of 4%, all in girls.87 Another study found uncircumcised boys to be at higher risk than circumcised boys.49 As discussed in Chapter 14, bagged urine specimens often reflect the periurethral flora. When there is no indication of the source of the fever, especially if the temperature is 103°F or higher, a catheterized urine for microscopic study and culture is reasonable. Although the yield is low,87 it is the young infant who is at highest risk for renal damage from unrecognized infection, especially if there is an underlying congenital urinary tract anomaly (Chapter 14).
Outpatient Blood Cultures
Studies of outpatients in the early 1970s showed that blood cultures were positive in a small percentage of children with fever, particularly those under 2 years of age with higher fever (104°F, 40°C) or leukocytosis (greater than 20,000/L).63 S. pneumoniae and H. influenzae were the most frequent species detected, but Group A streptococci, S. aureus, Salmonella, and meningococci were also detected, along with a number of skin contaminants. One emergency room study reported that 25% of the positive blood cultures were attributable to contaminants.64 In the newer study mentioned previously, however, only 2.1% of positive blood cultures were thought to be secondary to contamination.57 Using continuously monitored blood culture systems such as BACTEC may be helpful in differentiating true pathogens from contaminants: The mean time to positivity in patients with true pathogens was 15 hours; it was 31 hours in those with contaminants.57
In other studies, some of the children did not seem seriously ill or had seemingly trivial illnesses, but a high fever (140°F, 40°C) or a high leukocyte count (20,000/μL) was clearly a risk factor.
These studies led to the definition and recognition
of occult bacteremia. Many pediatricians in private practice have been skeptical that outpatient blood culture is superior to careful follow-up, especially because many bacteremic children do not develop serious illness rapidly or without clinical findings on follow-up examination. In one study of 4,151 visits to private practices, with 145 children aged 3–24 months deemed “at risk” for occult bacteremia, blood cultures, sedimentation rates, and WBC counts were seldom used, but hospitalizations were rare, and no deaths or notable sequelae were encountered.88
of occult bacteremia. Many pediatricians in private practice have been skeptical that outpatient blood culture is superior to careful follow-up, especially because many bacteremic children do not develop serious illness rapidly or without clinical findings on follow-up examination. In one study of 4,151 visits to private practices, with 145 children aged 3–24 months deemed “at risk” for occult bacteremia, blood cultures, sedimentation rates, and WBC counts were seldom used, but hospitalizations were rare, and no deaths or notable sequelae were encountered.88
Bacteremia with H. influenzae is certainly a potentially serious disease. One study of 69 children with culture-proven Hib bacteremia found that on follow-up, 36 (52%) of the children were still clinically ill; 17 developed meningitis, 5 got pneumonia, 3 had epiglottitis, 5 had cellulitis, 3 had septic arthritis, and 3 were persistently febrile. Even the patients who felt well at follow-up were apparently not safe; 3 (9%) of 33 were still bacteremic despite being afebrile, and 5 (15%) developed a secondary focus of infection.89 This is in sharp contrast to the 96% spontaneous cure rate observed with pneumococcal bacteremia in the retrospective emergency department study cited earlier.57
The criteria have not been entirely clarified for the use of blood cultures in the office of the private pediatrician or the walk-in clinic, which may deal with a different patient population. One of the best analyses of this dilemma deserves quotation: “When good observation or a well-functioning relationship with the medical system does not exist, the blood culture may serve an ‘administrative’ function by bringing to attention those infants in need of prompt recall and reevaluation.”90
For some physicians, “obtaining a blood culture binds anxiety” (by giving the feeling that one is ‘doing something’), but it is not by itself therapeutic for the infant.”90 At the present time, most investigators emphasize that blood cultures obtained selectively, using some clinical and laboratory criteria, do result in the detection of infants at higher risk of developing serious disease. However, it has also been noted that the same criteria for obtaining blood cultures can be applied for using outpatient antibiotic therapy until culture results are known.91 The efficacy of such treatment is unknown, and the patient must still be followed carefully. The rate of false-positive blood cultures (2% to 40%) and the cost of cultures also make them only another test, the value of which depends on the clinician’s judgment and experience with its use. Sloppy, injudicious, and unwise use and interpretation of blood cultures in the setting of fever can lead to enormous costs, both financial and psychological. In one extreme example, 41% of positive blood cultures contained only contaminants; despite this, phone calls, return visits, extra diagnostic procedures, and hospital admissions resulted, amassing a cost of $78,904, or $642 for every true pathogen isolated.92 It should be noted that, in most centers, the rate of false positive blood cultures is less than 5%.
Antibiotics in Febrile Outpatients
Antibiotics are usually not indicated in children over 2 years of age with fever without localizing signs, because most of these illnesses are viral. If an antibiotic is used and fever persists, there may be continued confusion and changing of antibiotics. In addition, antibiotics may disguise localizing signs of infection and sometimes allow unrecognized progression of tissue damage. Antibiotics do not reduce bacterial complications of acute viral diseases. In addition, antibiotics can be a cause of persistent fever and rarely may have serious, even life-threatening, toxicities.
Criteria for Antibiotic Use
In children younger than 2 years of age, it has been suggested that a nontoxic infant who meets the criteria for an outpatient blood culture merits antibiotic therapy.91,93 Some studies of antibiotics in febrile infants have been done. In the oldest studies, an injection of procaine-benzathine penicillin followed by oral penicillin V was better than a placebo for nonhospitalized infants at high risk of bacteremia.94 Oral amoxicillin was no different from a placebo in another study of febrile infants.95 A meta-analysis of all published trials that compared oral antibiotics (usually amoxicillin) with no treatment in children whose cultures eventually grew S. pneumoniae showed a modest benefit of oral antibiotics. A total of 656 cases of pneumococcal occult bacteremia were identified. The incidence of serious bacterial infections was 3.3% in the group given oral antibiotics versus 9.9% in those left untreated. Meningitis developed in 7 (2.7%) of 257 children in the untreated control group, but only in 3 (0.8%) of the 399 children given oral antimicrobials.96 The numbers concerning the incidence of meningitis were too small to reach statistical significance, but
a trend was demonstrated. It must be remembered that this study included only those with proven bacteremia, not a population of children with FWLS, the majority of whom do not have occult bacteremia.
a trend was demonstrated. It must be remembered that this study included only those with proven bacteremia, not a population of children with FWLS, the majority of whom do not have occult bacteremia.
It has become increasingly popular to give children who present with high fever and/or a WBC count over 15,000/μL an injection of intramuscular ceftriaxone. Although emergency department physicians could not agree about which patients need WBC counts, which patients need blood cultures, or which patients require other diagnostic evaluations, 75% of them agreed on IM ceftriaxone as their first choice for patients at high risk for occult bacteremia.97 The popularity of IM ceftriaxone in the setting of FWLS stems from a couple of studies: (1) The study that cited a 9.8% risk of progression to meningitis in children without therapy of occult bacteremia also found that none of 139 patients who received IM ceftriaxone developed meningitis.97a (2) A multicenter prospective trial of IM ceftriaxone versus oral amoxicillin for patients with occult bacteremia reported that there were three patients with persistently positive blood cultures and two children with positive spinal fluid cultures among those who were randomized to the oral amoxicillin arm, but none in the ceftriaxone group. The paper went on to state that probable or definite serious bacterial infections occurred in six children in the oral amoxicillin group, but in only three children in the ceftriaxone group. Finally, fever persisted longer in those who received oral amoxicillin.98
How do we account for these differences, if ceftriaxone is not, indeed, superior? In the first study, all the patients who developed meningitis had Hib infection. Occult bacteremia due to Hib, as mentioned earlier, is a disappearing disease. In the second study, the children in the parenteral group received 50 mg/kg of ceftriaxone, whereas the children in the oral treatment group received 20 mg/kg/dose of amoxicillin for 6 doses (60 mg/kg/d for 2 days). These dosages are probably not equivalent. In the era of increasing penicillin resistance among isolates of the pneumococcus, a larger dose of amoxicillin would be prudent to ensure levels that exceed the minimum inhibitory concentration of most isolates. A careful reading of the second study also reveals some problems with outcome assignment. One of the patients in the amoxicillin group grew S. pneumoniae from spinal fluid that was obtained before amoxicillin was administered. Additionally, two children in the ceftriaxone group had cerebrospinal fluid (CSF) pleocytosis at the time of follow-up; in the setting of proven bacteremia, these findings are indicative of bacterial meningitis, despite negative CSF cultures. Reassigning these patients gives a total of five serious bacterial infections (four meningitis) in the ceftriaxone group and five serious bacterial infections (two meningitis) in the amoxicillin group, results that surely cannot be construed to support the supposed superiority of ceftriaxone.98a
Moreover, the incidence of bad outcomes from occult bacteremia is decidedly lower in the post-Hib era. Thus, differences in efficacy between IM ceftriaxone and oral amoxicillin, even if they exist, are not likely to be clinically significant. One large meta-analysis comparing the two regimens concluded that the differences are so small that a study that included more than 7,500 bacteremic children (approximately 300,000 with FWLS) would be required to demonstrate superiority of one regimen over the other.98b Drawbacks to the widespread use of IM ceftriaxone, on the other hand, are obvious and include pain, expense, potentially serious drug reactions, and widespread increases in antibiotic resistance patterns of common bacteria. Giving IM ceftriaxone in this setting effectively asks the parents to judge whether they think their child is developing partially treated bacterial meningitis, a daunting task even for the trained professional.98a There is no support for the unfortunately widespread practice of administering IM ceftriaxone without obtaining blood cultures.
The problem becomes stickier still if one extends it to its true setting; that is, in clinical practice, the physician doesn’t know which children with fever are bacteremic and which are not. In this setting, the use of either IM ceftriaxone or oral amoxicillin is not convincingly effective. Both forms of treatment trend toward being beneficial, but statistical significance is not reached. A meta-analysis of empiric antibiotic therapy for children with fever reached the conclusion that approximately 414 children would need to be treated unnecessarily in order to prevent 1 serious bacterial infection.99 It is clear that widespread use of empiric antibiotics for children with FWLS is likely to treat many children who cannot possibly benefit from the therapy.
Recommendations
An expert panel met and published practice guidelines in 1993.100 Their recommendations were
based on a review of available literature and discussion of the issues. They recommended the following: (1) hospitalization and intravenous antibiotics for all children less than 28 days and for all toxic-appearing infants and children, (2) febrile infants between 28 and 90 days of age may be defined to be at low risk by specific clinical and laboratory criteria (Table 10-3) and, if so judged, can be managed as outpatients if close follow-up is ensured, (3) children 3–24 months old with fever less than 39°C without a source need neither antibiotics nor laboratory tests, (4) children 3–24 months of age with fever greater than 39°C and a WBC count of 15,000/μL or greater should be considered for blood culture and antibiotic treatment pending culture results, and (5) urine cultures should be obtained in all boys 6 months of age or less and all girls 2 years of age or less who are treated with antibiotics.
based on a review of available literature and discussion of the issues. They recommended the following: (1) hospitalization and intravenous antibiotics for all children less than 28 days and for all toxic-appearing infants and children, (2) febrile infants between 28 and 90 days of age may be defined to be at low risk by specific clinical and laboratory criteria (Table 10-3) and, if so judged, can be managed as outpatients if close follow-up is ensured, (3) children 3–24 months old with fever less than 39°C without a source need neither antibiotics nor laboratory tests, (4) children 3–24 months of age with fever greater than 39°C and a WBC count of 15,000/μL or greater should be considered for blood culture and antibiotic treatment pending culture results, and (5) urine cultures should be obtained in all boys 6 months of age or less and all girls 2 years of age or less who are treated with antibiotics.
The choice of empiric antibiotic for those who are judged to be at highest risk for true occult bacteremia should be either high-dose amoxicillin (80–150 mg/kg/day divided TID) or ceftriaxone 50 mg/kg as a single intramuscular dose, to be given after blood culture and possibly CSF culture is obtained. Follow-up examination is of utmost importance and should be scheduled for the next morning.
Hospitalization
Hospitalization is more likely to be indicated if the child is very young or appears “toxic” or if the parents are too anxious or too unreliable to be alert for changes in the general appearance as described earlier. Symptoms of dyspnea, somnolence, or areas of pain or tenderness also call for hospitalization.
Hospitalization need not be done routinely for the infant less than 2 months of age, as iatrogenic risks and financial costs argue against routine admission.45,101 Infants who are deemed to be at higher risk for severe infection because of prematurity, poor growth, or other parameters are routinely hospitalized. Clinical and laboratory criteria (Table 10-3) for identifying babies over 1 month of age who are at low risk for serious bacterial infection have been identified, studied, and even established as the standard of care in some hospital emergency departments.102,103 The Rochester criteria have a greater focus on the babies’ health history, whereas the Philadelphia criteria place more weight on clinical appearance and laboratory findings (Table 10-3).
Most pediatricians routinely hospitalize babies who develop fever during the first 28 days of life, probably because of a perception that the risk of severe infection is higher in these babies. Strictly speaking, this perception is true. There is no magic shift in immune function that occurs at the age of 29 days, however, and children between the ages of 28 days and 2 months with high fever remain candidates, on a case-by-case basis, for hospitalization. Some authors are attempting to apply the approach of screening with selective treatment even to babies less than 28 days of age. In one study, only one (0.8%) of 131 febrile neonates younger than 28 days who appeared well, had no focal physical examination findings, had a peripheral WBC count of between 5,000 and 15,000/μL, a neutrophil band form count of less than 5,000/μL, a spun urine specimen that had less than 10 WBCs per high-power field, and a C-reactive protein of less than 20 mg/dL had a bacterial infection.104 Further study along these lines will be required before the standard of care is altered. Most experts agree that babies less than 28 days old with fever should undergo a complete work-up for sepsis, be hospitalized, and be given intravenous antibiotics expectantly. Treatment is generally ampicillin plus either gentamicin or cefotaxime. If the reason for hospitalization is related more to parents’ ability to observe or return, then observation without antibiotics is satisfactory.
Fever of Unknown Origin (FUO)
Prolonged unexplained fever is a more precise diagnosis than FUO, particularly when the original definition of FUO is not observed. FUO is a convenient working diagnosis if properly defined. The review and classification of fever by Dechovitz and Moffet in 1968 was stimulated by the prevalent misuse of the diagnosis of FUO in children now classified as having fever without localizing signs.31
Fever of unknown origin was classically defined by three criteria:105
Documented fever of at least 38.4°C (101°F) rectally and usually higher.
Prolonged fever of at least 2 weeks’ duration in some definitions106 and at least 3 weeks’ in others.
Unexplained fever with no diagnosis after simple laboratory tests after 1 week of study in a hospital.
These criteria were developed for use in adults
and at a time when sophisticated radiographic screening techniques were not readily available. These criteria are neither practical nor particularly useful in the modern practice of pediatrics. In general, diagnoses are established much sooner now than they were in the past.
and at a time when sophisticated radiographic screening techniques were not readily available. These criteria are neither practical nor particularly useful in the modern practice of pediatrics. In general, diagnoses are established much sooner now than they were in the past.
Old lists of causes of FUO are more a guide to the utility of early studies than a true list of late-diagnosed diseases. Indeed, the subgroup here called fever with nonspecific signs, discussed later, has clues about anatomic areas to investigate and includes many diagnoses on the old list of causes of FUO.
The list of causes of FUO changes depending on the definition used for “prolonged.” If the duration of fever is set at 7 days, for example, one is likely to include many more cases of respiratory illnesses and other self-limited viral illnesses that lack classic features. If the duration is set to 3 weeks, as in the past, all self-limited infections will be excluded, and the percentage of patients with serious illnesses will increase. It seems to us that a reasonable clinical definition of FUO in childhood would be the presence of fever greater than 38.3°C (101°F) for more than 10 consecutive days in a patient without an obvious focus of infection by physical examination and screening laboratory evaluation.
It is useful to preserve the concept of a syndrome of fever that is documented, prolonged, and unexplained. Many other working diagnoses that describe this syndrome have been used, including fever (or pyrexia) of unexplained origin, fever of obscure origin, fever of undetermined origin, obscure fever, persistent perplexing pyrexia, and prolonged undiagnosed fever. Many of these terms lack an important component of this clinical picture, which is that the fever is prolonged. This is a problem with the currently used appellation, fever of unknown origin, as well, although the term is widely understood. In contrast, fever of recent onset should be regarded as a different syndrome, because it is usually benign and self-limited.
“Pseudo-FUO” has been used for patients with a history of fever that cannot be documented, often in families with stress, misinformation, or behavioral problems.107
Causes
The frequencies of the various possible causes of prolonged undiagnosed fever are considerably different in children than in adults, and children are not included in most reviews of fever in adults. There are only a few reviews to consult for the possible causes of prolonged fever in children.106,107,108,109 The possible causes are described following and are listed in Box 10-1.106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122
The text follows the sequence of the table. For completeness, some very rare possibilities are listed, which often are based on a few case reports. It may seem difficult to see how some of these possibilities were not diagnosed earlier, but retrospective analysis always seems simpler.
Juvenile Rheumatoid Arthritis (JRA)
One of the most common causes of prolonged fever in children is acute rheumatoid disease.109 Rheumatoid arthritis is defined by rheumatologists using rather strict criteria on the basis of definite arthritis involving multiple joints and lasting over a period of at least several months. In adults or older children, rheumatoid arthritis usually presents as a problem of arthritis. This form of rheumatoid arthritis occasionally resembles acute rheumatic fever or infectious arthritis. In young children, however, rheumatoid arthritis may present as a diagnostic problem of prolonged fever without definite arthritis. This form of the disease is commonly referred to as “systemic-onset juvenile rheumatoid arthritis” or Still’s disease. No specific laboratory test is available, and the diagnosis is a clinical one, often delaying therapy.
Any form of rheumatoid arthritis occurring in children younger than 16 years of age is called JRA.
JRA is especially difficult to identify when it has its onset during or at the end of another illness, as it often does, thus appearing to be a complication or continuation of the first illness.
Because acute JRA with a systemic form of onset is one of the most frequent causes of prolonged fever in children, and because the diagnosis usually must be based on clinical findings, it is discussed in detail later in a separate section.
Other Collagen Vascular Diseases
Systemic lupus erythematosus or acute rheumatic fever are a rare causes of prolonged unexplained fever. Usually, however, polyarthritis or polyarthralgia is prominent, as described in Chapter 16. In polyarteritis nodosa in children, abdominal pain and hypertension are usually present.110
BOX 10-1 Some Causes of Prolonged Unexplained Fever in Children
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree