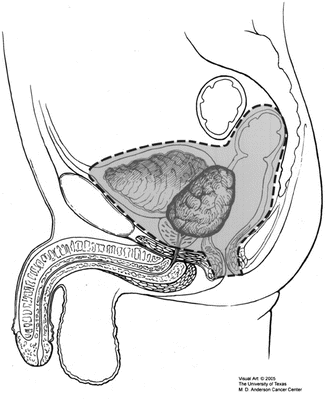
Fig. 14.1
(a) PET/CT scan axial, (b) PET/CT scan coronal, (c) MRI axial, and (d) MRI coronal. Note these images are all taken from the same patient at presentation, demonstrating the utility of PET in highlighting areas of active disease
High-resolution magnetic resonance imaging (MRI) with or without diffusion-weighted imaging (DWI) is the current standard of care for evaluation of pelvic resectability [1, 20, 21]. Good-quality T2-weighted images are essential (Fig. 14.1) [22], preferably accompanied by standardized reporting on involvement of any residual mesorectum, other pelvic viscera, pelvic bones, nerve roots, and major vascular structures [23, 24]. While some assessment can be made of residual superior rectal and pelvic sidewall nodal disease [25], it is worth noting that nodal size and appearance in the pelvis do not necessarily correlate directly with tumor positivity, especially in the context of previous surgery [26]. Dedicated bone nuclear medicine scans and brain imaging are required only if the patient has specific symptoms that warrant this and are not requested routinely [1].
Confirmation
Confirmation of rectal cancer recurrence is obtained by formal tissue biopsy and histological assessment. For intraluminal recurrences, the biopsy can be achieved using rigid or flexible endoscopy. For extra-luminal recurrence, radiologically guided percutaneous biopsy is performed. According to the Beyond TME Collaborative Consensus Statement, where tissue biopsy is not possible or is negative, serial enlargement of a PET-CT enhancing lesion or a serially rising CEA level can be accepted as sufficient proof of recurrence, but only after multidisciplinary team consensus [1]. At our institution, however, histological confirmation is mandatory prior to undertaking major extirpative surgery, radiotherapy, or chemotherapy treatment in this setting [27]. It is rare that biopsy of recurrence cannot be obtained and confirmation will prevent error.
Multidisciplinary Considerations
After the above assessment and investigations are performed, the patient’s case is reviewed at a multidisciplinary tumor board meeting, and the options for management are discussed (Fig. 14.2). Treatment options can be broadly categorized as: palliation or treatment with curative intent.
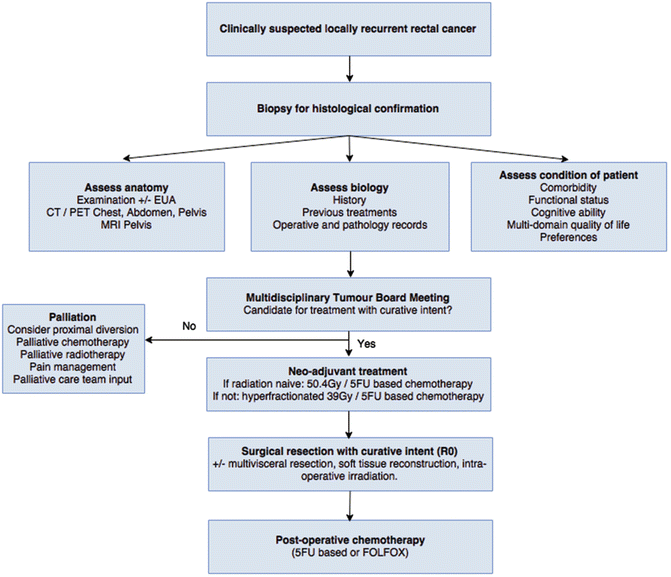

Fig. 14.2
Management algorithm for recurrent rectal cancer at the University of Texas MD Anderson Cancer Center (Adapted with permission from You et al.) [27]. EUA examination under anesthesia, Gy gray, 5FU 5-fluorouracil, FOLFOX 5FU, oxaliplatin, and leucovorin
Palliation may involve proximal fecal diversion if soiling or obstruction is an issue, but usually reserved for patients with limited distant metastatic disease load. A defunctioning loop sigmoid colostomy via a minimally invasive surgical approach is ideal if this is technically achievable. Palliative pelvic exenteration has been performed, but this should only rarely be considered in highly selected patients due to the morbidity of the surgery and no demonstrable benefit in terms of pain control or survival [12, 28, 29]. Chemotherapy and/or radiotherapy with palliative intent may also be considered. Involvement of the pain management team and dedicated palliative care services is important at an early stage, once the decision for palliation has been made. Recently, radiofrequency ablation under CT guidance has been described and appears to induce tumor necrosis and reduce pelvic pain [30].
Treatment with curative intent mandates surgical resection of all known disease, which can range from repeat total mesorectal excision to complete pelvic exenteration with multi-visceral resection, sacrectomy, and reconstruction. If the patient has not had pelvic radiotherapy previously, then neo-adjuvant long course chemoradiotherapy is given (50·4 Gy in 28 fractions and concurrent 5-Fluorouracil (5FU)/capecitabine). In situations where previous radiotherapy had been given, then hyperfractionated radiation therapy is recommended to limit late toxicity (39 Gy in 26 fractions of 1·5 Gy twice daily, with concurrent capecitabine on days of radiotherapy) [31, 32]. These regimens may improve long-term disease-free and overall survival outcomes after surgery, although tumor response rates are lower than in the primary rectal cancer neo-adjuvant setting [27, 33]. Restaging with repeat pelvic imaging should be performed 6–8 weeks after neo-adjuvant treatment, preferably with repeat DWI-MRI and PET-CT [17, 18]. The optimal timing of surgery is unclear, but 6–8 weeks after neo-adjuvant treatment is generally recommended based on data from primary rectal cancer treatment [1]. After surgery, all patients are offered adjuvant treatment with 5FU-based chemotherapy or combined treatment with 5FU, oxaliplatin, and leucovorin (FOLFOX).
Patient Selection for Surgery
“The conundrum for exenteration surgery in the past two decades has been: what can be done safely? Presently, the conundrum presently is not what can be done but what should be done?” [34] Patients with locally recurrent rectal cancer should only be offered surgery if all of the following criteria are fulfilled:
- 1.
Complete R0 resection is technically possible based on imaging.
- a.
Absolute technical contraindications to resection are unresectable extra-pelvic disease, para-aortic node involvement, bilateral sciatic nerve involvement, circumferential bone involvement, and lumbar spine involvement (above L5/S1) [1].
- b.
- a.
- 2.
There is no unresectable distant metastatic disease.
- 3.
The patient is medically fit to undergo the procedure.
- 4.
The patient is cognitively able to engage in a shared decision-making discussion and give reasonable informed consent [23].
Preparation for Surgery
Informed Consent
After multidisciplinary tumor board recommendations have been finalized, a face-to-face meeting between the primary colorectal surgeon and the patient accompanied by a support person (such as a close family member) is scheduled, and formal informed consent is obtained. There may be multiple options offered to the patient, and each of these needs to be discussed fully outlining the benefits and risks. The consent discussion should also include details of planned (and potentially unplanned) stomas and the possibility of the surgeon finding unexpected metastatic disease intraoperatively which is unresectable and would lead to the procedure being abandoned. As alluded to previously, a shared decision-making process on the part of the attending surgeon and the patient is required and should be clearly documented.
It is usual for the patient to also discuss their management with a medical oncologist, a radiation oncologist, and any other surgical services that are expected to be involved during the operation. Involvement of a cancer nurse specialist or care coordinator, an ostomy nurse, and relevant allied health staff such as cultural support workers and interpreters is prudent.
Medical Optimization
Given the magnitude of the surgery to be undertaken, the management of all relevant medical comorbidities should be optimized, with the help of perioperative care physicians and the involvement of anesthetists, preferably with experience in this type of surgery. In patients who require it based on screening assessments, preoperative nutritional support and pre-habilitation programs should be undertaken [41]. There is a high risk of intraoperative hemorrhage, and any anticoagulants the patient is on preoperatively should be managed accordingly.
Coordination of Multidisciplinary Team
Depending on the anatomy of the surgery to be performed, a multidisciplinary team of surgeons may be required. Anticipated involvement based on preoperative assessment should result in the patient being seen and consented by the relevant service ahead of time in the outpatient setting. This may result in requests for further imaging required for planning such as a CT angiogram to look at major vascular structures and the inferior epigastric vessels for use in future tissue flap reconstructions or renal excretion scans. Unanticipated need for other surgical services is also possible, but should be avoided by optimal planning, with relevant teams placed on standby ahead of time. The frequent involvement of the urinary bladder and distal ureters necessitates involvement of a dedicated urology service, the members of which will often perform a cystoscopy and place bilateral ureteric stents at the beginning of surgery if this has not been done already. Involvement of the iliac arteries or veins may necessitate reconstruction by a vascular surgeon and sacral, iliac bone, or pubic bone involvement resection and reconstruction by a neurologic or orthopedic surgeon. Finally, abdominal wall and perineal defects may require flap and mesh closure by plastic surgeons. The patient should be marked by an experienced stoma nurse before he or she changes into a hospital gown to facilitate assessment of stoma location in standing, supine, and sitting positions and when the patient is in his or her usual clothes. The patient should be marked bilaterally, and if they already have a stoma on one side, the other side should be marked. Given the lengthy nature of exenteration surgery, it is important that delays are minimized by effective coordination. All consultants should communicate their expectations, roles, and plans ahead of the day of operation.
Operating Room Setup
Some important elements of the operating room setup are a radiolucent operating table in the correct orientation with the ability to perform angiography; vascular loops and clamps; ureteric stents of different sizes; a selection of prosthetic, biological, and composite mesh of different sizes; and intraoperative radiotherapy equipment. Ideally, all of these tools should be made available for every case as their use may not have been anticipated prior to surgery being undertaken. Equipment requirements should be communicated to the nursing staff ahead of the procedure. Patient safety requires a high degree of teamwork among the professionals undertaking these procedures. Preoperative antibiotics and pharmacologic DVT prophylaxis are used. Blood product availability is confirmed. The nature of procedure and potential concerns are reviewed prior to the procedure.
Surgery
The primary goal of surgery is to achieve an R0 resection, with preservation of function and soft tissue coverage. Total pelvic exenteration is defined as the removal of the rectum, distal colon, genitourinary viscera including lower ureters, internal reproductive organs, draining lymph nodes, and pelvic peritoneum (Fig. 14.3). A sacrectomy may also be included. Anterior pelvic exenteration in the context of rectal cancer surgery is defined as the removal of the upper rectum, reproductive organs, and bladder, but sparing the lower rectum. Posterior pelvic exenteration is the removal of the reproductive organs and rectum, sparing the bladder [43]. Clearly the exact surgery undertaken will depend on the current anatomy and what has been done previously, but the following will present a general overview of salient points.
Patient Position
The patient is usually placed in modified Lloyd-Davies position , with provision for undertaking part of the procedure via a perineal or posterior approach if necessary. Calf compression devices and precautions against pressure areas are routinely used. A folded towel or roll may be placed under the sacrum to improve access to the perineum. If the patient already has a colostomy, this can be sutured, covered with a swab and plastic adhesive dressing so that it is excluded from the wound in the early stages of the procedure. Draping is performed taking into account not only the needs of the primary surgeon but also the optimal exposure and prepping for the other members of the multidisciplinary surgical team. For example, if vein harvest is anticipated for an interposition graft or patch, then the patient’s groin and thigh are prepped. Both thighs maybe prepped circumferentially to allow a lateral thigh or gracilis flap.
Technical Aspects
The first step is digital rectal examination and bimanual examination in women to reestablish tumor height, position, and fixity followed by an exploratory laparotomy. All four quadrants of the abdomen, including the liver, the diaphragm, and the small and large bowel and their mesentery, are visually inspected and palpated. A finding of unexpected metastatic disease which is unresectable should be confirmed with intraoperative frozen section, and the procedure should be abandoned and saved for palliative defunctioning stoma if deemed beneficial by the operating surgeon [44].
In the absence of unresectable metastatic disease, the next step is a complete adhesiolysis , noting that any small bowel adhesions in the region of the recurrent tumor are usually taken en bloc with the specimen. Next, inspection and palpation of the area of local recurrence is performed. Often local resectability is difficult to confirm at this stage, and the preoperative imaging is relied upon to determine this. It is important, however, to avoid making any irreversible decisions or operative maneuvers early in the procedure and before progress is made on mobilizing the tumor. Instead, identification of any “normal” or previously “untouched” planes and dissecting within these is usually the best start. Dissection then continues from easier and softer tissues toward more difficult and rigid areas, with wider margins taken as necessary. Identification of the ureters (which are often more medial in reoperative surgery than usual) and a decision regarding whether one or both can be preserved can often be the defining step in this operation. Ureteric catheters are a useful aid to assist in palpation and identification of the ureters which, if free, can be slung with a vascular loop and preserved. The site of previous inferior mesenteric artery transection is located, and if this was not taken high at the original surgery, then re-resection of is warranted and may provide a lead into the correct posterior plane if this is not invaded directly. Dissection is then extended distally as far as required posteriorly in the total mesorectal excision plane, although often there is little remaining mesorectum, and dissection therefore proceeds in the plane deep to the endopelvic fascia.
Preservation of the integrity of the anal sphincter complex is considered whenever possible based on adequacy of margins and anticipated acceptable functional outcomes. Anterior resection with primary colorectal or colo-anal anastomosis may be performed for high central recurrences where adequate distal margins can be attained; however, it should be noted that functional outcomes may be suboptimal in this setting. In areas of clear tumor invasion, wider excision will be necessary, and abdominoperineal resection is performed with end colostomy. Options for ureteric reconstruction include ureteroureterostomy with or without psoas hitch, ureteric reimplantation, or Boari flap repair. If the situation is not directly reconstructible, then the urologist may proceed with a radical cystectomy ± prostatectomy and subsequent ileal or colonic conduit reconstruction after the specimen is removed. [27].
In cases with extensive lateral pelvic sidewall involvement, iliac vessel identification and dissection are required. If the common, external veins are invaded by tumor, then en bloc resection and reconstruction by a vascular surgeon is required to achieve R0 resection [45]. The detailed technique of lateral pelvic compartment excision has been described in the literature [46]. First, the internal iliac artery and its branches are ligated, ideally distal to the superior gluteal artery to minimize buttock claudication and preserve supply to any potential gluteal flaps. Lymph node dissection is performed including nodal tissue overlying the aortoiliac bifurcation down to the origin of the internal iliac. Proximal and distal control of the internal iliac vein is achieved, and smaller branches are divided prior to division of the main trunk. The lateral and middle sacral vein branches are also ligated and divided, and dissection is continued along the pelvic sidewall bilaterally down to the pelvic floor, with the dissection completed from below if required. Extended lateral pelvic sidewall excision which includes resection of the sciatic nerve may also be necessary [47].
En bloc sacrectomy can also be performed if necessary with neurologic or orthopedic surgeon involvement. Resections above S3 require conversion to the prone position, whereas below S3 a combined abdominal/perineal approach can be used [48]. All gluteus muscular attachments to the posterior aspect of the coccyx and sacrum are dissected free up to a point above the planned site of sacral transection. The sacrococcygeal ligaments are divided off the sacrum, and the anterior sacrum is scored with diathermy at the level of planned transection. Division is possible with the patients still in the supine position using two osteotomes, one placed posterior to the sacrum to protect the skin and the other anteriorly for transection. For sacrectomy above the level of S3, temporary or permanent closure of the abdomen is required prior to turning the patient prone. A posterior midline incision is made from L5 down to the perineal scar or anus. Again the gluteus muscles are dissected away from the sacral attachments, and the sacrospinous and sacrotuberous ligaments are divided, taking care not to injure the sciatic and pudendal nerves which are seen at this point. If the piriformis is involved, this can be excised en bloc before the sacrum is transected at the appropriate level using osteotomes.
If possible, an omental pedicle flap based on the right gastroepiploic artery is fashioned and used to fill the dead space in the pelvis [49]. At least one intra-abdominal drain is often used and placed in the pelvis as well.
Intraoperative Radiotherapy (IORT)
Any margins which are not obviously macroscopically clear are confirmed with intraoperative frozen section. Intraoperative radiotherapy (IORT) is administered selectively with consultation with the radiation oncologist if any resection margins are microscopically positive (R1) based on intraoperative frozen pathology assessment or if the margins were negative but 2 mm or less [50]. At our center, IORT is administered using high-dose rate iridium-192 brachytherapy using a Harrison-Anderson-Mick applicator, with a dose of 10–15 Gy at 1 cm from the radiation source [27]. Level one evidence to support the use of IORT is not yet available [51], but comparative studies have demonstrated improved local control, disease-free survival, and overall survival with no increase in total complications [52–56].
Soft Tissue Reconstruction
There are two wounds that may require reconstruction: the anterior abdominal wall and the perineum, with the latter usually requiring greater consideration. The size of the defect depends on the extent of the resection (determines lateral dimensions) and the patient’s body habitus (determines depth). The complexity of the reconstruction can be compounded by the presence of stomas, ileal conduits, previous incisions and hernias, as well as vascular supply to tissue flaps or lack thereof. Close consultation with experience plastic surgical services is therefore required at all stages.
Flap closure of perineal defects reduces wound complications compared with attempts at primary closure, particularly after radiation treatment [57, 58]. It is possible to use absorbable or biological mesh to reconstruct the pelvic floor, but this technique may still require additional soft tissue coverage, as primary skin closure is not always possible without tension [59]. Selection of which flap to use is based on the size of the defect, the availability of tissue, the characteristics of the patient, and the local expertise available. The most common technique utilized for perineal reconstruction is the vertical rectus abdominis myocutaneous (VRAM) flap based on the inferior epigastric artery, which is a robust flap which usually affords significant tissue bulk [60]. Harvesting of this flap leaves a defect in the anterior abdominal wall which usually requires repair with synthetic mesh or component separation techniques [61]. Other options include gracilis muscle flaps [62], inferior gluteal artery myocutaneous (IGAM) flap [63], gluteal fold flaps [64], anterolateral thigh flaps [65], and full-thickness local advancement flaps [66]. In female patients, vaginal reconstruction can be attempted using modifications of the above flaps, or including local rotation flaps such a modified Singapore flap [67].
Postoperative Care
All patients are managed in a high dependency unit setting immediately after surgery and transitioned to standard ward care as possible. Principles of enhanced recovery after surgery are applied as closely as possible while taking into account the magnitude of surgery undertaken and the unique considerations that go along with this [68]. Median day stay after pelvic exenteration surgery for locally recurrent rectal cancer is 14.5 days [3]. It is critical to monitor for bleeding in the immediate postoperative period followed by the issues of poor wound healing or infection. Urinary tract complications require prompt management to avoid long-term renal deterioration.
Outcomes
Short Term
The complication rate after surgery for locally recurrent rectal cancer is very high, with a meta-analysis published in 2012 documenting an overall major complication rate of 51% [3]. Common complications included wound infection; intra-abdominal and pelvic collections; urinary tract infections; intestinal obstruction; fistulae; incisional, perineal, and para-stomal hernias; urological complications; renal failure; venous thrombosis; pulmonary embolus; and pressure ulcers [69]. In the same study, the median 30-day mortality rate was 4% [3]. When sacrectomy is performed, the morbidity rate increases corresponding to the level of sacrectomy [43].
Long-Term Survival
The median rate of R0 resection in the recent published literature is just over 60% (see Table 14.1) [3, 70]. Five-year overall and disease-free survival after aggressive therapy with curative intent are currently 30% and 36%, respectively, but these have been improving with time due to advances and standardization of adjuvant and neo-adjuvant treatment and better patient selection [27, 74]. Patients who previously received radiotherapy for their primary rectal cancer treatment have worse oncologic outcomes than those who were radiotherapy naive [75], as do patients with multiple sites of fixation in the pelvis [76], but the most important prognostic factor is the completeness of resection [3]. Patients with an R1 resection have an overall 5-year survival of approximately 20%, whereas R2 resection offers no survival benefit compared to palliative treatment [3]. In terms of median survival times, patients with an R0 resection survive on average 38 months longer than those undergoing R1 resection and 53.0 months longer than those undergoing R2 resection (median survival time approximately 1 year) [3].
Table 14.1
Summary of the most recent reported data on long-term survival after planned curative surgery for recurrent rectal cancer
Author | Demographics | Year | n | R0 (%) | 5-year OS (%) | 5-year DFS (%) |
|---|---|---|---|---|---|---|
Harris [70] | Five centers (UK, Australia, New Zealand) | 2015 | 533 | 59 | 28 | 37 |
You [27] | Single center (USA) | 2016 | 229 | 80 | 43 | 47 |
Alberda [71] | Single center (Netherlands) | 2015 | 165 | 55 | 32 | nr |
Neilsen [72] | Single center (Denmark) | 2015 | 115 | 61 | 30 | nr |
Selvaggi [16] | Three centers (Italy) | 2015 | 100 | 61 | 28 | 35 |
Denost [73] | Multiple sites (France)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|