Erectile Dysfunction and Diabetes
Ricardo Munarriz
Abdulmaged Traish
Irwin Goldstein
Male sexual dysfunctions are classified into dysfunctions of libido, problems with emission/ejaculation/orgasm, impotence, and priapism. Erectile dysfunction (ED) or impotence is the most common of the various sexual dysfunctions, and because multiple advances have been realized in understanding the physiologic and biochemical mechanisms involving penile erection and in clinical techniques of improving ED, this chapter will be devoted primarily to the sexual dysfunction of impotence.
Erectile dysfunction or impotence is the consistent inability to achieve or sustain an erection of sufficient rigidity to permit satisfactory sexual intercourse (1). It has been estimated from data collected in 1948 that 10 million American males, or approximately 1 in 10 men, have impotence (1,2). New epidemiologic research in a random, community-based population of aging men suggests that the prevalence of impotence among men 39 to 70 years of age is greater than 50% (3). Contemporary studies indicate that impotence afflicts over 30 million American men.
The prevalence of impotence is particularly high in certain groups of patients. In the previous study (3), aging, treated hypertension, treated heart disease, and treated diabetes were among several physiologic variables found to strongly predict impotence. The prevalence of self-assessed complete impotence was more than three times higher among men with diabetes than among men without diabetes. Impotence is an age-dependent disorder (2,4,5) that affects the diabetic male an average of 10 to 15 years earlier than it does his nondiabetic counterpart. Other studies have demonstrated this higher prevalence of impotence among men with diabetes than in the general male population. Depending on the investigators and the study population, the reported prevalence of impotence in diabetic men has ranged from 35% to 75% (6,7,8,9,10,11,12).
Impotence in men with diabetes develops insidiously over a period of months or years (9). Patients frequently describe diminished penile rigidity and reduced ability to sustain an erection. Impotence, however, is not always a late progressive complication of the disease but can occur early in its natural history. Libido may persist despite poor erectile performance (8,9).
ANATOMY OF THE PENIS
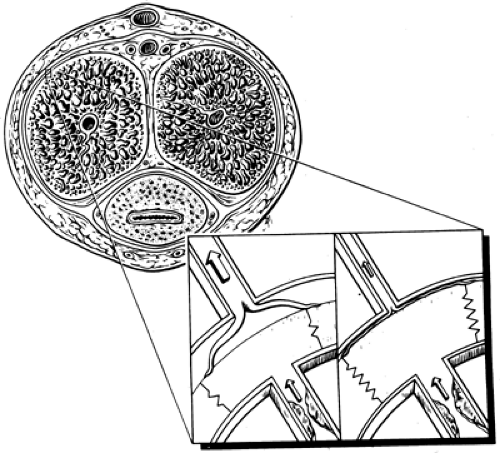
The penis has two paired corpora cavernosa and a corpus spongiosum, which surrounds the urethra and distally forms the glans penis. Each corpus cavernosum is surrounded by a thick fibrous sheath, the tunica albuginea, which is composed of wavy collagen and elastin that allows erectile tissue to expand and elongate. Formation of tunica plaques (Peyronie disease) can result in loss of tunica compliance, penile curvature, and
veno-occlusion dysfunction (4). The erectile tissue consists of multiple, interconnected lacunae lined by vascular endothelium. The walls of the lacunae, the trabeculae, are composed of thick bundles of smooth muscle and a fibroelastic frame (5).
veno-occlusion dysfunction (4). The erectile tissue consists of multiple, interconnected lacunae lined by vascular endothelium. The walls of the lacunae, the trabeculae, are composed of thick bundles of smooth muscle and a fibroelastic frame (5).
The internal pudendal artery enters the perineum through the Alcock canal and gives rise to four terminal branches (dorsal artery, cavernosal artery, bulbar artery, and the scrotal artery). Along with the nerves that run within the Alcock canal (pudendal nerves), the blood vessels in this region are vulnerable to compression injuries such as those that may occur during bicycle riding (13). It has been recently demonstrated that the dorsal artery interconnects with the cavernosal artery (14). This communication is responsible for the success of microvascular penile bypass surgery between the inferior epigastric artery (donor vessel) and the dorsal artery (recipient vessel) in patients with cavernosal artery occlusion. Last, accessory pudendal arteries provide additional blood flow to the corpora cavernosa. Injury to these arteries during radical retropubic prostatectomy may explain why some patients experience ED following successful nerve-sparing procedures (15).
Multiple muscular helicine arteries branch off each cavernosal artery and open directly into the lacunar spaces. Blood drains from the corporal bodies through subtunica venules located between the periphery of the erectile tissue and the tunica albuginea. Subtunica venules coalesce to form larger emissary veins that pierce the tunica albuginea (16,17).
The peripheral innervation of the penis consists of sympathetic nerves arising from the 11th thoracic to the second lumbar spinal cord segments and from parasympathetic and somatic nerves arising from the second, third, and fourth sacral spinal cord segments. Somatic innervation is via the pudendal nerve, which is composed of efferent fibers innervating the striated musculature of the perineum and of afferent fibers from the penile and perineal skin (18).
PHYSIOLOGY OF ERECTION
Central Mechanism of Erection
Penile erections are elicited by local sensory stimulation of the genital organs (reflexogenic erections) and by central psychogenic stimuli received by or generated within the brain (psychogenic erections) (18,19). Recently, specific regions of the brain (inferior temporal cortex, right insula, right inferior frontal cortex, and left anterior cingulated cortex) activated by visual evoked sexual arousal have been identified by positron emission tomography (20). The medial preoptic area and the paraventricular nucleus within the hypothalamus appear to integrate visual (occipital area), tactile (thalamus), olfactory (rhinencephalon), and imaginative (limbic system) input and send neural projections to the thoracolumbar sympathetic and sacral parasympathetic centers of the spinal cord (21). Dopamine and oxytocin are thought to play important roles in mediating the pre-erectile response in the medial preoptic area and the paraventricular nucleus respectively (22). In contrast, the nucleus paragigantocellularis (nPGi) in the brainstem exerts an inhibitory effect on sexual arousal (23). Nerves from the nPGi project to sacral segments of the spinal cord and release serotonin. This has been postulated as the reason specific serotonin reuptake inhibitors (SSRIs) depress sexual function. Because men treated with SSRI drugs most commonly exhibit delayed or blocked ejaculation, cases of premature ejaculation have also been successfully managed with SSRI treatment. The locus ceruleus also exerts inhibitory input via sympathetic nerves that interface with hypothalamic nuclei as well as with the spinal cord. Withdrawal of sympathetic input due to suppressed activity of the locus caeruleus during rapid eye movement (REM) sleep is thought to lead to episodes of nocturnal penile tumescence (22,24). The pudendal nerve, which is the afferent limb for reflexogenic erections, collects somatic sensation from the genital skin. The autonomic nerve fibers that arise from the sacral parasympathetic center (S2-S4) make up the efferent limb for this reflex, innervating the penile smooth muscle. Reflexogenic and psychogenic erectile mechanisms probably act synergistically in the control of penile erection (18,25,26,27,28,29,30,31).
Erection follows relaxation of arterial and trabecular smooth muscle (32). Dilation of the cavernosal and helicine arteries increases blood flow into the lacunar spaces. Relaxation of the trabecular smooth muscle dilates the lacunar spaces, accommodating a larger volume of blood and thus engorging the penis. The expansion of the relaxed trabecular walls against the tu nica albuginea compresses the plexus of subtunica venules (16,33,34). This results in increased resistance to the outflow of blood with increased lacunar space pressure, making the penis rigid. The reduction of venous outflow by the mechanical compression of subtunica venules is known as the corporal veno-occlusive mechanism (Fig. 59.1).
Contraction of penile smooth muscle results in detumescence. Activation of sympathetic constrictor nerves causes an increase in the tone of the smooth muscle of the helicine arteries and the trabeculae, resulting in a reduction of arterial inflow, a collapse of the lacunar spaces with decompression of subtunica
venules (16,33,34), an increase in venous outflow from the lacunar space, and a return of the penis to the flaccid state.
venules (16,33,34), an increase in venous outflow from the lacunar space, and a return of the penis to the flaccid state.
Peripheral Mechanisms of Erection
NEUROGENIC REGULATION OF PENILE ERECTION
Erectile function in the penis is regulated by autonomic (parasympathetic and sympathetic) and somatic (sensory and motor) pathways to the erectile tissues and perineal striated muscles. Three sets of peripheral nerves innervate the penis, the sympathetic nerves, the parasympathetic nerves, and the pudendal nerves.
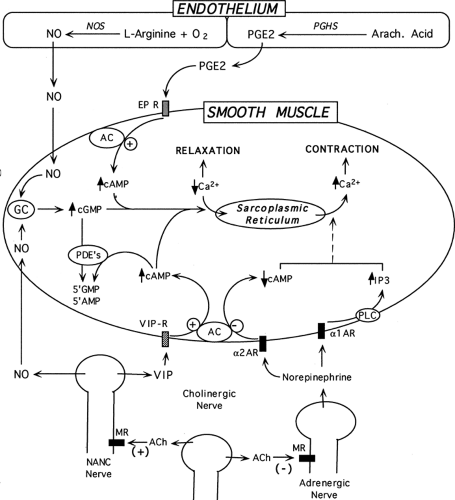
The sympathetic nerves (T10–L2), which are responsible for detumescence and maintenance of flaccidity, project to the corpora, as well as to the prostate and bladder neck via the hypogastric nerves. Postganglionic noradrenergic fibers pass posterolateral to the prostate in the so-called nerves of Walsh to enter the corpora cavernosa medially. Adrenergic tone is crucial in initiating detumescence and in maintaining the flaccid state of the penis, because the smooth muscle of the arteries and cavernosal trabeculae must remain actively contracted. Contraction of cavernosal trabecular smooth muscle in response to norepinephrine is mediated by α1-adrenergic receptors. Prejunctional α2-adrenergic receptors on adrenergic nerves inhibit neurotransmission and provide a self-regulating negative feedback loop for secreted norepinephrine. Cholinergic nerves act on prejunctional muscarinic receptors to also inhibit adrenergic nerve activity.
It is possible that adrenergic imbalance toward vasoconstriction impairs erection. While specific factors that contribute to this imbalance remain unknown, aging and/or associated disease states may cause selective upregulation of specific adrenergic receptor subtypes, resulting in higher efficacy for norepinephrine action. Since norepinephrine is a key modulator of erectile function, it is plausible that α-adrenergic receptor antagonists may prove useful in the treatment of ED. Clinical experience with drugs such as yohimbine and phentolamine have had varying efficacy in men with ED. The role of α-adrenergic receptors in the physiology of penile erection is reviewed more completely elsewhere (35,36).
The parasympathetic nerves, originating in the intermediolateral nuclei of the S2 to S4 spinal cord segments, provide the major excitatory input to the penis and are responsible for vasodilation of the penile vasculature and subsequent erection. Exiting through the sacral foramina, these nerves pass forward lateral to the rectum as the pelvic nerve and synapse in the pelvic plexus with postganglionic nonadrenergic, noncholinergic (NANC) nerve fibers, which travel within the cavernous nerves to the corpora cavernosa. Vasoactive intestinal peptide (VIP) and nitric oxide (NO) are two NANC neurotransmitters that are often co-localized in the same nerves in penile tissue. However, the role of VIP as a modulator of penile erection remains unclear because the experimental results with this peptide on erectile function are inconsistent. Intracavernosal administration of VIP in animals and humans has yielded varying results, ranging from no effect to partial tumescence to full erection. Furthermore, the lack of specific and effective antagonists for VIP hinders experimental investigation concerning its role in erectile function.
The primary mediator of NANC parasympathetic input is NO. The ability of NO, a highly reactive and unstable gas, to regulate a wide array of physiologic functions in mammals has become evident only within the last two decades. Along with carbon monoxide, NO is a unique primary effector molecule with the characteristics of an intracellular second messenger that defies previous classification schemes. It is apparently synthesized on demand with little or no storage, and it directly activates a soluble enzyme (guanylate cyclase) rather than a “traditional” receptor molecule. NO is produced by nitric oxide synthase (NOS), which uses the amino acid L-arginine and mo lecular oxygen as substrates to produce NO and L-citrulline. NO can readily cross plasma membranes to enter target cells, where it binds the heme component of soluble guanylate cyclase. This activation of guanylate cyclase stimulates the production of cyclic guanosine monophosphate (cGMP), with the resultant activation of the cGMP-dependent protein kinase, which regulates the intracellular events, leading to relaxation of trabecular smooth muscle. The levels of cGMP also are regulated by phosphodiesterases, which break down cGMP and terminate signaling. Sildenafil (Viagra) is a potent, selective, and reversible inhibitor of phosphodiesterase type 5, the major enzyme responsible for cGMP hydrolysis in penile erectile tissue (37). Inhibition of this enzyme leads to the increase in levels of intracellular cGMP and enhancement of the relaxation of smooth muscle in response to stimuli that activate the NO/cGMP pathway. This activity may explain the success of sildenafil in the treatment of male ED (38).
Recently, it has become evident that NO interacts directly with other cellular targets, including receptors, ion channels, and pumps, that may modulate the contractility of smooth muscle cells, independently of the cGMP pathway (39,40,41). Thus, NO has intracellular targets in addition to guanylate cyclase that may play a role in the regulation of vascular and trabecular smooth muscle contractility.
The activity of NANC nerves may be modulated by cholinergic nerves, which facilitate NANC relaxation by stimulating the synthesis and release of NO and other vasodilatory neurotransmitters such as VIP. Thus, the release of acetylcholine may coordinate withdrawal of adrenergic input and increase of NANC input by binding to prejunctional muscarinic receptors on adrenergic and NANC nerves (24). In certain disease states, such as diabetes, the ability of the corpus cavernosum to synthesize and release acetylcholine is diminished (42). Such processes may be responsible in part for the compromised erectile function associated with diabetes. Parasympathetic nerves are also vulnerable during surgical procedures, such as abdominoperineal resection of the rectum and radical prosta tectomy (15,43).
The pudendal nerves comprise motor efferent and sensory afferent fibers innervating the ischiocavernous and bulbocavernous muscles as well as the penile and perineal skin. Pudendal motor neuron cell bodies are located in the Onuf nucleus of the S2 to S4 segments. The pudendal nerve enters the perineum through the lesser sciatic notch at the posterior border of the ischiorectal fossa and runs in the Alcock canal (pudendal canal) toward the posterior aspect of the perineal membrane. At this point, the pudendal nerve gives rise to the perineal nerve, with branches to the scrotum and the rectal nerve supplying the inferior rectal region. The dorsal nerve of the penis emerges as the last branch of the pudendal nerve. It then turns distally along the dorsal penile shaft, lateral to the dorsal artery. Multiple fascicles fan out distally, supplying proprioceptive and sensory nerve terminals to the dorsum of the tunica albuginea and the skin of the penile shaft and glans penis.
NON-NEURONAL MODULATORS OF PENILE ERECTION
In addition to neurogenic mechanisms, local paracrine/autocrine factors, with vasoactive and/or trophic effects, profoundly influence the function of the smooth muscle in the penis. These include endothelins, prostanoids, NO, and oxygen.
Endothelin-1 (ET-1), a member of the endothelin family of peptides, is one of the most potent vasoconstrictors known at
this time. Similar to NO, the release of endothelin from the intimal lining of vascular compartments can be induced by shear stress. However, little is known about the physiologic or cellular mechanisms that regulate its production. In human corpus cavernosum, ET-1 is synthesized by the endothelium and elicits strong, sustained contractions of corpus cavernosum smooth muscle. Both major subtypes of endothelin receptors (ETA and ETB) have been identified in penile corpus cavernosum and are distributed on both the endothelium and the smooth muscle. It has also been suggested that endothelin may exert vasodilatory effects at low concentrations through a “super-high” affinity form of the ETB receptor potentially by stimulating NO production. However, the significance of this mechanism in penile erection remains unclear. In rabbit models of disease, ETB receptors in penile corpus cavernosum were upregulated in alloxan-induced diabetic rabbits and downregulated in hypercholesterolemic Watanabe rabbits. Additionally, elevated plasma endothelin levels have been reported in both diabetic and nondiabetic men with ED. Thus, endothelin may contribute to the maintenance of penile flaccidity by providing sustained tone to the trabecular smooth muscle, and alterations in endothelin production may result in impaired erectile function. Several selective antagonists of endothelin receptor subtypes have been developed, but their efficacy and safety in the treatment of ED have not been fully evaluated.
this time. Similar to NO, the release of endothelin from the intimal lining of vascular compartments can be induced by shear stress. However, little is known about the physiologic or cellular mechanisms that regulate its production. In human corpus cavernosum, ET-1 is synthesized by the endothelium and elicits strong, sustained contractions of corpus cavernosum smooth muscle. Both major subtypes of endothelin receptors (ETA and ETB) have been identified in penile corpus cavernosum and are distributed on both the endothelium and the smooth muscle. It has also been suggested that endothelin may exert vasodilatory effects at low concentrations through a “super-high” affinity form of the ETB receptor potentially by stimulating NO production. However, the significance of this mechanism in penile erection remains unclear. In rabbit models of disease, ETB receptors in penile corpus cavernosum were upregulated in alloxan-induced diabetic rabbits and downregulated in hypercholesterolemic Watanabe rabbits. Additionally, elevated plasma endothelin levels have been reported in both diabetic and nondiabetic men with ED. Thus, endothelin may contribute to the maintenance of penile flaccidity by providing sustained tone to the trabecular smooth muscle, and alterations in endothelin production may result in impaired erectile function. Several selective antagonists of endothelin receptor subtypes have been developed, but their efficacy and safety in the treatment of ED have not been fully evaluated.
Nitric oxide is synthesized and released not only by the NANC nerves but also by the vascular endothelium. Vasodilators such as acetylcholine and bradykinin act by binding their respective membrane receptors and increasing intracellular Ca2+ within endothelial cells. Physical stimuli, such as shear stress, are also known to enhance NO production in endothelium. In the penis, shear-induced NO production by endothelium is most likely to occur during the onset of erection, when blood flow into the cavernosal bodies is rapidly increased. The mode of action of endothelium-derived NO is identical to that of nerve-derived NO, as described in the previous section (Fig. 59.2).
Prostaglandins (PG) are prostanoids (as are eicosanoids), which are 20-carbon derivatives produced by the action of cyclooxygenases on the common precursor arachidonic acid in both endothelial and smooth muscle cells of the corpora cavernosa. Prostanoids act locally and exert both trophic and tonic effects in an autocrine and paracrine manner. Although the precise physiologic role of prostaglandins in penile erection remains poorly defined, experimental evidence indicates that they may play an important role in the regulation of the production of extracellular matrix. Further, the antiplatelet-aggregating effects of PGI2 (prostacyclin), similar to those of NO, may be important in preventing coagulation of blood, since blood flow within the cavernosal bodies is negligible during full penile tumescence. The five primary active prostanoid compounds in the penis are the prostaglandins PGD2, PGE2, PGF2α, PGI2, and thromboxane A2 (TXA2). Prostanoids can induce both relaxation and contraction in penile corpus cavernosum. PGE is the only endogenous prostaglandin that appears to elicit relaxation of human trabecular smooth muscle; the others cause constriction or have no effect on smooth muscle tone. There are five major groups of prostanoid receptors, termed DP, EP, FP, IP, and TP, which mediate the effects of PGD, PGE, PGF, PGI, and thromboxane, respectively. The multifunctional, dose-dependent effects of prostanoids may be explained by the coupling of receptor subtypes and isoforms to different second messenger systems. Clinically, PGE1 (alprostadil) has been developed as the first U.S. Food and Drug Administration (FDA)-approved intracavernosal injectable drug for the treatment of ED.
Oxygen tension plays an active role in regulating penile erection. Measurements of cavernosal blood Po2 in human volunteer subjects indicate that oxygen tensions change rapidly from venous (∼35 mm Hg) to arterial (∼100 mm Hg) levels during the transition of the penis from the flaccid to the erect state. Maintenance of constant oxygen tension is a critical imperative in most tissues of the body. The penis is the only organ that changes from venous to arterial oxygen tensions during the course of its normal function. This transition is the basis of a unique regulatory mechanism that takes advantage of key synthetic enzymes that utilize molecular oxygen as a cosubstrate. NO synthase and prostaglandin synthase are two well-studied examples of a class of enzymes known as dioxygenases. At low oxygen tension, measured in the flaccid state of the penis, the synthesis of NO is inhibited, preventing relaxation of trabecular smooth muscle. This inhibition of NO production is probably necessary for the maintenance of penile flaccidity. Following vasodilation of the resistance arteries, the increase in arterial flow raises oxygen tension. In the oxygen-enhanced environment, autonomic dilator nerves and the endothelium are able to synthesize NO, mediating relaxation of trabecular smooth muscle. The synthesis of prostanoids is similarly regulated in the flaccid versus the erect state. Therefore, oxygen tension may regulate the types of vasoactive substances present in the vascular bed. At low oxygen tension, norepinephrine- and endothelin-induced contraction may predominate, while at high oxygen tension, NO and prostaglandins are produced because of the availability of molecular oxygen required for their synthesis.
PATHOPHYSIOLOGY OF DIABETES-RELATED ERECTILE DYSFUNCTION
Although impotence in men with diabetes may be primarily psychogenic, several reports have documented that a primarily organic origin is more common (8,9). In patients with diabetes, the erectile disorder is rarely reversible, lending support to an organic cause (11,44). Organic impotence can be differentiated from psychogenic impotence by monitoring nocturnal erections associated with REM. Men with diabetes have been found to have a decrease in such REM-associated erections (45), lending support to an organic basis of their impotence.
Vasculopathy and neuropathy are common complications associated with the natural history of diabetes mellitus. It is hypothesized that cavernosal artery insufficiency, corporal veno-occlusive dysfunction, and/or autonomic neuropathy are the major organic pathophysiologic mechanisms leading to persistent erectile impairment in men with diabetes mellitus (46). The role of hormonal abnormalities in the pathophysiology of organic-based impotence is controversial.
Neurogenic Erectile Dysfunction
Penile autonomic neuropathy has a major role in the pathophysiology of ED in men with diabetes. The incidence of peripheral and autonomic neuropathy is significantly higher in impotent than in potent men with diabetes (8,47). Erectile failure is a common feature of diabetic autonomic neuropathy (48) and may precede the appearance of neuropathy (49).
Until recently, clinical tests of the integrity of the autonomic nerves to the corpora have been performed exclusively by indirect testing, such as tests of nocturnal penile tumescence. Since patients with diabetes commonly have associated hemodynamic abnormalities, indirect testing is not reliable as a technique for accurate documentation of cavernosal nerve integrity (50).
The presence of bladder areflexia and bladder or bowel dysfunction provides indirect support for the impairment of the motor efferent autonomic cavernosal nerves, since the bladder and penis receive autonomic innervation from a common origin (8,51). Ellenberg (8) found that 82% of impotent patients with diabetes had evidence of neuropathic bladder by cystometric diagnosis, whereas only 10% of age-matched potent patients with diabetes had bladder involvement. Vascular reflexes such as beat-to-beat variation in heart rate provide another indirect measurement of autonomic parasympathetic neuropathy. Several studies have also documented abnormal vascular reflexes in the impotent patient with diabetes (52).
The possibility of direct testing of the autonomic innervation of the corpora is now being investigated by recording the electrical activity of the corporal smooth muscle with the use of intracavernosal electromyographic needles or surface electrodes on the penile shaft (53). With use of single-potential analysis, waveforms of a defined duration, amplitude, and polyphasicity can be recorded in healthy subjects (54). In patients with peripheral neuropathy secondary to diabetes, the single-potential analysis of cavernous electrical activity may reveal potentials that are of abnormal duration and amplitude (54,55). Because this technique is at the initial development phase and is available in only a few centers, further investigations will be needed for its validation as a test of the autonomic nervous system.
Several tests are used in the evaluation of the presence of neuropathy in the sensory afferent nerves from the penile skin
and the motor efferent nerves to the perineal skeletal musculature. These tests include perineal electromyography, sacral latency testing, evaluation of dorsal nerve somatosensory-evoked potential, and testing of vibration-perception sensitivity (56,57,58,59). An abnormal result in somatic testing may suggest, but does not prove, the co-existence of autonomic neuropathy in the corpora cavernosa. Faerman et al. (60) reported morphologic alterations in the unmyelinated nerves in the corpus cavernosum of impotent men with diabetes. Ultrastructural studies of penile nerves in rats with long-term streptozotocin-induced diabetes reveal axonal degeneration with loss of axonal filaments, tubules, and mitochondria (61). The most prominent finding we observed in the penile nerves of impotent patients with diabetes was a thickening of the Schwann and perineurial cell basement membranes (62).
and the motor efferent nerves to the perineal skeletal musculature. These tests include perineal electromyography, sacral latency testing, evaluation of dorsal nerve somatosensory-evoked potential, and testing of vibration-perception sensitivity (56,57,58,59). An abnormal result in somatic testing may suggest, but does not prove, the co-existence of autonomic neuropathy in the corpora cavernosa. Faerman et al. (60) reported morphologic alterations in the unmyelinated nerves in the corpus cavernosum of impotent men with diabetes. Ultrastructural studies of penile nerves in rats with long-term streptozotocin-induced diabetes reveal axonal degeneration with loss of axonal filaments, tubules, and mitochondria (61). The most prominent finding we observed in the penile nerves of impotent patients with diabetes was a thickening of the Schwann and perineurial cell basement membranes (62).
The nerves in patients with diabetes can exhibit biochemical abnormalities, which are subject to some degree of improvement with strict glycemic control (63). Excessive nonenzymatic glycosylation of myelin proteins and polyol pathway activity and abnormal metabolism of myoinositol and its phospholipid derivatives have been proposed as possible biochemical mechanisms in the pathogenesis of diabetic neuropathy (64). In addition, neural ischemic insult has been proposed as a possible mechanism for diabetic polyneuropathy (65). It is now known that hyperglycemia induces decreases in endoneurial blood flow and nerve conduction velocities (66).
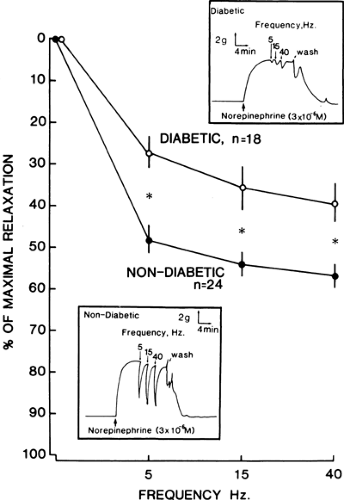
Melman et al. (67) were the first to report diminished levels of norepinephrine in the tissues of patients with diabetes. Norepinephrine depletion was most severe in patients with insulin-dependent diabetes. These results were corroborated by Lincoln et al. (68), who also found diminished levels of norepinephrine and a marked reduction in acetylcholinesterase-positive fibers in corpus cavernosum tissue of patients with diabetes. These findings suggest the possibility of a depletion in the number of cholinergic nerves in corpus cavernosum of patients with diabetes. Impotent men with diabetes have a functional impairment of the neurogenic dilator mechanism of penile smooth muscle (69) (Fig. 59.3). Corporal tissue from impotent men with diabetes accumulates and releases less acetylcholine than does corporal tissue from impotent men without diabetes (70). Because relaxation of penile smooth muscle is necessary for erection and the cholinergic neuroeffector system facilitates this smooth muscle relaxation, the dysfunction of penile cholinergic nerves may be contributory to the development of impotence in men with diabetes. The duration of diabetes is negatively correlated to the ability of the cholinergic nerves to synthesize acetylcholine (70). Therefore, patients with long-standing diabetes are more likely to present with penile autonomic neuropathy.
Vasculogenic Erectile Dysfunction
Vascular disease, of either large or small vessels, is a major contributor to the morbidity and mortality of diabetes (73,74). Diabetic microangiopathy produces alterations and decompensation of local microvascular blood flow (75). There is progressive venule dilation, periodic arteriolar vasoconstriction, and sclerosis of the walls of the arterioles, capillaries, and venules (76). Endothelial cell metabolism and function, thickness of the basement membrane of the vessel wall, oxygen transport, blood-flow properties, and hemostasis also are altered in diabetic microangiopathy (77). Similarly, large-vessel disease is strongly associated with diabetes. Intimal, medial, and luminal changes observed in obliterative atherosclerosis have been well-documented (78). There is growing evidence supporting a role of hyperglycemia in increasing levels of diacylglycerol (79,80), which induces activation of protein kinase C (PKC) (79,81). Activation of this enzyme leads to vascular dysfunction by disrupting NO signaling, which is thought to be a factor contributing to the vascular and neural abnormalities seen in patients with diabetes. In addition, hyperglycemia induces decrease in Na+-K+-ATPase activity (82). A reduction in the activity of this enzyme may enhance vascular smooth muscle vasoconstriction and impaired veno-occlusion. PKC activation has been postulated to mediate the hyperglycemia-induced decrease in Na+-K+-ATPase activity. Finally, PKC activation may increase the expression of vascular endothelial growth factor (VEGF) and vascular permeability factor (VPF). These factors have been implicated in the pathogenesis of diabetic macular edema and diabetic retinopathy (79,81).
Atherosclerotic vascular disease and ED are strongly related. Arteriosclerosis is the most common organic disorder leading to impotence (83). Among men with clinically significant peripheral arterial disease, 40% to 50% complain of impotence, and in 80% of these cases, the primary cause of the impotence is organic (84). ED develops when more than 50% of the major
arterial supply to the penis is involved in atherosclerotic occlusive disease (85). Atherosclerosis has also been observed to have an adverse effect on the ultrastructure of corpus cavernosum in 40% to 45% of men with diabetic impotence; as the smooth muscle cell content decreases, the severity of symptoms and clinical findings increases (86,87,88,89).
arterial supply to the penis is involved in atherosclerotic occlusive disease (85). Atherosclerosis has also been observed to have an adverse effect on the ultrastructure of corpus cavernosum in 40% to 45% of men with diabetic impotence; as the smooth muscle cell content decreases, the severity of symptoms and clinical findings increases (86,87,88,89).
Postmortem examinations of impotent diabetic men have revealed numerous penile arterial vascular abnormalities, including fibrous proliferation of the intima, medial fibrosis, calcification, narrowing, and obliteration of the lumen. Such vascular alterations in the penile arteries impede blood flow to the cavernous bodies at the time of erection and are thus in part responsible for the ED (90).
Impotent men with diabetes may also have associated vascular risk factors, such as cigarette smoking, hypertension, and hyperlipidemia (1). Cigarette smoking is a statistically significant independent risk factor in the development of angiographically confirmed atherosclerotic arterial occlusive disease to the hypogastric-cavernous arterial bed. Five, ten, and twenty pack-year (1 pack, or 20 cigarettes, per day for 1 year) histories of exposure to cigarette smoking are associated with 15%, 30%, and 70% incidence, respectively, of arterial occlusive disease within the common penile artery (90,91). Hypertension also was noted in 45% of impotent men, while hyperlipidemia or other disturbances of lipid metabolism were found in 40% to 50% of impotent men (84).
Diabetes-associated vascular disease affects the physiology of erection by many routes at the level of large inflow vessels, the penile microvasculature, the lacunar space endothelium, and the penile fibroelastic frame. One mechanism of diabetes-induced, atherosclerosis-associated ED is the lowering of arterial perfusion pressure and arterial inflow to the lacunar spaces of the corpora cavernosa (92,93,94). The clinical consequences of these hemodynamic changes are a diminished rigidity of the erect penis and a prolongation of the time to maximal erection. Another mechanism is interference with corporal veno-occlusion. In all men with organic impotence, the incidence of corporal veno-occlusive dysfunction may be as high as 86% (95).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree