Epithelial ovarian, fallopian tube, and peritoneal cancer
Jonathan S. Berek, MD, MMS, FASCO  Michael L. Friedlander, MD, MBChB, PhD
Michael L. Friedlander, MD, MBChB, PhD  Robert C. Bast Jr. MD
Robert C. Bast Jr. MD
Overview
Ovarian cancer is one of the most treatable solid tumors, as the majority will respond temporarily to surgery and cytotoxic agents. The disease, however, frequently persists and recurs in 70% of cases, having the highest fatality-to-case ratio of all the gynecologic cancers.1, 2 Ovarian cancer is neither a common nor a rare disease, with a lifetime risk of 1 in 70 and a prevalence in the postmenopausal population in the United States of 1 in 2500, which impact strategies for early detection and prevention. Germ line mutations of BRCA1 and BRCA2 are associated with 10–15% of ovarian cancers and increase the risk of ovarian cancer dramatically. Factors that contribute to persistent ovulation increase the risk in women with sporadic disease, whereas use of oral contraceptives decreases the risk of disease post menopause. While epithelial ovarian cancers have been thought to arise from the ovarian surface epithelium or in the lining of inclusion cysts beneath the ovarian surface, many high-grade serous “ovarian” carcinomas as well as peritoneal cancers are now believed to arise in the fimbriae of fallopian tube, rather than the ovary or peritoneum.3–10 Epithelial ovarian cancers can exhibit serous, endometrioid, mucinous, or clear cell histotypes. Low-grade type I ovarian cancers grow slowly, can evolve from tumors of low malignant potential, bear Ras mutations in a majority of cases, express wild type TP53, often are detected in early stage (I–II), and respond less frequently to platinum- and taxane-based therapy. High-grade type II ovarian cancers grow rapidly, arise from precursors with TP53 mutations, are driven by DNA copy number changes, are diagnosed in late stage (III–IV), and frequently respond to combination chemotherapy. Owing to a lack of specific symptoms or an effective screening strategy, more than 70% of ovarian cancers are diagnosed in an advanced stage (III–IV). Primary treatment of epithelial ovarian cancer involves cytoreductive surgery and 6–8 cycles of carboplatin and paclitaxel. For those women with small volumes of disease after surgery, intraperitoneal administration of chemotherapy improves survival. Recurrent disease cannot be cured with currently available agents, but survival can be prolonged with combinations of cytotoxic agents including retreatment with paclitaxel and carboplatin. Palliative agents include liposomal doxorubicin, gemcitabine, topotecan, bevacizumab, pemetrexed, and etoposide. Experimental therapy for low-grade cancers involves MEK inhibitors, whereas trials of PI3K pathway inhibitors and PARP inhibitors are underway in patients with high-grade epithelial ovarian cancers. Combinations of targeted agents will be required.
Incidence, etiology, and epidemiology
Incidence
Ovarian, fallopian tube, and peritoneal cancers are predominantly diseases of postmenopausal women, with only 10–15% of all cases diagnosed premenopause.1–3 The median age for diagnosis of epithelial cancers from these three sites ranges between 60 and 65 years. Less than 1% of epithelial cancers are found in women younger than 30 years of age, and most ovarian malignancies in these younger patients are germ cell tumors (GCTs) (see Chapter 105).2 The prevalence of ovarian cancer among postmenopausal women in the United States is 40 per 100,000 or 1 in 2500. The lifetime risk for a woman to develop ovarian cancer in the United States is approximately 1 in 70 (1.4%), compared to 1 in 8 or 9 for breast cancer. The prevalence of ovarian cancer in the general population impacts substantially on strategies for prevention and early detection. In the absence of better markers for increased risk, strategies for prevention must have few, if any, serious adverse effects and screening strategies must be highly specific. A strong hereditary component contributes to development of the disease in at least 10% of cases with inherited germ line mutations in BRCA1 and BRCA2 being the most common with only a small percentage of cases related to mutations in mismatch repair (MMR) genes or p53.
In 2015, in the United States, almost 22,000 new cases of ovarian cancer and more than 14,000 deaths are expected.1 There is a trend toward improved survival for ovarian cancer.1, 2 On the basis of Surveillance, Epidemiology, and End Results (SEER) data in the United States, the 5-year survival for all stages combined increased from 33.6% in 1975 to 45.9% in 2003 to 2011.2 Using statistical models for analysis, rates for new ovarian cancer cases have been falling on average 1% per year over the past 10 years. Death rates have been falling on average 1.6% per year over the same period. The death rate decreased 22% from 10 per 100,000 women per year in 1976 to 7.8 per 100,000 per women per year in 2010.2 Ovarian cancer rates are highest in women aged 55–64 years (median age 63 years), and deaths are highest in people aged 75–84 years (median age 71 years).2
The incidence of ovarian cancer varies in different geographic locations throughout the world. Western countries, including the United States and the United Kingdom, have an incidence of ovarian cancer that is 3–7 times greater than in Japan, where epithelial ovarian tumors are considered rare.10, 11 In Asia, the incidence of GCTs of the ovary appears to be somewhat higher than in the West. Japanese immigrants to the United States, however, exhibit a significant increase in the incidence of epithelial ovarian cancer at a rate approaching that of white women from the United States. The incidence of epithelial tumors is about 1.5 times greater in whites than in blacks.
Etiology and epidemiology
In the past, most epithelial ovarian cancers were thought to arise from a single layer of epithelial cells that covers the ovarian surface or from the epithelial cells that line “inclusion cysts” immediately beneath the ovarian surface. Over the past decade, this view has been modified. Of all epithelial ovarian cancers, approximately 10–15% arise in women with germ line mutations of BRCA1 and BRCA2 and most of these are high-grade serous neoplasms. Up to 80% of these high-grade serous “ovarian cancers” associated with BRCA1/2 mutations are derived from the fimbriae of the fallopian tube, accounting for at least 10% of all epithelial ovarian cancers. Some 20% of high-grade serous cancers coat the ovary rather than grow from it and have been termed “primary peritoneal carcinomas.” Most of these high-grade serous primary peritoneal cancers are likely to arise from fimbriae of the fallopian tube, although some may be derived from developmental remnants of the secondary Müllerian system.12 Primary peritoneal carcinoma explains how “ovarian cancer” can arise in a patient whose ovaries, but not fallopian tubes, were surgically removed many years earlier.13–15 Thus, at least a third, and possibly a majority of high-grade serous cancers arise from the fallopian tube, although the remainder are still of ovarian origin. High-grade serous cancers of the ovary, fallopian tube carcinomas, and peritoneal carcinomas should be regarded as a single disease entity and managed with a common approach. In this regard, fallopian tube cancer is now included in the same FIGO (International Federation of Gynecology and Obstetrics) staging system with ovarian cancer, as discussed in the following paragraph.4
The causes of ovarian cancer are not well understood.16, 17 Cigarette smoking has been linked to mucinous ovarian cancers but not to the more common serous carcinomas.17 Case–control studies have also pointed to an association of white race, high-fat diet, and galactose consumption with a higher incidence of the disease.16, 18 Prior reproductive history and the number of ovulatory cycles are associated with development of the disease, with low parity, infertility, early menarche, and late menopause increasing the risk.16, 19, 20 Fertility-enhancing drugs, such as clomiphene citrate, and gonadotropins used for ovulation induction have been thought to increase the risk of ovarian cancer (ROC), but the data have not been consistent and have not adequately distinguished the influence of infertility per se from the use of fertility-stimulating agents.21–24 A pooled analysis of eight case–control studies that included 5207 cases and 7705 controls found an association of fertility-stimulating drugs with serous borderline tumors but not with invasive ovarian cancers.24 Many case–control and cohort studies have failed to link hormone replacement therapy to an increased risk of epithelial ovarian cancer.25 A large cohort study had reopened controversy regarding this issue.26 Among 44,241 postmenopausal women in the Breast Cancer Detection Demonstration Project, 329 developed ovarian cancer. Women who had received estrogen replacement therapy only for more than 10 years without progestin were at increased risk of developing ovarian cancer. By 20 years, the relative risk (RR) was 3.2-fold. This is supported by a recent meta-analysis, which included individual data from just over 12,000 postmenopausal women, 55% of whom had used hormone therapy, who developed ovarian cancer. Among women last recorded as current users, risk was increased even with <5 years of use (RR 10.43, 95% confidence interval [CI] 10.31–10.56; p < 00.0001). Combining current-or-recent use resulted in a RR of 10.37 (95% CI 10.29–10.46; p < 00.0001); this risk was similar in European and American prospective studies and for estrogen-only and estrogen–progestagen preparations but differed across the four main histological subtypes, being definitely increased only for the two most common types, serous (RR 10.53, 95% CI 10.40–10.66; p < 00.0001) and endometrioid (10.42, 10.20–10.67; p < 00.0001). The authors concluded that the increased risk may be causal and that women who use hormone therapy for 5 years from around age 50 years have about one extra ovarian cancer per 1000 users.27
Prevention
As parity is inversely related to the ROC, having at least one child reduces the RR by 30–40% and use of oral contraceptives for 5 or more years reduces the risk by 50%.21 Women who have had two children and have used oral contraceptives for 5 or more years have as much as 70% reduction in risk. To date, oral contraceptive medication is the only documented method of chemoprevention for ovarian cancer, and it can be recommended to women for this purpose. When counseling patients regarding birth control options, this important benefit of oral contraceptive use should be emphasized. This is also important for women with a strong family history of ovarian cancer in the absence of BRCA1 or BRCA2 mutation.28 Surgical prevention is important, but its use depends critically on identifying women at sufficient risk to justify prophylactic salpingo-oophorectomy with or without total abdominal hysterectomy.
Genetic predisposition
Hereditary ovarian cancer
The ROC is significantly higher than that of the general population in women with a family history of breast or ovarian cancer as well as in families with Lynch syndrome.29–45 Although most epithelial ovarian cancer is sporadic, at least 10–13% of patients with epithelial ovarian cancer have a germ line mutation in either BRCA1 or BRCA2.42
BRCA1 and BRCA2
Most hereditary ovarian cancer results from mutations in the BRCA1 gene, which is located on chromosome 17, with a smaller fraction of familial ovarian cancers associated with mutations in BRCA2, which is located on chromosome 13. Both genes mediate DNA repair. BRCA1-associated ovarian cancers generally occur in women approximately 10 years earlier than those with nonhereditary tumors.36, 42 As the median age of epithelial ovarian cancer is 62–63 years, a woman with a first- or second-degree relative who had early onset ovarian cancer may have a higher probability of having a BRCA1 or BRCA 2 mutation. There is, however, no significant family history in 44% (95% CI, 35.8–52.2%) of mutation-positive women, which underscores the importance of offering mutation testing to all women with high-grade serous ovarian cancer diagnosed under the age of 70 irrespective of family history, and this is reflected in current guidelines.44
There is a higher carrier rate of BRCA1 and BRCA2 mutations in women of Ashkenazi Jewish descent, Icelandic women, and in other ethnic groups.35, 36 There are three specific founder mutations that are carried by the Ashkenazi population: 185delAG and 5382insC on BRCA1, and 6174delT on BRCA2. The carrier rate of at least one of these mutations for a patient of Ashkenazi Jewish descent is 1 in 40 or 2.5%, which is considerably higher than the general Caucasian population. The increased risk is a result of the founder effect—that is, a higher rate of mutations that have occurred within a specific population group that was geographically or culturally isolated in the past in which one or more of the ancestors carried the mutant gene.
A combined analysis of 22 studies unselected for family history has found that women who have a germ line mutation in the BRCA1 gene have a lifetime ROC of 39% (18–54%), and the risk has been calculated to be 11% (2.4–19%) in women with a BRCA2 mutation.45 Women with a BRCA1 or BRCA2 mutation have a risk of breast cancer as high as 65% and 45%, respectively. The breast cancers typically occur at a young age and may be bilateral. There is a higher incidence of triple-negative (ER-, PR-, HER2-) breast cancers in women with BRCA1 mutations. A large consortium found genetic risk modifiers on 4q32.2 and 17q21.31 that significantly increased the risk of developing ovarian cancer in BRCA1-mutation carriers, which may explain the variable risks that have been reported.46
Lynch syndrome
There are other less common genetic causes of ovarian cancer. Lynch syndrome, which is also known as the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, confers increased risk for colorectal cancer and a wide range of other malignancies (e.g., endometrial, ovarian, and gastric cancer) as a result of a germ line MMR genetic mutation.39 The mutations that have been associated with this syndrome are MSH2, MSH6, MLH1, PMS1, and PMS2. The risk of endometrial cancer equals or exceeds that of colorectal cancer in women with Lynch Syndrome. The diagnosis of gynecologic cancer precedes that of colorectal cancer in over half the cases, making gynecologic cancer a “sentinel cancer” for Lynch syndrome. The lifetime ROC in women with Lynch Syndrome has been estimated at approximately 6–12%. The mean age at diagnosis is 42.7–49.5 years. There is a higher risk of endometrioid and clear cell subtypes, and the majority of cases are diagnosed in stage I or II.
The management of women at high risk for ovarian cancer
The management of a woman with a strong family history of epithelial ovarian cancer must be individualized and will depend on her age, reproductive plans, and the estimated level of risk. A thorough pedigree analysis is important. A geneticist should evaluate the family pedigree for at least three generations. Decisions about management are best made after careful study of the pedigree and, whenever possible, verification of the histologic diagnosis of the family members’ ovarian cancer as well as the age of onset and other tumors in the family. Although recommended by the National Institutes of Health Consensus Conference on Ovarian Cancer47 , the value of screening with transvaginal ultrasonography and CA125 has not been established in women at high risk. The findings of two prospective studies of annual transvaginal ultrasonography and CA125 screening suggest a very limited benefit, if any, of screening high-risk women.48, 49
Data derived from a multi-institutional consortium of genetic screening centers has suggested that the use of oral contraceptives is associated with a lower ROC in women who have a BRCA1 or BRCA2 mutation,50 but this has not been confirmed.51 Tubal ligation may also decrease the ROC in patients with a BRCA1 but not BRCA2 mutation, but the protective effect is not nearly as strong as risk-reducing bilateral salpingo-oophorectomy (BSO).52
The value of prophylactic risk-reducing BSO in these patients has been well documented.53–58 Occult ovarian/fallopian tube cancers detected at the time of risk-reducing BSO have been reported in many studies with wide variability in reported prevalence ranging from 2.3% to 23%. The performance of a prophylactic salpingo-oophorectomy reduces the risk of BRCA-related gynecologic cancer by 96%.56 There remains a small risk of subsequently developing a peritoneal carcinoma. The risk of developing peritoneal carcinoma was 0.8% and 1%, respectively, in two series.54, 55 As discussed earlier, many so-called ovarian cancers arise from the fallopian tube, and it is essential that women having prophylactic surgery have their fallopian tubes removed as well and that these are carefully assessed by the pathologist as it is easy to miss small cancers or precursor lesions, particularly in the fimbrial end of the fallopian tube.6 Prophylactic salpingo-oophorectomy in premenopausal women also reduced the risk of developing subsequent breast cancer by 50–80%.54, 55
Grann et al.59 reported the application of Markov modeling—that is, quality-adjusted survival estimate analysis—in a simulated cohort of 30-year-old women who tested positive for BRCA1 or BRCA2 mutations. The analysis predicted that a 30-year-old woman could prolong her survival beyond that associated with surveillance alone by 1.8 years with tamoxifen, 2.6 years with prophylactic salpingo-oophorectomy, 4.6 years with both tamoxifen and prophylactic salpingo-oophorectomy, 3.5 years with prophylactic mastectomy, and 4.9 years with both prophylactic surgeries. Quality-adjusted life expectancy was estimated to be prolonged by 2.8 years for tamoxifen, 4.4 years with prophylactic salpingo-oophorectomy, 6.3 years for tamoxifen and prophylactic salpingo-oophorectomy, 2.6 years with mastectomy, and 2.6 years with both operations. This has been supported by a study of women with BRCA1 and BRCA2 mutations which found that risk-reducing mastectomy was associated with a lower risk of breast cancer, risk-reducing BSO was associated with a lower ROC, and that there was an improvement in all-cause mortality, breast cancer-specific mortality as well as ovarian cancer-specific mortality.60
The survival of women who have a BRCA1 or BRCA2 mutation and develop ovarian cancer is longer than that for those who do not have a mutation. In one study, the median survival for mutation carriers was 53.4 months compared with 37.8 months for those with sporadic ovarian cancer from the same institution.61 These findings have recently been confirmed in a population-based study from Israel in which Chetrit et al.62 reported that among Ashkenazi women with ovarian cancer those with BRCA1 and BRCA2 mutations had an improved long-term survival (38% vs 24% at 5 years). This may result from intrinsic growth properties or from a better response to chemotherapy.
Recommendations
Current recommendations are that all women under the age of 70 with a nonmucinous epithelial ovarian, fallopian tube, or peritoneal cancer should undergo testing for BRCA1 to BRCA2 mutations.63 The recommendations for management of women at high risk for ovarian cancers are summarized as follows40, 41, 47, 51–59, 64 :
- 1. Women who appear to be at high risk for ovarian and or breast cancer should undergo genetic counseling; if there is a probability of 10% or greater of having a BRCA mutation, they should be offered genetic testing for BRCA1 and BRCA2.
- 2. Women who wish to preserve their reproductive capability or delay prophylactic surgery should undergo periodic screening by transvaginal ultrasonography and CA125 every 6 months, although the efficacy of this approach has not been established and is not based on evidence that this reduces mortality.
- 3. Oral contraceptives should be recommended to young women at increased risk.
- 4. Women who do not wish to maintain their fertility or who have completed their family should undergo PBSO. In women who have a strong family history of breast or ovarian cancer, annual mammographic and magnetic resonance imaging (MRI) breast screening should be performed commencing at age 30 years, or younger if there are family members with documented very early onset breast cancer.
- 5. Women with a documented Lynch syndrome should be counseled about prophylactic hysterectomy and oophorectomy after childbearing, in view of the risk of both endometrial and ovarian cancer. Although there are no definitive studies to support screening, endometrial sampling and transvaginal ultrasound of the ovaries may be considered from ages 30 to 35. Colonoscopy is recommended every 1–2 years starting from ages 20 to 25 or 10 years younger than the youngest person diagnosed in the family.39, 65, 66
Molecular, cellular, and clinical biology
Similar to most epithelial cancers, more than 90% of epithelial ovarian cancers arise from the progeny of a single cell, that is, ovarian cancer is generally a clonal disease.67 Despite origin from a single cell, ovarian cancers are markedly heterogeneous at a molecular, cellular, and clinical level. A number of genetic abnormalities are observed in ovarian cancers (Table 1)68 that activate or inactivate different genes. The function of several tumor suppressor genes has been lost (Table 2)68 by deletion, loss of heterozygosity, inactivating mutation (BRCA1, BRCA2, and TP53), promoter methylation (ARHI), histone modification, or loss of processed miRNAs. Abnormalities in miRNA processing with decreases in Dicer and Drosha have been found in 60% and 51% of ovarian cancer associated with a poor outcome.69 Oncogenes have been amplified, transcriptionally overexpressed and/or mutationally activated (Table 3).68 At a cellular level, the fraction of proliferating cells can vary from 1% to 90%.70 Ovarian cancers vary in histotype exhibiting the serous, endometriod, mucinous and clear cell variants described in the following paragraphs, related at least in part to the aberrant expression of HOX genes associated with normal gynecologic development.71
Table 1 Genetic and epigenetic abnormalities in epithelial ovarian cancer
| Activating events | |
| Amplification by CGH | 1q22 (RAB25), 3q26 (PKCiota, EVI1, PIK3CA), 5q31 (FGF-1), 8q24 (MYC), 19q (PI3Kp85, AKT2), 20p, 20q13.2 (BTAK) |
| Mutationa | K-Ras, BRAF, CTNNB1, CDKN2A, PIK3CA, KIT, MADH |
| Hypomethylation | BORIS, CLDN-4, IGF2, MCI, SAT2, SNCG |
| Histone modification | cyclin B1, GATA4, GATA6, p21/WAF1 |
| miRNA | BAP1, DLK1, MSX2, PTEN, SIP1, VEGFA, ZEB1/2 |
| Inactivating events | |
| Deletion by CGH | 4q, 5q, 16q, 17p, 17q; Xp, Xq |
| LOH | (>50 %): 17p13, 17q21 ( >30 %): 1p, 3p, 5q, 5q, 6q, 7q, 8q, 9p,10q,11p, 13q, 18q, 19p, 20; Xp |
| Mutation | ARID1A, TP53, Rb1a, APC, BRCA1, BRCA2, CDK12, NF1, PTEN, PP2R1A |
| Promoter methylation | APC, ARHI, ANGPTL, ARLTS1, BRCA1, DAPK, FBX032, H-CADHERIN, hMLH1, HOXA10, HOXA11, Hsulf-1, ICAM-1, LOT-1, MCJ, MUC2, MYO188, OPCML, PACE-4, PALP-B, PAR-4, PEG3, p16, p21, RASSF1, SOCS1, SOCS2, SPARC, TMS/ASC, TUBB3, 14-3-3σ |
| Histone modification | Adam 19, GATA4, GATA6, RASSF1 |
| miRNA | BCL2, FGF2, MMP13, PAR8, c-SRK, VEGFA |
| Inhibition of growth by chromosome transfer | 2, 3, 7, and 22 |
Table 2 Putative tumor suppressor genes in epithelial ovarian cancer
| Gene | Chromosome | Downregulated or inactivated | Mechanisms of downregulation | Function |
| ARHI (DIRAS3) | 1p31 | 60% of all histotypes | Imprinting; LOH; promoter methylation; transcription downregulated by E2F1 and E2F4 | 26 kDa GTPase; inhibits proliferation and motility; induces autophagy and dormancy; upregulates p21; inhibits Cyclin D1, PI3K, Ras-MAP, Stat3 |
| ARID1A | 1p35.5 | 49% of clear cell and 30% of endometrioid histotypes | Mutation | Chromatin remodeling |
| RASSF1A | 3p21 | — | Hypermethylation | Inhibits proliferation and tumorigenicity in many different cancers. Interacts with Ras inhibiting downregulating cyclin D and signaling through JNK, stabilizes microtubules, and regulates spindle checkpoint and fas- and TNF-induced apoptosis |
| DLEC1 | 3p22.3 | 73% | Promoter hypermethylation and histone hypoacetylation | 166 kDa cytoplasmic protein that inhibits anchorage-dependent growth |
| SPARC | 5q31 | 70–90% decreased expression; 9% lost | Transcription, hypermethylation | 32 kDa Ca++ binding protein; prevents adhesion |
| DAB-2 (DOC2) | 5q13 | 58–85% lost | Transcription | 105 kDa protein binds GRB2 preventing Ras/MAP activation, prevents c-fos induction and decreases ILK activity, contributing to anoikis and inhibiting proliferation and anchorage-independent growth and tumorigenicity |
| LOT-1 (ZAC1) | 6q25 | 39% | Imprinting; hypermethylation LOH; transcription downregulated by EGF, TPA | 55 kDa nuclear zinc finger protein inhibits proliferation and tumorigenicity |
| RPS6KA2 | 6q27 | 64% | Monoallelic expression in ovary; LOH | 90-kDa ribosomal S6 serine threonine kinase that inhibits growth, induces apoptosis, decreases pERK and cyclin D1, and increases p21 and p27 |
| PTEN (MMAC-1) | 10q23 | 3–8% mutated; expression lost in 27%, particularly in endometrioid and clear cell histotypes | Promoter methylation; LOH; mutation | PI3 phosphatase; decreases proliferation, migration, and survival; decreases cyclin D and increases p27 |
| OPCML | 11q25 | 56–83% | Promoter methylation; LOH; mutation | GPI-anchored IgLON family member; induces aggregation; inhibits proliferation and tumorigenicity |
| BRCA2 | 13q12-13 | 3–6% | Mutation; LOH | Binds RAD51 in repair of DNA double-strand breaks (DSBs) |
| ARLTS1 | 13q14 | 62% | Promoter methylation | ADP ribosylation factor induces apoptosis |
| WWOX | 16q23 | 30–49%, particularly in mucinous and clear cell histotypes | LOH; mutation | Decreases anchorage-independent growth and tumorigenicity; mouse homolog required for apoptosis |
| TP53 | 17p13.1 | 50–70% overall; 96% of high-grade serous histotype | Mutation | 53-kDa nuclear protein induces p21 with cell cycle arrest promoting DNA stability; induces apoptosis |
| OVCA1 | 17p13.3 | 37% | LOH | 50 kDa protein; decreases proliferation and clonogenicity; decreased Cyclin D1 |
| BRCA1 | 17q21 | 6–8% | Mutation; LOH; promoter methylation | E3 ubiquitin ligase that participates directly in repair of DNA DSBs through homologous recombination; regulates c-Abl; induces TP53, androgen receptor, estrogen receptor and c-Myc |
| PEG3 | 19q13 | 75% | Imprinting; LOH; promoter methylation; transcription | Induces TP53-dependent apoptosis |
| PPP2R1A | 19q13.44 | 7% of clear cell histotype | Mutation | Protein phosphatase 2 regulatory subunit inhibits proliferation |
Candidate tumor suppressor genes with preliminary reports in the literature also include APC, BRMS1, CTGF, EPB41L3, MAP2K4, MKK4, RNF43, RP36RA7, PINX1, SFRP4, SLIT2, SOX11, TUSC3, and 53BP1.
Table 3 Oncogenes associated with epithelial ovarian cancer
| Oncogenes | Chromosome | Amplified (%) | Overexpressed (%) | Mutated (%) | Function |
| Rab25 | 1q22 | 54 | 80–89 | — | Cytoplasmic GTPase/apical vessel trafficking |
| Evi-1 | 3q26 | — | — | — | Transcription factor |
| eIF-5A2 | 3q26 | — | — | — | Elongation factor |
| PKCi | 3q26 | 44 | 78 | — | Cytoplasmic serine-threonine kinase |
| PIK3CA (PI3K p110α) | 3q26 | 9–80 | 32 | 8–12 | Cytoplasmic lipid kinase |
| FGF-1 | 5q31 | — | 51 | — | Growth factor for cancer and angiogenesis |
| Myc | 8q24 | 20 | 41–66 | — | Transcription factor |
| EGFR | 7p12 | 11–20 | 9–28 | <1 | Tyrosine kinase growth factor receptor |
| Notch-3 | 9p13 | 20–21 | 62 | — | Cell surface growth factor receptor |
| K-Ras | 12p11-12 | 5–53 | 30–52 | 2–24 | Cytoplasmic GTPase |
| HER-2 | 17q12-21 | 6–11 | 4–12 | — | Tyrosine kinase growth factor receptor |
| p85 PI3K | 19q | — | — | — | Cytoplasmic lipid kinase |
| Cyclin E | 19q12 | 12–53 | 42–63 | — | Cyclin |
| AKT2 | 19q13.2 | 12–27 | 12 | Cytoplasmic serine-threonine Kinase | |
| BTAK/Aurora A | 20q13 | 10–15 | 48 | — | Nuclear serine-threonine kinase/activates telomerase |
Additional targetable genes with low or high gain of copy number in >20% of high-grade serous ovarian cancers include AKT1, AKT3, CDK2, IL8RB, EPCAM, ERBB3, FGFR2, HDAC4, HSP90AB1, HSP90B1, IGF1, IGFR1, LPAR3, MAP3K6, MAPK15, MAPKAPK2, MAPKAPK5, MECOM, MSTN, MTOR, NCAM1, NOS1, NOS3, PIK3CD, POLB, POLE, RHEB, RICTOR, PPS6KC1, RAPTOR, SKI1, STAT1, STAT4, TERT, TGFB1, TGFB2, TGFBR3, TNFRSF9, and VEGFA.
Biologically and clinically, there is a major distinction between low-grade (type I) and high-grade (type II) epithelial ovarian cancers.72 Low-grade serous cancers (comprising <10% of ovarian epithelial malignancies) are thought to develop from borderline tumors, to present in early stage (I–II) and to depend on mutations of Ras (>50%), PIK3CA (30%), and PTEN (10%), as well as upon expression of the insulin-like growth factor receptor (IGFR), which responds to IGF produced by the tumor stroma. High-grade serous cancers (comprising 60% of all ovarian epithelial malignancies) are thought to develop from histologically normal ovarian and fallopian tube epithelial cells, to present in late stage (III–IV), and to depend on amplification of multiple wild-type oncogenes and the functional loss of tumor suppressor genes. While TP53 is rarely mutated in low-grade type I cancers, it is mutated in nearly all high-grade serous type II cancers. When germ line and somatic mutations of BRCA1/2 are associated with epithelial ovarian cancer, the cancers are generally high grade. Defects in homologous recombination DNA repair deficiency (BRCAness) are associated with up to half of high-grade cancers, but few, if any, low grade cancers, possibly accounting for the fact that high-grade cancers are more sensitive to platinum-based chemotherapy than low-grade cancers. Current strategies are being developed to exploit this deficiency using PARP inhibitors in the presence and absence of BRCA1/2 mutations. The PI3-kinase pathway is a potential target in both type I and type II cancers and is activated in at least half of high-grade cancers.73
The Tumor Cancer Genome Atlas (TCGA) project has sequenced DNA from more than 300 high-grade serous ovarian cancers and aside from TP53 (98%) and BRCA1/2 (15–20%) only a few genes are mutated more than 1% of the time (NF1, RB1, CSMD3, and CDK12).74 Consequently, low-grade cancers are driven by mutations and high-grade cancers by DNA copy number abnormalities.
Clear cell ovarian cancers have mutations of ARID1A (49%),75 a chromatin-processing enzyme and PP2R1A (6%), a phosphatase. Endometriod ovarian cancers also have mutations of ARID1A (30%). Among the nonepithelial ovarian cancers, 97% of granulosa cell tumors (see the following discussion) exhibit a characteristic mutation, 402C→G (C134W) in FOXL2, a gene encoding a transcription factor known to be critical for granulosa-cell development.76
Ascites formation results from increased leakage of proteinaceous fluid from capillaries under the influence of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) produced by ovarian cancers and from inhibition of fluid outflow through diaphragmatic lymphatics that have been blocked by metastatic disease.77 Studies of the immunobiology of the peritoneal cavity suggest that it may function as an immunoprivileged site, with elevated levels of suppressive molecules and growth factors. Angiogenesis in ovarian cancer has been shown to depend on multiple factors including VEGF and IL-8. The presence of multiple angiogenic factors can explain, in part, development of resistance to bevacizumab therapy. Autophagy and tumor dormancy are regulated by ARHI (DIRAS3) an imprinted tumor suppressor gene that is downregulated in 60% of ovarian cancers of low and high grade.78, 79 The upregulation of ARHI and high prevalence of autophagy in dormant, drug-resistant cancer cells in positive second look specimens after primary surgery and chemotherapy, suggest that autophagy could be a target in ovarian cancer.80
Upregulation and aberrant glycosylation of extracellular mucins have provided markers for monitoring disease. MUC-1 is a mucin expressed by more than 80% of ovarian cancers.81 In transformed cells, aberrant glycosylation exposes peptide determinants recognized by murine monoclonal antibodies that have been used for serotherapy. CA125 is also a mucin (MUC16) associated with cells that line the coelomic cavity during embryonic development. CA125 is shed from 80% of epithelial ovarian cancers82, 83 and can be measured using the murine monoclonal antibody OC125. Regression and progression of disease tend to correlate well with falling or rising CA125 levels. The precise function of the glycoprotein is unknown,84–86 but knockout of murine MUC16 does not affect the development or fertility of mice.87 In cancer cells, CA125 expression is upregulated transcriptionally and 80% of the CA125 is cleaved and shed. Interaction of CA125 is with mesothelin at the peritoneal surface and is likely to be the first point of contact for ovarian cancer cells metastasizing within the peritoneal cavity.
Classification and pathology
Primary ovarian cancers are classified according to the structures of the ovary from which they are derived.88 As noted above, most have thought to be derived from the epithelial cells that cover the ovarian surface or that line inclusion cysts, although, as described earlier, this concept has recently been challenged by recognition that many high-grade serous cancers arise from the fimbriae of fallopian tubes. These cells are ultimately derived from the coelomic epithelium of mesodermal origin and share cytologic markers with mesothelium. Germ cell malignancies constitute the next most common group and the least common tumors are derived from ovarian stromal cells (see Chapter 105). Granulosa-Theca tumors are derived from the specialized connective tissue of the ovary and are the least common (see Chapter 105).
Epithelial malignancies account for 85–90% of ovarian cancers. The majority of epithelial lesions are seen in patients who are 40 years of age or older. Under the age of 40 years, epithelial malignancies are uncommon, and most malignancies seen in women under the age of 30 years are of germ cell origin. The histologic types of the epithelial tumors are listed in Table 4. The majority of lesions, about 75%, are of the serous type, followed by the mucinous, endometrioid, clear cell, mixed, Brenner, and undifferentiated histologies.11
Table 4 Epithelial ovarian tumors
| Histologic type | Cellular type |
1. Serous
| Endosalpingeal |
2. Mucinous
| Endocervical |
3. Endometrioid
| Endometrial |
4. Clear cell “mesonephroid”
| Müllerian |
5. Brenner
| Transitional |
6. Mixed epithelial
| Mixed |
7. Undifferentiated
| |
8. Unclassified
|
Invasive histotypes
Serous carcinomas may have a complex admixture of cystic and solid areas with extensive papillations, or they may contain a predominantly solid mass with areas of necrosis and hemorrhage (Figure 1). The poorly differentiated tumors may have some areas with a papillary pattern, but other portions may be indistinguishable from the other histologic patterns described in the following paragraphs (Figure 2). Stage I or II lesions are most frequently unilateral, with about 10–20% involving both ovaries. Conversely, about 50–70% of stage III serous carcinomas are bilateral.11
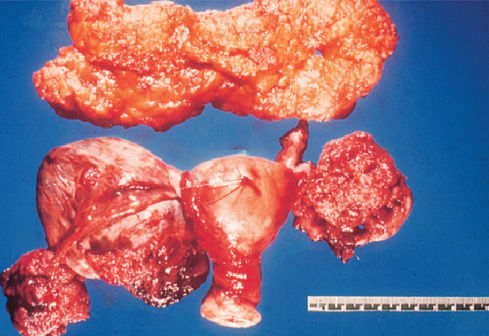
Figure 1 Serous cystadenocarcinoma gross with omentum.
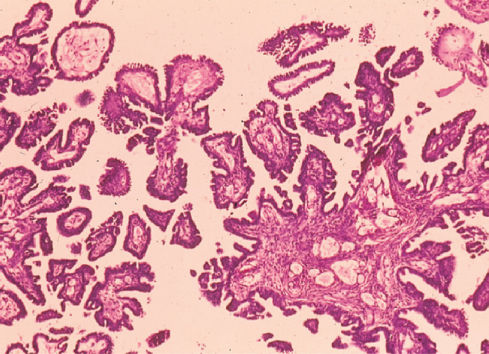
Figure 2 Poorly differentiated serous carcinoma of ovary.
Mucinous tumors tend to be large, with many masses over 20 cm in diameter (Figure 3). The histologic pattern resembles uterine endocervical glands. The lesions frequently contain areas of hemorrhage, necrosis, and various quantities of mucin. These tumors are bilateral in 10–20% of cases. Occasionally, mucin is secreted into the peritoneal cavity and produces a condition known as pseudomyxoma or myxoma peritonei. A mucocele of the appendix may also be seen in conjunction with this tumor.
Figure 3 Mucinous cystadenocarcinoma.
Endometrioid carcinomas of the ovary resemble typical carcinomas of the endometrium. These tumors may be seen with synchronous endometrial carcinoma, and when they are, both lesions may be of low stage. Rarely, endometrioid carcinomas may arise in conjunction with pelvic endometriosis, resulting from malignant transformation of a benign process (Figure 4).11 Similar to previous endometrial cancers, endometrioid ovarian cancers are associated with inactivating mutations of PTEN with consequent activation of PI3 kinase signaling. Bilaterality is seen in 10–15% of stage I and II disease and in about 30% of stage III.
Figure 4 Endometrioid carcinoma.
Ovarian clear cell carcinomas have abundant intracellular glycogen that is removed during histopathologic processing. About one-fourth of clear cell tumors are associated with endometriosis. Clear cell tumors are only rarely bilateral.11
Brenner tumors are uncommon, representing less than 1% of all epithelial malignancies. Mixed epithelial tumors may contain small areas of Brenner tumor histology, which have a histologic pattern similar to that of transitional cell. Malignant Brenner tumors are unilateral.11
Borderline tumors
Borderline tumors, or those of low malignant potential, are important to differentiate from those that are frankly invasive. The treatment and prognosis for borderline lesions are considerably different from those for invasive malignancies. Borderline tumors tend to remain confined to a single ovary at the time of diagnosis and also tend to occur in younger, premenopausal women (Figure 5). They may be confused with a well-differentiated invasive ovarian cancer, and the treatment for the two may be different. Thus, in a young patient who has a lesion confined to the ovary, which is suspected of being an epithelial ovarian cystadenocarcinoma, a borderline tumor must be excluded because bilateral oophorectomy, hysterectomy, and chemotherapy are unnecessary in these patients. In women under the age of 40 years, about 60–70% of nonbenign ovarian neoplasms are borderline, whereas in women over 40 years, only 10% are borderline.11, 89 Histologic criteria for borderline tumors include (1) the presence of epithelial cell proliferation with a “piling up” of cells, the so-called pseudostratification; (2) cytologic atypia, but with rare mitoses; and (3) no evidence of stromal invasion. Borderline tumors tend to remain confined to the ovary but may be associated with peritoneal disease, which represents either dissemination or the multifocal evolution of the disease. In those rare patients with peritoneal involvement, death can occur by progressive intestinal obstruction.
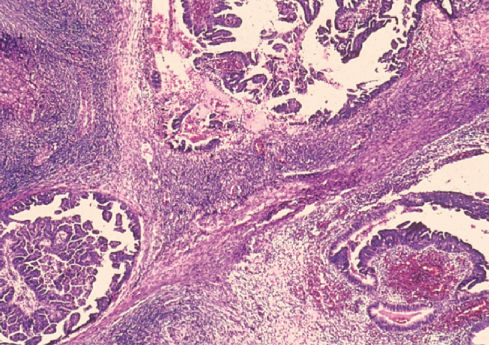
Figure 5 Borderline serous tumor.
Peritoneal carcinomas
Epithelial malignancies that coat the surface of the ovary and peritoneum are referred to as peritoneal carcinomas.14 These cancers are distinct from the very rare peritoneal mesotheliomas that exhibit a different natural history, as well as response to chemotherapy.90 The cells of the peritoneum have the ability to recapitulate any of the histologic patterns seen in ovarian cancers, although serous carcinomas occur most frequently and the other histologic types are rarely seen. Many of these high-grade serous primary peritoneal cancers are likely to arise from fimbriae of the fallopian tube, although some may be derived from developmental remnants of the secondary Müllerian system.12
Recognition of peritoneal carcinomas explains the occurrence of ovarian cancer after oophorectomy.91 In addition, peritoneal cancers can involve the surface of the ovaries without ovarian enlargement. Thus, ovaries can be innocent bystanders in a process originating in the peritoneal cavity. Therapeutically, peritoneal malignancy should be treated as one would manage an epithelial ovarian cancer.
Patterns of spread
Ovarian epithelial tumors spread primarily by direct exfoliation and implantation of cells throughout the peritoneal cavity but also metastasize via the lymphatic and hematogenous routes. GCTs (see Chapter 105) have a greater predilection for spread via the retroperitoneal lymphatics, which must be evaluated carefully when staging those tumors that appear to be confined to the ovary.11, 92
Exfoliated ovarian cancer cells spread directly to the pelvic and abdominal peritoneal surfaces and tend to follow the path of circulation of peritoneal fluid from the right pericolic gutter cephalad to the right hemidiaphragm. At primary surgery, the parietal and visceral peritoneum can be studded with dozens to hundreds of metastatic nodules. The intestinal mesenteries can become involved by peritoneal metastases. Adhesions form between loops of small intestine producing mechanical obstruction, even though involvement of the lumen of the intestine by direct extension is uncommon. The intestinal dysfunction can also result from involvement by tumor of the myenteric plexus, the autonomic innervation of the intestine that is found in the mesentery. This condition has been referred to as “carcinomatous ileus.” Large pelvic masses can compress the rectum producing colonic obstruction.
Spread via the lymphatics is common in epithelial ovarian cancer. Apparent stage I and II tumors have retroperitoneal lymphatic dissemination in about 5–10% in most series, whereas lymphatic dissemination in stage III has been reported to be as high as 42–78% in carefully explored patients.93 Most of these lymph nodes are not enlarged but are microscopically positive for malignant cells. Spread through the retroperitoneal and diaphragmatic lymphatics can result in metastasis to the supraclavicular lymph nodes on the left and right, respectively. Bloodborne metastasis of ovarian cancer is uncommon at diagnosis and is often a late finding in the disease. Hematogenous dissemination at the time of diagnosis to the parenchyma of the liver or lung is seen in a minority of patients. In advanced recurrent disease, parenchymal metastases are seen more frequently in the parenchyma of the lung and even of the brain.
Clinical symptoms
Some patients with ovarian cancers confined to the ovary are asymptomatic, but the majority will have nonspecific symptoms that do not necessarily suggest an origin in the ovary. In one survey of 1725 women with ovarian cancer, 95% recalled symptoms before diagnosis, including 89% with stage I/II disease and 97% with stage III/IV disease.94 Seventy percent had abdominal or GI symptoms, 58% pain, 34% urinary symptoms, and 26% pelvic discomfort. At least some of these symptoms could have reflected pressure on the pelvic viscera from the enlarging ovary. Goff et al. have developed an ovarian cancer symptom index and reported that symptoms associated with ovarian cancer were pelvic/abdominal pain, urinary frequency/urgency, increased abdominal size or bloating, and difficulty eating or feeling full when they were present for less than 1 year and occurred >12 days a month. The index had a sensitivity of 56.7% for early ovarian cancer and 79.5% for advanced-stage disease.95 Interestingly, a population-based study from Australia found that there did not appear to be a significant difference in the duration of symptoms or the nature of symptoms in patients with early as opposed to advanced-stage ovarian cancer.96, 97
Metastatic ovarian cancer is rarely asymptomatic. In addition to the GI and urinary symptoms noted in early-stage disease, formation of ascites can produce an increase in abdominal girth. Pleural effusion may lead to dyspnea as the first complaint. Acute symptoms, such as those of adnexal rupture or torsion, are uncommon. Vaginal bleeding is also an uncommon symptom in postmenopausal women, although premenopausal patients may present with irregular or heavy menses. Detection of an adnexal mass by pelvic examination can permit the early diagnosis of ovarian cancer. As malignancy is rare and the majority of palpable adnexal masses are benign, an enlarged ovary discovered on pelvic examination is not likely to be an ovarian malignancy. In premenopausal women, ovarian cancer is uncommon and represents less than 7% of all adnexal masses.11 Even in postmenopausal women, 70–80% of adnexal tumors are benign. In some patients who complain primarily of abdominal symptoms, however, a pelvic examination frequently is omitted and the tumor missed. Signs of advanced disease include abdominal distention and a fluid wave consistent with ascites. These signs are nonspecific and can be associated with many conditions arising in the abdominal cavity, especially malignancies of other primary sites or carcinomatosis from metastatic tumors of the GI tract and breast.
Diagnosis
The diagnosis of ovarian cancer is usually made at laparotomy, but occasionally at laparoscopy. If a pelvic mass is suspicious and the most likely diagnosis is ovarian cancer, surgery should not be unnecessarily delayed. In premenopausal patients, however, simple cystic ovarian lesions can be observed over a period of 1–2 months. Lesions that are essentially mobile, are unilateral, and have a smooth contour are much less likely to be neoplastic, and are unlikely to be malignant. In premenopausal patients with cystic lesions of less than 8 cm, attempted suppression with oral contraceptives is indicated. In women who are definitely postmenopausal, cystic masses larger than 5 cm should be removed unless they represent a chronic finding. Those masses that regress in size can be managed with continued observation, whereas those that persist or enlarge must be evaluated surgically. Conversely, patients whose lesions are irregular, predominantly solid, and somewhat immobile should undergo an exploratory laparotomy.
The preoperative evaluation of patients can be aided by the use of blood biomarkers. Three algorithms have been developed for distinguishing malignant from benign pelvic masses. Ultrasound, CA125, and menopausal status have been combined to create a risk of malignancy index (RMI) that has achieved a sensitivity of 71–88% with a reciprocal specificity of 97–74% for predicting the presence of ovarian cancers in women with pelvic masses.98 An OVA1 panel including CA125, apolipoprotein A1, transthyretin, transferrin, and B2-microglobulin combined with imaging and menopausal status provides 92% sensitivity at 42% specificity in postmenopausal women and 85% sensitivity at 45% specificity for premenopausal women.99 The negative predictive value for women judged at low risk is 94%–96%. Similar sensitivity and higher specificity have been attained with a risk of malignancy index (ROMA) calculated from CA125 and HE4 values combined with menopausal status alone, without imaging.100 In an initial trial of patients referred to academic centers, the sensitivity for predicting a malignant pelvic mass was 93%, specificity 75%, and negative predictive value 94%. In a subsequent community-based trial, a sensitivity of 94%, specificity of 75%, and negative predictive value of 99% were attained.101 The ROMA has been shown superior to the RMI.100 Both the OVA1 and the ROMA panels have been approved for use by the US FDA. Utilization of these panels could assure that women with ovarian cancer receive optimal surgery. At present, less than half of women in the United States with ovarian cancer have their initial operation with a gynecologic oncologist trained to perform optimal cytoreductive surgery.
Ultrasonographic signs of malignancy include an adnexal pelvic mass with areas of complexity, such as irregular borders; multiple echogenic patterns within the mass; and dense, multiple irregular septae. Bilateral tumors are more likely to be malignant, although the individual characteristics of the lesions are of greater significance. Transvaginal ultrasonography may have a somewhat better resolution than transabdominal ultrasonography for adnexal neoplasms. Newer techniques using Doppler color-flow imaging may enhance the specificity of ultrasonography for demonstrating findings consistent with malignancy.
Radiographic techniques, including abdominal radiographs, computed tomography (CT) scans, PET-CT (positron emission tomography) scans, and nuclear MRI, are not useful before the surgical diagnosis of ovarian cancer. The preoperative evaluation of patients who have a suspicious pelvic mass can omit these studies when blood chemistries and enzymes suggest normal hepatic and pancreatic function. In patients with ascites and no pelvic mass, however, a CT or MRI may be useful in identifying other potential sites of origin. Paracentesis is not recommended because of the frequency of metastatic implantation and growth in the needle tract. Liver–spleen scans, brain scans, and bone scans are unnecessary unless specific symptoms suggest metastasis to these sites.
In premenopausal women, radiographic studies of the intestines are not required unless there is the finding of occult blood in the rectum or there are symptoms indicating upper or lower intestinal obstruction. A barium enema or endoscopy is appropriate in postmenopausal patients. Mammography should be performed to exclude primary breast cancer, which can coexist with ovarian cancer or spread to the ovaries. Cervical cytology should be performed, although ovarian cancer cells are unlikely to exfoliate through the uterus to the cervix. In patients with irregular or heavy menses, an endometrial biopsy should be performed to exclude primary endometrial pathology.
The differential diagnosis of an adnexal mass includes a variety of functional changes of the ovary, benign neoplasms of the reproductive tract, and inflammatory lesions of these organs. A hydrosalpinx, endometriosis, and pedunculated uterine leiomyomata can simulate an ovarian neoplasm. Nongynecologic diseases, such as inflammatory processes of the colon and rectum, must be excluded.
Screening
There is no well-established strategy for early detection of ovarian cancer. Discovery of a pelvic mass on routine physical examination can lead to surgery before the dissemination of a malignancy, but conventional diagnosis detects less than 20% of patients in stage I. Given the prevalence of ovarian cancer in the postmenopausal population, any screening strategy must be highly specific (>99.6%) as well as highly sensitive for early-stage disease (>75%) to achieve a PPV of 10% (i.e., 10 laparotomies for each case of ovarian cancer detected). Two approaches have been evaluated for early detection of ovarian cancer: ultrasonography and serum tests such as CA125.
Ultrasonography
Transvaginal sonography (TVS) has proved superior to transabdominal sonography (TAU) for the detection of a pelvic mass. In three large studies that screened 66,620 women with TVS, 565 operations were performed to detect 45 ovarian cancers, 34 of which were invasive.102–104 Overall, the sensitivity for early-stage disease was 78%, but the specificity fell just short of that required for a PPVof 10% with 12 operations per case of ovarian cancer detected. The most promising single study achieved a PPV of 9.9%. Confirmatory tests with Doppler ultrasound have not proved consistent, but additional studies with 3-D power Doppler are underway to improve specificity in distinguishing malignant from benign ovarian abnormalities.
CA125
CA125 is elevated in 50–60% of patients with stage I and in 90% with stage II ovarian cancer.105 CA125 levels can rise 10–60 months before diagnosis with an average estimated lead time of 1.9 years before diagnosis of disease in all stages.106 In the Prostate, Lung, Colorectal and Ovarian (PLCO) screening trial, 37,500 postmenopausal women had an annual CA125 and TVS for 3 years.107 If either were abnormal, women were referred to a gynecologist. CA125 alone had a PPV of 3.7%; TVS alone had a PPV of 1%. If both were abnormal, the PPV rose to 23.5%, but 60% of the invasive ovarian cancers would have been missed. Thus, the specificity for a single determination of CA125 or a single TVS is not adequate to screen a population at average risk, but specificity can be improved with a two-stage strategy that utilizes CA125 followed by ultrasound in a subset of women with elevated CA125. Use of CA125 to trigger ultrasound has been evaluated in trials in Sweden and in the United Kingdom.108, 109 The latter randomized 22,000 women to conventional surveillance or to annual CA125 with TAU if the value were elevated. When TAU was abnormal, surgery was undertaken. Among 10,985 women screened, 29 operations were performed to detect 6 cancers, providing a PPV of 21%. During 7 years of follow-up, 10 more cancers were diagnosed in the screened group. Over the same intervals, 21 ovarian cancers were diagnosed in the control group. Median survival in the screened group (72.9 months) was significantly greater (p = 0.0112) than that in the control group (41.8 months).
Risk of ovarian cancer (ROC) algorithm
A rising CA125 is a more specific indicator of ovarian cancer. Analyzing serum samples stored from screening studies in Stockholm and in the United Kingdom with an improved CA125 II assay, it has been possible to improve the specificity of CA125 as a screening tool by following the values of an individual over time.110, 111 Elevated CA125 levels in women without ovarian cancer remain static or decrease with time, whereas levels associated with ovarian malignancy tend to rise. This finding has been incorporated into an algorithm that uses age, rate of change of CA125, and absolute levels of CA125 to calculate an individual’s “risk of ovarian cancer” (ROCA, risk of ovarian cancer algorithm). Patients at sufficient risk undergo TVS. Over the past 15 years, a trial has been conducted with the ROCA in the United States coordinated by the MD Anderson SPORE in Ovarian Cancer. Some 18 patients have undergone surgery based on the algorithm. Six have had benign disease, two have had borderline tumors (stage I) and ten have had invasive ovarian cancers with seven in early stage (I–II) and three in stage III. Only three operations have been required to detect each case of ovarian cancer.112 Currently, accrual to a larger trial has been completed in the United Kingdom that includes 200,000 postmenopausal women who were randomized to three groups: a control group (∼100,000) that has been followed with conventional pelvic examinations; a second group (∼50,000) that had annual TVS; and a third group (∼50,000) that had CA125 determined at least annually. On the basis of the ROCA, patients in the third group were referred for TVS and/or surgery. Women were screened for 3 years and followed for 7 years. Results from the initial 3 years of the “prevalence phase” of accrual suggested that a higher fraction of early stage disease could be detected (48%) with the ROCA and that no more than 3–4 operations would be required for each case detected.113 Results of the study will become available in late 2015, but it is already apparent that use of the ROCA doubled the number of screen-detected ovarian cancers compared to use of an arbitrary threshold for CA125.114 Results from the first year of screening suggest that the ROCA followed by TVS will have substantially higher specificity and no less sensitivity than annual TVS, but data in subsequent years will be required to determine whether screening improves survival.
Whatever the outcome of the current trial in the United Kingdom, strategies based on CA125 alone are not likely to exceed a sensitivity of 80%, as CA125 is not expressed by 20% of epithelial ovarian cancers. Greater sensitivity might be attained through the use of multiple serum markers in combination, provided that specificity was not compromised. Two other biomarkers—HE4 and CA72.4—detect a fraction of patients (15%) missed by CA125. Autoantibodies against TP53 can also detect 18% of patients missed by CA125 and provide 13–33 months of lead time.
Current recommendations for screening women at average risk
The application of screening techniques other than pelvic examination for ovarian cancer in the entire female population is unwarranted at this time. The sensitivity and specificity of ultrasound or CA125 alone are inadequate. Use of annual CA125 followed by TVS appears more promising, but application of this approach outside of a research study will depend on the outcome of the UKCTOCS trial. If a significant improvement in survival and mortality are observed, the MD Anderson trial has shown that this approach is feasible in the United States.
Current recommendations for screening women at high risk
Although ultrasound and CA125 screening have been advocated for women at increased genetic ROC, the efficacy of surveillance to reduce mortality or detect cancers at an earlier stage is unproved. Many of the occult cancers found after PBSO (prophylactic bilateral salpingo-oophorectomy) have been in the fimbrial end of the fallopian tube, and this consistent finding suggests that ultrasound of the ovaries is unlikely to detect cancers at an early stage in women at high genetic risk. Screening can be problematic because this high-risk population generally includes premenopausal women who have a higher incidence of false-positive CA125 elevations and ultrasound abnormalities. In these high-risk populations, initial screening trials using ultrasound alone or in combination with color-flow Doppler were associated with high false-positive rates (2.5–4.9%). The current trend among those who support screening is to combine ultrasound every 6–12 months with CA125 every 3–6 months.
There are five prospective studies where combined screening has been undertaken in high-risk populations.33, 115–118 In three screening programs involving a total of 1228 women with a family history of ovarian cancer, no invasive ovarian cancer was detected and false-positive rates have ranged from 0.4% to 3.9%.33, 48, 49, 115–119 In one of the remaining two studies, one case of ovarian cancer was detected on screening 137 high-risk women with a false-positive rate of 0.7%; in the other study, nine ovarian cancers were detected in screening 180 women with a false-positive rate of 3.9%.117, 118 The findings of two prospective studies of annual transvaginal ultrasound and CA125 screening in 888 BRCA1 and BRCA2 mutation carriers in the Netherlands and 279 mutation carriers in the United Kingdom are not encouraging and suggest a very limited benefit of screening in high-risk women.48, 49 Therefore, it is unlikely that annual screening will reduce mortality from ovarian cancer in BRCA1/2 mutation carriers. Women in the high-risk population who request screening should be counseled about the current lack of evidence for the efficacy for either CA125 or sonography as well as the associated false-positive rates. Many will still opt for screening despite the risks and limitations of the available strategies.
Staging
Ovarian, fallopian tube and peritoneal malignancies are staged according to the new FIGO system of 2014 (Table 5) that is based on the findings at surgical exploration. A preoperative evaluation should exclude the presence of extraperitoneal metastases. A thorough surgical exploration is important because subsequent treatment will be determined by the stage of disease. In patients whose exploratory laparotomy does not reveal any macroscopic evidence of disease by inspection and palpation of the entire intra-abdominal space, a careful search for microscopic spread must be undertaken. In an earlier series in which patients did not undergo careful surgical staging, the overall 5-year survival for patients with apparent stage I epithelial ovarian cancer was only about 60%.4 Survival rates of 90–100% have been reported for properly staged patients found to have stage IA or IB disease.4
Table 5 FIGO staging of ovarian, fallopian tube, and peritoneal cancer (2014)
| Stage I: Tumor confined to ovaries or fallopian tube(s) | T1-N0-M0 |
| IA: Tumor limited to one ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings | T1a-N0-M0 |
| IB: Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings | T1b-N0-M0 |
| IC: Tumor limited to one or both ovaries or fallopian tubes, with any of the following | |
| IC1: Surgical spill | T1c1-N0-M0 |









