Epithelial ovarian cancer has the highest fatality-to-case ratio of all the gynecologic malignancies, because more than two-thirds of patients have advanced disease at diagnosis (1,2). It presents a major surgical challenge, requires intensive and often complex therapies, and is extremely demanding of the patient’s psychological and physical energy. High-grade serous carcinomas, which are the most common, are now believed to be related etiologically to fallopian tube and peritoneal cancer and most appear to arise from the fimbria of the fallopian tube (3–11).
There are nearly 22,000 new cases of ovarian cancer annually in the United States, and more than 14,000 women can be expected to succumb to their illness (1). It is the fifth most common cancer in women in the United States after cancers of the lung, breast, colon, and uterus. It accounts for 4% of all female cancers and 31% of cancers of the female genital tract. Ovarian cancer is the fourth most common cause of death from malignancy in women (1). A woman’s risk at birth of having ovarian cancer some time in her lifetime is nearly 1.4%, and the risk of dying from ovarian cancer is almost 1% (2). The number of new cases per 10,000 women per year was 12.5 in 2010 (2).
There is a trend toward improved survival for ovarian cancer (1,2). Based on Surveillance, Epidemiology, and End Results (SEER) data in the United States, the 5-year survival for all stages combined increased from 33.6% in 1975 to 44.2% in 2003 to 2009 (2). Using statistical models for analysis, rates for new ovarian cancer cases have been falling on average 1% per year over the last 10 years. Death rates have been falling on average 1.6% per year over the same period. The numbers of new cases, deaths, and 5-year relative survival trends are presented in Figure 11.1. The death rate decreased 22% from 10 per 100,000 women per year in 1976 to 7.8 per 100,000 per women per year in 2010 (2). Ovarian cancer rates are highest in women aged 55 to 64 years (median age 63 years), and deaths are highest in people aged 75 to 84 years (median age 71 years) (2). The percentage of new ovarian cancer cases by age group and the percentage of deaths by age group are presented in Figure 11.2.
Classification
Approximately 90% of ovarian cancers are derived from cells of the coelomic epithelium or modified mesothelium (3). The cells are a product of the primitive mesoderm, which can undergo metaplasia. Neoplastic transformation can occur when the cells are genetically predisposed to oncogenesis or exposed to an oncogenic agent.
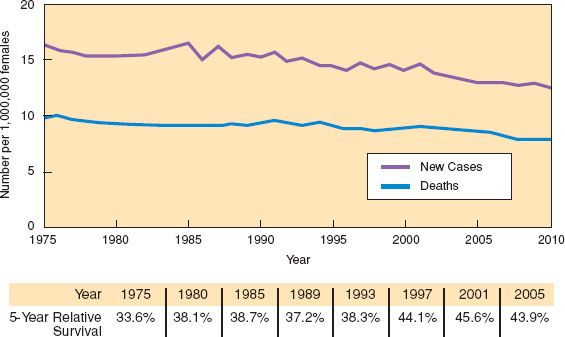
Figure 11.1 Ovarian cancer: New cases, deaths, and 5-year relative survival. SEER 9 Incidence and U.S. Mortality 1975–2010, all races, females. Rates are age adjusted. SEER Cancer Statistics Factsheets: Ovary Cancer. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/statfacts/html/ovary.html
Epithelial ovarian cancers are thought to arise either from the fimbriated end of the fallopian tube or from a single layer of cells that covers the ovary or lines cysts immediately beneath the ovarian surface. These latter cells are generally quiescent, but proliferate following ovulation to repair the defect created by rupture of a follicle.
There are at least two different molecular pathways that lead to the development of ovarian cancers, and these develop into tumors that have quite distinct biologic behaviors and probably different cells of origin (4). There are those tumors (so-called type I tumors) that arise from ovarian surface epithelium and müllerian inclusions, either from endosalpingiosis or invagination of the ovarian surface epithelium during repair of ovulation or implantation of cells from endometrium. This process typically involves a relatively slow and multistep pathway and accounts for many early-stage cancers such as endometrioid, clear cell, mucinous, and low-grade serous cancers. In contrast, the more common high-grade serous cancers (type II) have a phenotype that resembles the fallopian tube mucosa, and they commonly have p53 mutations. These tumors appear to develop rapidly, and are almost always at an advanced stage at presentation. Many appear to arise in the fallopian tube (4).
Pathology
Invasive Cancer
Approximately 75–80% of epithelial cancers are of the serous histologic type, with the majority being high-grade cancers. Less common subtypes are mucinous (10%), endometrioid (10%), clear cell, Brenner, and undifferentiated carcinomas (3). Each tumor type has a histologic pattern that recapitulates the epithelial features of a section of the lower genital tract. For example, the serous pattern has an appearance similar to that of the glandular epithelium lining the fallopian tube, and it is now appreciated that many high-grade serous epithelial cancers originate in fimbriae of the distal fallopian tubal epithelium (5–11). High-grade serous cancers commonly involve both the ovaries and tubes and it can be difficult to ascertain the site of origin (7–11). Mucinous tumors resemble the endocervical glands, and the endometrioid tumors resemble the endometrium. More specific details of the histopathology are discussed in Chapter 5.
There is increasing evidence to demonstrate that many high-grade serous ovarian cancers arise in the fallopian tube (7–11), a situation that may have been obscured by an overly rigid WHO definition of fallopian tube cancer. Molecular and genetic evidence supports this etiology, as well as the finding of carcinoma in situ in the tubal epithelium that appears to be the precursor of high-grade serous carcinomas (4).

Figure 11.2 Ovarian cancer: Percentage of cases by A: age group, and B: percentage of deaths by age group. U.S. 2006–2010, all races, females. SEER Cancer Statistics Factsheets: Ovary Cancer. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/statfacts/html/ovary.html
It is now believed that most high-grade serous cancers of the ovary, fallopian tube carcinomas, and peritoneal carcinomas should be regarded as a single disease entity. Although a substantial proportion of cancers appear to arise in the fimbriated end of the fallopian tube, this site cannot account for all of these cancers, and a proportion is thought to be derived from components of the secondary müllerian system (12).
In histologic features and clinical behavior, fallopian tube carcinoma is the same as ovarian cancer; thus, the management is the same. Almost all fallopian tube cancers are of “epithelial” origin, most frequently of serous histology. Rarely, sarcomas have also been reported. The fallopian tubes are frequently involved secondarily from other primary sites, most often the ovaries, endometrium, gastrointestinal tract, or breast (1). They may be secondarily involved in peritoneal cancer.
Peritoneal serous carcinomas are identical pathologically to serous cancers arising in the fallopian tube or ovary and have the same biologic behavior as well as response to chemotherapy. They are thought to arise from müllerian remnants in the coelomic epithelium or alternatively to arise in the fimbria of the fallopian tube. In most cases there may be microscopic or small macroscopic cancer on the surface of the ovary and extensive disease in the upper abdomen, particularly in the omentum. Peritoneal carcinoma also explains how “ovarian cancer” can arise in a patient whose ovaries were surgically removed many years earlier, although in some patients this may be due to the fallopian tubes not being removed or due to an ovarian remnant (13–15).
Based on these data, patients with epithelial ovarian, fallopian tube, and peritoneal cancer are now staged by the same International Federation of Gynecology and Obstetrics (FIGO) system, and evaluated and treated in the same manner. The discussion that follows regarding ovarian cancer includes patients with fallopian tube and peritoneal cancer.
Borderline Tumors
An important group of tumors to distinguish is the tumors of low malignant potential, also called the borderline tumors (16–19). Borderline tumors are usually confined to the ovary at diagnosis, occur predominantly in premenopausal women, and are associated with a very good prognosis. They are diagnosed most frequently between the ages of 30 and 50 years, while invasive carcinomas occur more commonly between the ages of 50 and 70 years (3). Although uncommon, peritoneal implants may occur and may be either noninvasive or invasive. Invasive implants are associated with a higher likelihood of progression, which can lead to intestinal obstruction and death (17–19).
Clinical Features
As noted above, the peak incidence of invasive epithelial ovarian cancer is ages 55 to 64 years with a median of age 63 years (2,3,20) (Fig. 11.2). The age-specific incidence of this disease rises precipitously from 20 to 80 years of age and subsequently declines (21). By contrast, the average patient age of those with borderline tumors is approximately 46 years (3,12). Eighty percent to 90% of ovarian cancers, including borderline forms, occur after the age of 40 years.
The chance that a primary epithelial tumor will be of borderline or invasive malignancy in a patient younger than age 40 years is approximately 1 in 10, but after that age, it rises to 1 in 3 (3). Less than 1% of epithelial ovarian cancers occur before the age of 20, two-thirds of ovarian malignancies in such patients being germ cell tumors (3,21). Approximately 30% of ovarian neoplasms in postmenopausal women are malignant, whereas only about 7% of ovarian epithelial tumors in premenopausal patients are frankly malignant (3).
Epidemiology
Ovarian cancer has been associated with low parity and infertility (22). Although there has been a variety of epidemiologic variables correlated with ovarian cancer—such as increased risk with talc use, galactose consumption, and decreased risk with tubal ligation and the oral contraceptive pill usage (see Chapter 6)—none has been so strongly correlated as prior reproductive history and duration of the reproductive career (22–24). Early menarche and late menopause increase the risk of ovarian cancer (23). These factors and the relationship of parity and infertility to the risk of ovarian cancer have led to the hypothesis that suppression of ovulation may be an important factor. Theoretically, the surface epithelium undergoes repetitive disruption and repair. It is thought that this process might lead to a higher probability of spontaneous mutations that can unmask germ line mutations or otherwise lead to the oncogenic phenotype (see Chapter 1).
In a cohort study of more than 1.1 million Norwegian women, a positive association was found between body mass index (BMI), height, and risk of ovarian cancer, particularly of the endometrioid type in women younger than 60 years (25). Women who had a very high BMI and were clinically obese in adolescence and childhood had a relative risk of 1.56 of developing ovarian cancer compared with women with a medium BMI.
There has been considerable controversy as to whether fertility-enhancing drugs increase the risk of ovarian cancer. In a meta-analysis of eight case-control studies (26), there were 5,207 women with cancer compared with 7,705 controls. The relative risk (RR) of fertility drug exposure for ovarian cancer was 0.97—that is, the use of the drugs was not associated with an increased risk. However, in the same cohort, nulliparity (compared with multiparity greater than 4) carried a RR of 2.42, and infertility per se for 5 years or longer (compared with shorter than 1 year) carried a RR of 2.7. These results support the hypothesis that the higher risk in these women is related to infertility, independent of fertility drug use.
Most case-control and cohort studies have failed to link hormone replacement therapy to an increased risk of epithelial ovarian cancer (27). A large cohort study has reopened controversy regarding this issue (28). Among 44,241 postmenopausal women in the Breast Cancer Detection Demonstration Project, 329 developed ovarian cancer. Women who had received estrogen replacement therapy only for more than 10 years without progestin were at increased risk. By 20 years, the relative risk was 3.2-fold.
The incidence of ovarian cancer varies in different geographic locations throughout the world. Western countries, including the United States and the United Kingdom, have an incidence of ovarian cancer that is three to seven times greater than in Japan, where epithelial ovarian tumors are considered rare (3). In Asia, the incidence of germ cell tumors of the ovary appears to be somewhat higher than in the West. Japanese immigrants to the United States exhibit a significant increase in the incidence of epithelial ovarian cancer, the rate eventually approaching that of white US women. The incidence of epithelial tumors is about 1.5 times greater in whites than in blacks (1).
Prevention
As parity is inversely related to the risk of ovarian cancer, having at least one child is protective of the disease, with a risk reduction of 0.3 to 0.4. The oral contraceptive reduces the risk of epithelial ovarian cancer (22). Women who use the oral contraceptive for 5 or more years reduce their relative risk to 0.5—that is, there is a 50% reduction in the likelihood of developing ovarian cancer. Women who have had two children and have used the oral contraceptive for 5 or more years have a relative risk of ovarian cancer as low as 0.3, or a 70% reduction (29). Therefore, the oral contraceptive pill is the only documented method of chemoprevention for ovarian cancer, and it should be recommended to women for this purpose. When counseling patients regarding birth control options, this important benefit of the oral contraceptive should be emphasized. This is also important for women with a strong family history of ovarian cancer.
Fenretinide, a retinoid, was thought to be a chemoprophylactic agent for ovarian cancer (30). However, in a prospective, randomized, placebo-controlled trial, there was no difference in the incidence or survival from ovarian cancer after 5 years (31). The Gynecology Oncology Group (GOG) initiated a confirmatory trial, but it was closed because of poor accrual.
As many high-grade serous epithelial tumors arise in the fallopian tube and because there is a higher rate of tubal carcinoma in women with BRCA1 and BRCA2 mutations, it is essential that risk-reducing prophylactic surgery includes the removal of both ovaries and both fallopian tubes. The performance of a prophylactic salpingo-oophorectomy will significantly reduce, but not eliminate, the risk of ovarian and fallopian tube cancer (14,15) because the entire peritoneum is potentially at risk and serous cancers may arise in the secondary müllerian system (19). The ovaries provide protection from cardiovascular disease and osteoporosis, so prophylactic bilateral salpingo-oophorectomy should not be routinely performed in premenopausal women at low risk for ovarian cancer.
Screening
There is no proven effective method of screening for ovarian cancer (see Chapter 10). Routine annual pelvic examinations are not considered to be an effective method of screening (32), and given the false positive results for both CA125 and transvaginal ultrasonography, particularly in premenopausal women, these tests are not cost-effective and have not been found to detect ovarian cancer at an early stage. Recent advances in transvaginal ultrasonography (33–39) have been reported by some groups to have a very high (>95%) sensitivity for the detection of early-stage ovarian cancer, although this test alone might result in up to 15 unnecessary laparotomies for every ovarian cancer detected (40). Transvaginal color-flow Doppler to assess the vascularity of the ovarian vessels may be a useful adjunct to ultrasonography (34–36).
In the future, new markers or technologies may improve the specificity of screening, but proof of this will require large prospective studies (37–47). Even screening in women at higher risk of ovarian cancer because of a strong family history or known BRCA mutation has not been found to be effective. These women should be advised to have risk reducing bilateral salpingo-oophorectomy.
CA125 screening has previously been thought to allow the earlier diagnosis of epithelial ovarian cancer (40–47). However, screening trials have not found a lower incidence of stage I tumors in women at population risk. For example, in the Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial, which included an annual CA125 and transvaginal ultrasound, the stage distributions were similar to the study group, with stage III and IV cancers comprising the majority of cases in both the screened group (77%) and usual care group (78%).
CA125 has been cloned, and although the function of the molecule is unclear, the elucidation of the MUC16 gene and its control may enhance the understanding of this important marker in ovarian cancer (48–50). Binding of MUC16 to mesothelin appears to mediate cell adhesion and facilitates peritoneal metastasis in animal models (51–53). Data suggest that the specificity of CA125 is improved when the CA125 levels are followed over time (47–57). The risk of ovarian cancer (ROC) algorithm might help to improve the efficacy of screening (57), although this was not confirmed even with every 4-month CA125 levels in the UKFOCCS trial (58).
A number of novel markers for ovarian cancer have been identified in recent years, including mesothelin, a 110-kd fragment of EGFR (sEGFR), and HE4 (59–65). Multiplex assays can measure more than 50 biomarkers with a few hundred microliters of serum (66), and using this technology, a combination of CA125, HE4, sEGFR, and soluble vCAM-1 has distinguished 90% of stage I ovarian cancer patients from 98% of healthy controls. The use of surface-enhanced laser desorption and ionization (SELDI) with subsequent resolution by mass spectroscopy has demonstrated a pattern of low molecular weight moieties that has been reported to distinguish sera from ovarian cancer patients from those of healthy individuals (67); however, methodologic issues have been raised regarding these data (68,69).
Genetic Risk for Epithelial Ovarian Cancer
Hereditary Ovarian Cancer
The risk of ovarian cancer is higher than that of the general population in women with a family history of breast or ovarian cancer as well as in families with Lynch syndrome (70–86). Although most epithelial ovarian cancer is sporadic, at least 10–14% of patients have a germ line mutation in BRCA1 or BRCA2 (73,85,86). Further discussion of germ line mutations and their biology is presented in Chapter 1.
BRCA1 and BRCA2
Most hereditary ovarian cancer results from mutations in the BRCA1 gene, which is located on chromosome 17 (72), with a smaller proportion associated with mutations in BRCA2, which is located on chromosome 13 (47). Although these appear to be responsible for most hereditary ovarian cancers, it is likely that there are other, as yet undiscovered, genes that also predispose to ovarian or breast cancer or both (81).
In the past, it had been thought that there were two distinct syndromes associated with a genetic risk: site-specific hereditary ovarian cancer and hereditary breast or ovarian cancer syndrome. It is now accepted that these groups represent a continuum of mutations of BRCA1 and BRCA2, with different degrees of penetrance within a given family (49,57). There are other less common genetic causes of ovarian cancer, and there is a higher risk of ovarian and endometrial cancer in women with the Lynch syndrome, which was also known as the hereditary nonpolyposis colorectal cancer syndrome (HNPCC syndrome) (82).
The mutations are autosomal dominant, and thus a complete family history and pedigree analysis, including both maternal and paternal sides of the family, must be evaluated (76). There are numerous distinct mutations that have been identified on each of these genes, and the mutations may have different degrees of penetrance, which may explain the preponderance of either breast cancer, ovarian cancer, or both, in any given family. A combined analysis of 22 studies unselected for family history has found that women who have a germ line mutation in the BRCA1 gene have a lifetime risk of ovarian cancer of 39% (18–54%), and the risk has been calculated to be 11% (2.4–19%) for women with a BRCA2 mutation (73,74,80). In this study, women with a BRCA1 or BRCA2 mutation had a risk of breast cancer of 65% and 45%, respectively (87). These figures are somewhat lower than the estimates based on multiple case families, which may be enriched for mutations of higher risk. A large consortium found genetic risk modifiers on 4q32.2 and 17q21.31 that significantly increased the risk of developing ovarian cancer in BRCA1-mutation carriers, which may explain the variable risks that have been reported (88).
Hereditary ovarian cancers, in particular, BRCA1-associated ovarian cancers, generally occur in women approximately 10 years younger than those with nonhereditary tumors (73,84). As the median age of epithelial ovarian cancer is 62 to 63 years, a woman with a first- or second-degree relative who had early onset ovarian cancer may have a higher probability of having a BRCA 1 or BRCA 2 mutation. For example, a recent Australian population based study found that 22.2% of women diagnosed before age 50 years carried a BRCA mutation, compared with 12.1% of those older than 50 years. There was no potentially significant family history in 44% (95% CI, 35.8–52.2%) of mutation-positive women, which underscores the importance of offering mutation testing to all women with ovarian cancer under the age of 70 irrespective of family history (89).
Women with BRCA mutations are also at increased risk of breast cancer. The breast cancers typically occur at a young age and may be bilateral. There is a higher incidence of triple-negative breast cancers in women with BRCA1 mutations.
Founder Effect
There is a higher carrier rate of BRCA1 and BRCA2 mutations in women of Ashkenazi Jewish descent, Icelandic women, and in other ethnic groups (78,79,81). There are three specific founder mutations that are carried by the Ashkenazi population: 185delAG and 5382insC on BRCA1, and 6174delT on BRCA2. The carrier rate of at least one of these mutations for a patient of Ashkenazi Jewish descent is 1 in 40 or 2.5%, which is considerably higher than the general Caucasian population. The increased risk is a result of the founder effect—that is, a higher rate of mutations that have occurred within a specific population group within a defined geographic area.
Pedigree Analysis
The risk of ovarian cancer depends on the number of first- or second-degree relatives with a history of epithelial ovarian carcinoma or breast cancer, and on the age of onset. The degree of risk is difficult to determine precisely unless a full pedigree analysis is performed, and all patients should be referred to a familial cancer service for genetic counseling.
Risch et al. (85) reported that the hereditary proportion of invasive ovarian tumors was approximately 13%, and was as high as 18% in the large subgroup of women with high-grade serous ovarian cancers. This was independent of family history. Similar findings have been reported in a smaller study from Poland (86), and confirmed in a large prospective Australian study (89). These findings have major implications for genetic testing, and argue for consideration of BRCA genetic testing in all patients with high-grade serous cancers irrespective of the pedigree and family history. Recent Australian guidelines recommend genetic testing for all women diagnosed with nonmucinous ovarian cancer under the age of 70.
Lynch Syndrome
Lynch syndrome is defined as a hereditary predisposition to colorectal cancer and a wide range of other malignancies (e.g., endometrial, ovarian, and gastric cancer) as a result of a germ line mismatch repair (MMR) genetic mutation (82). The mutations that have been associated with this syndrome are MSH2, MSH6, MLH1, PMS1, and PMS2. The risk of endometrial cancer equals or exceeds that of colorectal cancer in women with Lynch Syndrome. The diagnosis of gynecologic cancer precedes that of colorectal cancer in over half the cases, making gynecologic cancer a “sentinel cancer” for Lynch syndrome.
The lifetime risk of ovarian cancer in women with Lynch Syndrome has been estimated at approximately 6–12%. The mean age at diagnosis is 42.7 to 49.5 years. There is a higher risk of endometrioid and clear cell subtypes, and the majority of cases are stages I or II at diagnosis. It is important to take a full family history in all patients, but family history alone cannot be relied on to identify cases (90).
Management of Women at High Risk for Ovarian Cancer
The management of a woman with a strong family history of epithelial ovarian cancer must be individualized and will depend on her age, her reproductive plans, and the estimated level of risk. A thorough pedigree analysis is important. A geneticist should evaluate the family pedigree for at least three generations. Decisions about management are best made after careful study of the pedigree and, whenever possible, verification of the histologic diagnosis of the family members’ ovarian cancer as well as the age of onset and other tumors in the family.
The value of testing for BRCA1 and BRCA2 has been clearly established, and guidelines for testing now exist (76,83,84). The American Society of Clinical Oncologists has provided guidelines that emphasize careful evaluation by geneticists, careful maintenance of medical records, and a clear understanding of how to counsel and manage these patients. There remain concerns of the impact on insurability, how the results will be interpreted, and how the information will be used within a specific family—for example, to counsel children.
Although there are some conflicting data, the outcomes of women with breast cancer and with germ line mutations in BRCA1 or BRCA2 are comparable to women with sporadic tumors (75,91,92). Women with breast cancer who carry these mutations are at a greatly increased risk of ovarian cancer, as well as of a second breast cancer. The lifetime risk of ovarian cancer is 54% for women who have a BRCA1 mutation and 23% for those with a BRCA2 mutation; for the two groups together, there is an 82% lifetime risk of breast cancer (84).
Although recommended by the National Institutes of Health Consensus Conference on Ovarian Cancer (93), the value of screening with transvaginal ultrasonography and CA125 has not been established in women at high risk. The findings of two prospective studies of annual transvaginal ultrasonography and CA125 screening in 888 BRCA1 and BRCA2 mutation carriers in the Netherlands and 279 mutation carriers in the United Kingdom are not encouraging, and suggest a very limited benefit, if any, of screening even in high-risk women (94,95). Despite annual gynecologic screening, Hermsen et al. (94) reported that a high proportion of ovarian cancers in BRCA1 and BRCA2 carriers were interval cancers, and the majority of all cancers diagnosed were at an advanced stage. Similar findings were reported by Woodward (95). Therefore, it is unlikely that annual screening will reduce mortality from ovarian cancer in BRCA1 and BRCA2 mutation carriers (94,96).
This important question has also been addressed in GOG 199, a study of screening with annual transvaginal ultrasonography and CA125 ROCA compared to prophylactic bilateral salpingo-oophorectomy. Study accrual was completed in November 2006, with 2,605 participants enrolled: 1,030 (40%) in the surgical cohort and 1,575 (60%) in the screening cohort (97). Five years of prospective follow-up ended in November 2011 (see Chapter 10).
Data derived from a multi-institutional consortium of genetic screening centers has suggested that the use of the oral contraceptive pill is associated with a lower risk of ovarian cancer in women who have a BRCA1 or BRCA2 mutation (98). In women who had taken the oral contraceptive pill for five or more years, the relative risk of ovarian cancer was 0.4, or a 60% reduction in incidence. Another study failed to confirm this finding (99). Tubal ligation may also decrease the risk of ovarian cancer in patients with a BRCA1 but not BRCA2 mutation in one study, but the protective effect is not nearly as strong as risk-reducing bilateral salpingo-oophorectomy (100).
The value of prophylactic risk-reducing bilateral salpingo-oophorectomy in these patients has been well documented (101–106). Occult ovarian/fallopian tube cancers detected at the time of risk-reducing bilateral salpingo-oophorectomy have been reported in many studies with wide variability in reported prevalence ranging from 2.3–23%. This may reflect selection bias as well as inadequate pathologic review of the fallopian tubes and ovaries (102). Domchek et al. (102) estimated the prevalence of occult cancers in a prospective cohort of 647 BRCA1/2 mutation carriers from 18 centers (PROSE consortium) who underwent risk-reducing bilateral salpingo-oophorectomy between 2001 and 2008. An occult cancer was detected in 16 of 647 women (2.5%). Thirty-eight percent of women had stage I cancer versus none of the women in the PROSE database diagnosed with ovarian cancer outside of screening. Ovarian and fallopian tube tissues removed at major genetic referral centers were significantly more likely to have been examined completely by pathologists compared to specimens obtained at nonreferral centers (75% vs. 30%, p < 0.001), and the authors commented that an unacceptably high proportion of pathologic examinations did not adequately examine ovaries and fallopian tubes obtained at risk-reducing bilateral salpingo-oophorectomy (RRSO) (102).
The performance of a prophylactic salpingo-oophorectomy reduces the risk of BRCA-related gynecologic cancer by 96% (104). There remains a small risk of subsequently developing a peritoneal carcinoma, a tumor that may also have a higher predisposition in women who have mutations in the BRCA1 and BRCA2 genes. In these series, the risk of developing peritoneal carcinoma was 0.8% and 1%, respectively (102,103). Prophylactic salpingo-oophorectomy in premenopausal women reduced the risk of developing subsequent breast cancer by 50–80% (102,103).
The role of hysterectomy is more controversial. Although most studies show no increase in the rate of uterine and cervical tumors, there are some isolated reports of an increased risk of papillary serous tumors of the endometrium (107). Women on tamoxifen are at higher risk for benign endometrial lesions (e.g., polyps) and have a twofold higher risk of endometrial cancer. In selected patients who have completed childbearing, the performance of a prophylactic hysterectomy in conjunction with salpingo-oophorectomy may be considered on an individual basis.
Grann et al. (108) reported the application of Markov modeling—that is, quality-adjusted survival estimate analysis—in a simulated cohort of 30-year-old women who tested positive for BRCA1 or BRCA2 mutations. The analysis predicted that a 30-year-old woman could prolong her survival beyond that associated with surveillance alone by 1.8 years with tamoxifen, 2.6 years with prophylactic salpingo-oophorectomy, 4.6 years with both tamoxifen and prophylactic salpingo-oophorectomy, 3.5 years with prophylactic mastectomy, and 4.9 years with both prophylactic surgeries. Quality-adjusted life expectancy was estimated to be prolonged by 2.8 years for tamoxifen, 4.4 years with prophylactic salpingo-oophorectomy, 6.3 years for tamoxifen and prophylactic salpingo-oophorectomy, 2.6 years with mastectomy, and 2.6 years with both operations. This has been supported by a study of women with BRCA1 and BRCA2 mutations which found that risk-reducing mastectomy was associated with a lower risk of breast cancer, risk-reducing bilateral salpingo-oophorectomy was associated with a lower risk of ovarian cancer, and that there was an improvement in all-cause mortality, breast cancer-specific mortality as well as ovarian cancer-specific mortality (109).
The survival of women who have a BRCA1 or BRCA2 mutation and develop ovarian cancer is longer than that for those who do not have a mutation. In one study, the median survival for mutation carriers was 53.4 months compared with 37.8 months for those with sporadic ovarian cancer from the same institution (110). These findings have recently been confirmed in a population-based study from Israel in which Chetrit et al. (111) reported that among Ashkenazi women with ovarian cancer, those with BRCA1 and BRCA2 mutations had an improved long-term survival (38% vs. 24% at 5 years). This may result from distinct clinical behavior or from a better response to chemotherapy.
Recommendations
Current recommendations are that all women under the age of 70 with a nonmucinous epithelial ovarian, fallopian tube, or peritoneal cancer should undergo testing for BRCA1 to BRCA2 (112). The recommendations for management of women at high risk for ovarian cancers are summarized below (83,84,93,98–108):
1. Women who appear to be at high risk for ovarian and or breast cancer should undergo genetic counseling; if there is a probability of 10% or greater of having a BRCA mutation, they should be offered genetic testing for BRCA1 and BRCA2.
2. Women who wish to preserve their reproductive capability or delay prophylactic surgery should undergo periodic screening by transvaginal ultrasonography every 6 months, although the efficacy of this approach has not been established.
3. Oral contraceptives should be recommended to young women before a planned family.
4. Women who do not wish to maintain their fertility or who have completed their family should be recommended to undergo prophylactic bilateral salpingo-oophorectomy. The majority of BRCA1-related ovarian cancers occur in women after the age of 40, and BRCA2 ovarian cancers are more likely in postmenopausal women. The risk of ovarian cancer under the age of 40 is very low. The potential risk should be clearly documented and preferably established by BRCA1 and BRCA2 testing. These women should be counseled that this operation does not offer absolute protection, because peritoneal carcinomas may occasionally occur (102,103). A prophylactic hysterectomy is acceptable, and the option should be discussed with these patients.
5. In women who have a strong family history of breast or ovarian cancer, annual mammographic and magnetic resonance imaging (MRI) screening should be performed commencing at age 30 years, or younger if there are family members with documented very early onset breast cancer.
6. Women with a documented Lynch syndrome should be counseled about prophylactic hysterectomy and oophorectomy after childbearing, in view of the risk of both endometrial and ovarian cancer. Although there are no definitive studies to support screening, endometrial sampling and transvaginal ultrasound of the ovaries may be considered from ages 30 to 35. Colonoscopy is recommended every 1 to 2 years starting from age 20 to 25 or 10 years younger than the youngest person diagnosed in the family (82,90,113).
Symptoms
The majority of women with epithelial ovarian cancer have vague and nonspecific pelvic, abdominal, and menstrual symptoms (114–118). Goff et al. (119) recently developed an ovarian cancer symptom index and reported that symptoms associated with ovarian cancer were pelvic or abdominal pain, urinary frequency or urgency, increased abdominal size or bloating, and difficulty eating or feeling full. These symptoms were particularly suspicious when they were present for less than 1 year and lasted longer than 12 days a month. The index had a sensitivity of 56.7% for the diagnosis of early ovarian cancer and 79.5% for advanced-stage disease.
A study from the Royal Hospital for Women in Sydney compared 100 patients with early-stage epithelial ovarian cancer with 100 patients with advanced-stage disease. Ninety percent of women with early disease and 100% with advanced disease reported at least one symptom. With early disease, abdominal pain was reported by 51% and abdominal swelling by 32%. With advanced disease, abdominal swelling was reported by 62% and abdominal pain by 40%. Seventy percent of patients with early disease and 69% of those with advanced disease reported symptoms of less than 3 months duration. Patients with tumors less than 5 cm in diameter were three times more likely to have advanced disease. Patients with grade 1 tumors were 40 times more likely to have early-stage disease when compared to patients with grade 3 tumors (120). These findings were confirmed in a population-based study from Australia in which there did not appear to be a significant difference in the duration of symptoms or the nature of symptoms in patients with early as opposed to advanced-stage disease (121). These two studies reinforce the concept that early- and late-stage ovarian cancer are biologically different entities, and argue against the widely held misconception that ovarian cancer is diagnosed at an early stage because the symptoms are recognized earlier than in patients with more advanced disease (96,120–122).
Of historical interest is the so-called “classic triad” of symptoms and signs associated with fallopian tube cancer: watery vaginal discharge (hydrops tubae profluens), pelvic pain, and a pelvic mass. This triad is noted in fewer than 15% of patients (123).
Signs
The most important sign is the presence of a pelvic mass on physical examination. A solid, irregular, fixed pelvic mass is highly suggestive of an ovarian malignancy. If, in addition, an upper abdominal mass or ascites is present, then the diagnosis of ovarian cancer is almost certain. Because the patient usually reports abdominal symptoms, she may not be subjected to a pelvic examination, and the presence of a tumor may be missed. Pleural effusions commonly occur in association with ascites, and very occasionally in the absence of ascites in patients with advanced disease.
Diagnosis
The diagnosis of an ovarian cancer requires histologic examination of a resected ovary. The preoperative evaluation of the patient with an adnexal mass is outlined in Figure 11.3.
Ultrasonographic signs of malignancy include an adnexal pelvic mass with areas of complexity such as irregular borders; multiple echogenic patterns within the mass; and dense, multiple, irregular septae. Bilateral tumors are more likely to be malignant, although the individual characteristics of the lesions are of greater significance. Transvaginal ultrasonography may have a somewhat better resolution than transabdominal ultrasonography for adnexal neoplasms (33,37–39), and Doppler color-flow imaging may enhance the specificity (34–36).

Figure 11.3 Preoperative evaluation of the patient with an adnexal mass. RMI, Risk of Malignancy Index.
The size of the lesion is of importance. If a complex cystic mass is more than 8 to 10 cm in diameter, the probability is high that the lesion is neoplastic, unless the patient has been taking clomiphene citrate or other agents to induce ovulation (115). In the premenopausal patient, a period of observation is reasonable, provided it is not clinically suspicious (i.e., it is mobile, mostly cystic, unilateral, and of regular contour). Generally, an interval of no more than 2 months should be allowed for observation. If the lesion is not neoplastic, it should remain stable or regress. If a mass increases in size or complexity, it must be presumed to be neoplastic and removed surgically.
In postmenopausal women with unilocular cysts measuring 8 to 10 cm or less and normal serial CA125 levels, expectant management is acceptable, and this approach may decrease the number of surgical interventions (124–126). Premenopausal patients whose lesions are clinically suspicious (i.e., large, predominantly solid, relatively fixed, or irregularly shaped) should undergo laparotomy, as should postmenopausal patients with complex adnexal masses of any size.
Before the planned exploration, the patient should undergo routine hematologic and biochemical assessments. A preoperative evaluation in a patient older than 40 years should include a radiograph of the chest. An abdominal and pelvic computed tomographic (CT) or MRI scan is of limited value in patients with a definite pelvic mass (127–131). Patients with ascites and no pelvic mass should have a CT or MRI scan to look particularly for liver or pancreatic tumors (128). The value of positron emission tomography (PET) scans is being evaluated but may contribute to the specificity of the CT scan findings (130,131).
The preoperative evaluation should exclude other primary cancers metastatic to the ovary. A colonoscopy is indicated in selected patients with symptoms and signs suspicious for colon cancer. This would include any patient who has evidence of frank or occult blood in the stool or a recent history of diarrhea or constipation. A gastroscopy is indicated if there are upper gastrointestinal symptoms such as nausea, vomiting, or hematemesis (132). Bilateral mammography is indicated if there is any breast mass, and patients who have irregular menses or postmenopausal bleeding should have an endometrial biopsy and an endocervical curettage to exclude the presence of endometrial or endocervical cancer metastatic to the ovary.
Differential Diagnosis
Ovarian epithelial cancers must be differentiated from benign neoplasms and functional cysts of the ovaries (40,133,134). A variety of benign conditions of the reproductive tract—such as pelvic inflammatory disease, endometriosis, and pedunculated uterine leiomyomata—can simulate ovarian cancer. Nongynecologic causes of a pelvic tumor, such as an inflammatory or neoplastic colonic mass must be excluded (115). A pelvic kidney can simulate ovarian cancer.
The risk of malignancy index (RMI), first described by Jacobs in 1990, is one method of differentiating between benign and malignant masses (133). Utilization of this index may facilitate better triage of suspicious pelvic masses to gynecologic oncologists. The RMI incorporates the menopausal status, an ultrasonic score, and the serum CA125 level.
In an analysis of 204 consecutive patients with an ovarian mass seen at the Royal Hospital for Women in Sydney, an RMI of <200 correctly identified 83 of 108 (77%) benign ovarian masses (133). An RMI of >200 correctly identified 11 of 19 (58%) borderline ovarian tumors and 70 of 77 (91%) invasive ovarian cancers. An RMI of >200 had a sensitivity of 84%, specificity of 77%, positive predictive value of 76%, and a negative predictive value of 85% for the detection of both borderline and invasive ovarian tumors (133).
Patterns of Spread
Ovarian epithelial cancers spread primarily by exfoliation of cells into the peritoneal cavity, but also by lymphatic and hematogenous dissemination.
Transcoelomic The most common mode of dissemination is by exfoliation of cells that implant on the peritoneal surfaces. The cells tend to follow the circulatory path of the peritoneal fluid, which moves with the forces of respiration from the pelvis, up the paracolic gutters, especially on the right, along the intestinal mesenteries, to the right hemidiaphragm. Therefore, metastases are typically seen on the posterior cul-de-sac, paracolic gutters, right hemidiaphragm, liver capsule, the peritoneal surfaces of the intestines and their mesenteries, and the omentum. The disease seldom invades the intestinal lumen but progressively agglutinates loops of bowel, leading to a functional intestinal obstruction. This condition is known as carcinomatous ileus.
Lymphatic Lymphatic dissemination to the pelvic and para-aortic lymph nodes is common, particularly in advanced-stage disease (135–138). Spreading through the lymphatic channels of the diaphragm and through the retroperitoneal lymph nodes can lead to dissemination above the diaphragm, especially to the supraclavicular lymph nodes (135).
Burghardt et al. (137) performed systematic pelvic and para-aortic lymphadenectomy on 123 patients and reported that 78% of patients with stage III disease had metastases to the pelvic lymph nodes. In another series (138), the rate of positive para-aortic lymph nodes was 18% in stage I, 20% in stage II, 42% in stage III, and 67% in stage IV.
Hematogenous Hematogenous dissemination at the time of diagnosis is uncommon, with spread to vital organ parenchyma, such as the lungs and liver, in only some 2–3% of patients. Most patients presenting with disease above the diaphragm have a right pleural effusion. Systemic metastases are seen more frequently in patients who have survived for some years. Dauplat et al. (139) from UCLA reported that distant metastasis consistent with stage IV disease ultimately occurred in 38% of the patients whose disease was originally intraperitoneal. Sites of hematogenous spread and their median survivals were as follows.
• Parenchymal lung metastasis in 7.1%, median survival 9 months
• Subcutaneous nodules in 3.5%, 12 months
• Malignant pericardial effusion in 2.4%, 2.3 months
• Central nervous system in 2%, 1.3 months
• Bone metastases in 1.6%, 4 months
Significant risk factors for distant metastases were malignant ascites, peritoneal carcinomatosis, large metastatic disease within the abdomen, and retroperitoneal lymph node involvement at the time of initial surgery.
Prognosis
The outcome of patients after treatment can be evaluated in the context of prognostic factors, which can be grouped into pathologic and clinical factors.
Pathologic Factors
The morphologic and histologic pattern, including the architecture and grade of the lesion, are important prognostic variables (140–145). In general, stage for stage, histologic type is not of prognostic significance, with the exception of clear cell and mucinous carcinomas, which are associated with a worse prognosis than the other histologic types when diagnosed at an advanced stage (143,144,146–148). Traditionally, stage I clear cell cancers have been thought also to have a high risk of recurrence, but more recent data have challenged this belief and Stage IA clear cell cancers appear to have a good prognosis, and probably do not benefit from adjuvant chemotherapy (148,149).
Histologic grade, as determined either by the pattern of differentiation or by the extent of cellular anaplasia and the proportion of undifferentiated cells, seems to be of prognostic significance (144,150). However, studies of the reproducibility of ovarian cancer grading have shown a high degree of intraobserver and interobserver variation (145). Because there is significant heterogeneity of tumors and observational bias, the value of histologic grade as an independent prognostic factor has not been clearly established.
Clinical Factors
In addition to FIGO stage, the extent of residual disease after primary surgery, the volume of ascites, patient age, and performance status are all independent prognostic variables (149,151–165). Among patients with stage I disease, Dembo et al. (152) showed, in a multivariate analysis, that tumor grade and “dense adherence” to the pelvic peritoneum had a significant adverse impact on prognosis, whereas intraoperative tumor spillage or rupture did not. A subsequent study by Sjövall et al. (153) confirmed these findings. A multivariate analysis of these and several other studies was performed by Vergote et al. (155), who reported that poor prognostic variables for early-stage disease were the tumor grade, capsular penetrance, surfaces excrescences, and malignant ascites, but not iatrogenic rupture. More recent studies have supported these findings and reported that the most important prognostic factors in patients with early-stage ovarian cancer include substage, grade, age, positive cytology, dense adherence, capsular rupture, and histologic subtype (156).
FIGO reported a statistically significant improvement in survival for all stages from 29.8% for the interval 1976 to 1978 to 49.7% for the interval 1999 to 2001 (157). The 5-year survival rate for carefully staged patients with stage IA disease is about 90%, while it is 70–80% for stage IC. The 5-year survival for stage II disease is about 70%, 45–50% or stage IIIA, 40% for stage IIIB, 30–35% for stage IIIC, and 15–20% for stage IV (157). The percentage of patients by stage at the time of diagnosis is shown next to the 5-year survival by stage in Figure 11.4, and the survival by substage is presented in Figure 11.5. The 5-year survival of patients with stage III disease and microscopic residual after cytoreductive surgery is 63.5%, compared with 32.9% for those with optimal residual disease, and 25% for those with suboptimal residual disease. Patients whose Karnofsky’s index (KI) is low (76) have a significantly shorter survival than those with a KI > 70 (158). For stages I and II disease, the 5-year survival rate for grade 1 cancer is about 90%, compared with approximately 80% for grade 2 and 70–75% for grade 3 (Fig. 11.6). For patients with stages III and IV disease, the 5-year survivals for grades 1, 2, and 3 are about 60%, 30%, and 25%, respectively (157,159) (Fig. 11.7). Survival of patients with borderline tumors is excellent, with stage I lesions having a 98% 10-year survival (16,18,63). When all stages of borderline tumors are included, the 5-year survival rate is 87% (157).

Figure 11.4 Survival of patients with epithelial ovarian cancer by stage. The percentage of patients diagnosed at a particular stage (green bars) is shown next to the 5-year survival by stage (blue bars). Data from Heintz APM, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. 26th Annual Report on the Results of Treatment in Gynaecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S161–S192, with permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree