Established risk factors
Risk factors that need further study
Cigarette smoking
Dietary factors:
Fruit and vegetables
Alcohol
Obesity
Reproductive factors and other hormones:
Oral contraceptive pills
Parity
Hypertension
Occupational exposures:
Asbestos, cadmium, hydrocarbons, gasoline, trichloroethylene
Inherited susceptibility
Analgesics
1.3.1 Cigarette Smoking
Cigarette smoking is considered a causal risk factor for renal cell carcinoma by both the International Agency for Research on Cancer and the US Surgeon General. Most case–control [13–15] and cohort studies [16–19] have reported significant associations between cigarette smoking and increased rates of renal cell carcinoma, with relative risks greater than 30 %. Studies have also shown significant dose–response trends with the number of cigarettes smoked [14, 20]. This observation, together with the decline in risk following cessation, supports causation between cigarette smoking and RCC [14, 15, 21]. A meta-analysis of 24 studies showed that compared with lifetime nonsmokers, smoking increased renal cell carcinoma risk by 54 % among men and 22 % among women [22]. A clear dose–response pattern of risk was apparent for men and women, with risk doubling among men and increasing 1.6-fold among women who were heavy smokers (>21 cigarettes per day). There was a significant 15–30 % reduction in RCC risk 10–15 years after smoking cessation, which was observed in both sexes.
The mechanism of carcinogenesis through cigarette smoke may be mediated by one of the constituents, N-nitrosodimethylamine, a nitroso compound. Renal cell carcinoma patients were shown to have a higher level of DNA damage in their peripheral blood lymphocytes induced by a tobacco-specific N-nitrosamine compared to control subjects [23]. In addition, this compound has caused renal tumors in several animal species. A further study revealed N-nitrosodimethylamine induced clear cell renal tumors in rats with VHL mutations suggesting a possible molecular pathway from tobacco smoking to RCC [24, 25]. Genetic alterations frequently found in RCC such as deletions in chromosome 3p were also shown to be more common in cultured peripheral blood lymphocyte cells from renal cell carcinoma patients than control subjects after being treated with benzo[α]pyrene diol epoxide, a major constituent of cigarette smoke [26].
NAT2, a gene encoding the N-acetyltransferase 2 enzyme that is involved in the metabolism of arylamine in tobacco smoke, has been evaluated in a few studies of renal cell carcinoma. Smoking-related RCC risk was higher in individuals with slow acetylator genotype for NAT2 than rapid acetylators [27]. This suggests that NAT2 is an underlying susceptibility marker for RCC that can exacerbate RCC risk in combination with risk factors such as cigarette smoking. In addition to carcinogens in tobacco smoke, cigarette smoking is hypothesized to increase renal cell carcinoma risk through chronic tissue hypoxia caused by smoking-related conditions such as chronic obstructive pulmonary disease and exposure to carbon monoxide [28]. There is also evidence to suggest that passive exposure to cigarette smoke among nonsmokers as well as occasional smoking may increase the risk of renal cell carcinoma.
1.3.2 Obesity
The increasing prevalence of obesity is likely to account in part for the rising incidence of renal cell carcinoma. It has been estimated that over 40 % of renal cell carcinomas in the United States and over 30 % in Europe may be attributable to being obese and overweight [29–33]. The cumulative evidence from analytical epidemiologic studies is most consistent for obesity to be a risk factor for RCC in both women and men. A quantitative review of published studies showed that increased BMI was strongly associated with increased risk of RCC among men and women, after controlling for confounding factors [29]. A dose-dependent relationship exists as described in a meta-analysis of data from prospective observational studies which estimated that the risk of developing renal cell carcinoma increased 24 % and 34 % for men and women, respectively, for every 5 kg/m2 increase in body mass index (BMI) [34].
Several plausible mechanisms by which obesity influences renal cell carcinoma development have been hypothesized, but the actual pathophysiology has not been fully elucidated. Obesity may promote changes in the hormonal milieu by altering circulating levels of estrogen and other steroid hormones, or elevated levels of insulin-like growth factor-I (IGF-I), which could in turn contribute to the development of renal cell carcinoma by affecting renal cell proliferation and growth [31, 35–37]. In obese individuals lipid peroxidation is increased leading to oxidative stress through the formation of DNA adducts which may promote the development of RCC [38].
1.3.3 Hypertension
Hypertension can be the result of renin-producing tumors as well as from treatment of RCC with tyrosine-kinase inhibitors [41, 42]. Sufficient evidence from cohort studies has accumulated linking hypertension reported at baseline to subsequent renal cell carcinoma incidence [43–45].
Dose–response relations between measured blood pressure level and renal cell carcinoma risk have been reported [16, 46–49]. Compared to individuals with normal blood pressure, those with the highest blood pressure (100 mmHg diastolic pressure or 160 mmHg systolic pressure) were found to have twofold or higher risk. In a cohort of Swedish men with sequential blood pressure measurements during follow-up, the risk of RCC further increased among those whose blood pressure increased above the baseline level and reduced among those whose blood pressure declined over time [16]. This data suggests that hypertension could be a factor in renal cell carcinoma development, and the risk can be modified with better control of blood pressure.
In the United States, national surveys indicate that the prevalence of hypertension in the population has been increasing along with the number and types of medications used to treat hypertension. Most epidemiologic studies of antihypertensive drugs and renal cell carcinoma risk have found that diuretic use, a causal factor candidate in early studies, is not an independent risk factor and adjustment for high blood pressure appears to eliminate any excess risk associated with diuretic use [43, 49–51]. In a population-based evaluation of various antihypertensive medications in Denmark, excess risk of renal cell carcinoma was observed only during short-term follow-up, and risks were reduced to insignificant levels five or more years after the baseline [50]. Also in this study, no particular type or class of antihypertensive medication was consistently associated with renal cell carcinoma risk.
The association between hypertension and renal cell carcinoma risk has been shown to be independent of the effects of excess body weight and cigarette smoking [16, 43, 45, 47, 48, 52]. Individuals who are both obese and hypertensive have greater risk of developing renal cell carcinoma than those who have only one of these conditions [16, 48, 53].
The biologic mechanism underlying the association between hypertension and renal cell carcinoma risk has yet to be elucidated. Among the hypotheses proposed are lipid peroxidation and the formation of reactive oxygen species, which are elevated in hypertensive individuals and are thought to play a role in renal cell carcinoma development [38]. Chronic renal hypoxia accompanies hypertension and leads to the upregulation of hypoxia-inducible factors. In animal models this has been shown to increase proximal tubular cell proliferation and glomerular hypertrophy and may be a mediator in kidney oncogenesis [54–56].
1.3.4 Genetics
Renal cell carcinoma occurs in both sporadic and hereditary forms. However, sporadic renal cell carcinomas have been shown to have a familial predisposition, with a recent meta-analysis indicating a greater than twofold risk among individuals having a first-degree relative diagnosed with kidney cancer [57]. A study evaluating familial aggregation among RCC patients in Iceland demonstrated a two- to threefold increase in RCC risk for first-degree relatives and a 1.6-fold increased risk for third-degree relatives [58]. The interplay of exposures to environmental risk factors and genetic susceptibility of exposed individuals is believed to influence the risk of developing sporadic renal cell carcinoma.
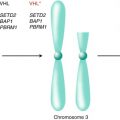
Hereditary renal cell carcinoma tends to occur earlier in life than sporadic forms of the disease and often involves bilateral, multifocal tumors [59]. Only about 3–4 % of renal cell carcinomas are explained by inherited predisposition of familial cancer syndromes, most notably the von Hippel–Lindau (VHL) syndrome. This syndrome is characterized by alterations in the VHL tumor suppressor gene, located on chromosome 3p, which predisposes to the clear cell subtype of renal cell carcinoma. The carcinogenesis pathway involves the VHL protein forming an ubiquitin ligase complex with proteins including elongin C, elongin B, and Cul-2. This complex targets the hypoxia-inducible factor (HIF)-1α pathway for degradation [60–62]. The HIF regulates multiple downstream genes via the mitogen-activated protein kinase (MAPK) and mTOR pathways whose expression is increased when the VHL gene is inactivated. These genes include the vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), which are critical in the pathway for tumorigenesis and are targets for therapeutic approaches for the treatment of renal cell carcinoma [63, 64]. Clinically, VHL is an autosomal dominant disorder characterized by clear cell RCC, retinal hemangiomata, cerebellar and spinal hemangioblastomas, pheochromocytomas, and pancreatic neuro-endocrine tumors [65].
There are other rare forms of renal cell carcinoma that have an inherited susceptibility (Table 1.2). Only a very small proportion of renal cell carcinoma patients are known to occur in families with these rare syndromes. Hereditary papillary carcinoma is an autosomal dominant syndrome where patients are at risk of developing bilateral multifocal type 1 papillary renal carcinoma, often at a late age of onset at 50–70 years [66]. Activation of a proto-oncogene, MET at 7pq31, is the inciting event, which activates downstream signaling cascades inducing cell proliferation and differentiation [67]. Birt–Hogg–Dubé syndrome is caused by abnormalities in the folliculin (FLCN) gene, an autosomal dominant tumor suppressor gene [68, 69]. Affected persons are at risk to develop cutaneous fibrofolliculomas, pulmonary cysts, spontaneous pneumothoraces, and renal tumors [70]. Renal lesions are bilateral and multifocal. The histological subtypes are usually chromophobe, oncocytic, or mixed [71]. Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) is a rare condition characterized by cutaneous and uterine leiomyomas [72]. Type II papillary renal cell carcinoma has been associated with HLRCC, with an onset of 30–50 years of age. These renal cancers are usually unilateral and often aggressive leading to death from metastatic disease within 5 years of diagnosis [73]. A mutation in the fumarate hydratase gene located on chromosome 1, an autosomal dominant tumor suppressor gene, leads to transcriptional upregulation of HIF target genes [74]. Some families with clear cell cancer have a balanced translocation involving chromosome 3 [75]. Tuberous sclerosis is an autosomal dominant disorder characterized by hamartomas in various organs. Other features can include epilepsy and cutaneous manifestations such as hypomelanotic macules, facial angiofibromas, shagreen patches, and ungual fibromas [76]. Tumor suppressor genes TSC1 and TSSC2 encoding hamartin and tuberin, respectively, are involved in regulation of the mTOR pathway and have been linked to tuberous sclerosis [77]. Renal manifestations include multifocal clear cell renal cancers and angiomyolipomas which can be large requiring surgical removal [78]. Hereditary paraganglioma (HPG) is an autosomal condition caused by a mutation in genes encoding mitochondrial succinate dehydrogenase (SDHB) [79]. There are reports of an increased incidence of clear cell renal cancer in two families with HPG because of a SDHB mutation although other histologies have also been described [80, 81]. Genetics plays an integral role in the inherited susceptibility of renal cell carcinoma; however, it has been shown that the majority of non-inherited clear cell carcinomas are associated with inactivation of the VHL gene through mutation or promoter hypermethylation [59]. Another example of a non-inherited genetic mutation is Xp11 translocation renal cell carcinoma (RCC), an RCC subtype that was introduced in 2004 as a genetically distinct entity into the World Health Organization classification of renal tumors. It accounts for at least one-third of pediatric RCCs and for 15 % of RCCs in patients <45 years of age. It is characterized by Xp11.2 translocations, which induces gene fusions involving the TFE3 transcription factor gene. Xp11 translocation RCC usually has a mixed papillary architecture with nested patterns of clear and/or eosinophilic cells and calcified foci. Patients with metastatic Xp11 translocation RCC have presented at an advanced stage and are usually afflicted by a short and aggressive disease course [82, 83].
Table 1.2
Inherited syndromes associated with renal cell carcinoma
Syndrome | Genetic inheritance | Prevalence | Histology | Incidence | Mean age at diagnosis | Clinical features |
|---|---|---|---|---|---|---|
Von Hippel–Lindau (VHL) [68] | VHL (3p25-26) Autosomal dominant | 1 in 36,000 | Cysts Clear cell | 25–45 % | 40 | Retinal and CNS hemangioblastomas Pheochromocytomas Pancreatic cysts Pancreatic neuroendocrine tumors |
MET (7q31) Autosomal dominant | Rare | Papillary type I | Unknown | >50 | None | |
FLCN (17p11.2) Autosomal dominant | >60 families from various populations | Chromophobe Oncocytic Clear cell Papillary Mixed (chromophobe/oncocytic) | 38 % | 48 | Fibrofolliculoma Trichodiscoma Acrochordon Lung cysts Pneumothorax | |
FH (1q42–43) Autosomal dominant | >100 families from various populations | Papillary type 2 Collecting duct | 2–16 % | 44 | Cutaneous and uterine leiomyomas | |
TSC1 (9q34) TSC2 (16p13) Both AD | 1 in 6,000 | Angiomyolipoma Renal cysts Clear cell Chromophobe Oncocytoma Papillary | 2–3 % | 36 | Epilepsy Mental retardation Adenoma sebaceum Hypomelanotic maculae Shagreen patch Fibrous plaques Ungual fibroma Dental pits Cardiac rhabdomyoma Periventricular hamartomas (tubers) | |
SDHB (1p36) SDHC (1q21) SDHD (11q23) All AD | Unknown | Clear cell | 3 cases in 2 families | 30 | Paragangliomas Pheochromocytomas |
Detection of hereditary forms of RCC relies on the clinician to appropriately recognize individuals with potentially inherited forms of cancer. Since the diagnosis of hereditary syndromes can have far-reaching consequences for the patients and their families, it has now been suggested that clinicians refer patients with early-onset kidney cancer (age 46 years or younger) for genetic counseling and germline mutation testing [84].
1.3.5 Hormonal and Reproductive Factors
Reproductive and hormonal factors may play a role in renal cell carcinoma development in susceptible individuals. Tissue from renal cell carcinoma patients has been shown to express steroid hormone receptors and luteinizing hormone-releasing hormone receptors [85, 86]. In animal studies, estrogen treatment has been shown to enhance the development of renal cell carcinoma, whereas removal of the ovaries reduced neoplastic renal changes [87]. An increased risk of renal cell carcinoma has been associated with parity among women in several studies. Compared with nulliparous women, the risk of renal cell carcinoma increased 40–90 % among women who had given birth [88–90]. A Swedish study found a significant 15 % increase in risk with each additional birth, after controlling for age at first birth among parous women [90]. An inverse association with age at first birth has also been reported, with highest risk among women who gave multiple births at a relatively young age [91]. Mechanisms underlying the observed association with parity are unclear, although pregnancy-induced hypertension and renal stress may play a role. Associations with other reproductive-related factors, including the use of oral contraceptives, which in some studies has been shown to be protective, and hormone replacement therapy, are not consistently observed [53, 92, 93].
1.3.6 Occupational and Environmental Exposures
Generally, renal cell carcinoma is not considered an occupational disease, but it has been linked to some occupations and industrial exposures. Trichloroethylene (TCE), a chlorinated solvent used as a degreaser in metal industries and as a general solvent, has been the most extensively studied risk factor for renal cell carcinoma. Three studies were initiated in response to a cluster of renal cell carcinoma cases observed in a plant in Germany. All of these studies reported elevated relative risks for renal cell carcinoma associated with TCE exposure [94]. Although not statistically significant, aerospace workers with airborne TCE exposures above 50 ppm were at a near twofold risk of kidney cancer mortality compared with workers exposed to lower levels [95]. In contrast, no association was reported in a small cohort study of TCE-exposed workers in Denmark and another retrospective cohort mortality study of workers exposed to chlorinated organic solvents in Taiwan [96, 97]. Given the methodological challenges including the complexities of TCE pharmacokinetics, co-exposure to other solvents, various study limitations, and the lack of association in some reports, further studies are warranted before causality is implicated [98–102]. Environmental carcinogen exposures may be linked to tumor DNA alterations. RCC patients with high, cumulative exposures of trichloroethylene have been shown to have more frequent somatic VHL mutations. A German study reported that VHL mutations were found in 33 of 44 RCC patients with TCE exposure. Of the 33 patients with VHL mutations, 14 had multiple VHL mutations and 13 had the same C to T substitution in codon 81 [103]. Genes encoding the glutathione S-transferase (GST) enzymes, including GSTM1, GSTT1, and GSTP1, have been studied in relation to renal cell carcinoma risk [104–112]. The GST enzymes are active in the detoxification of polycyclic aromatic hydrocarbons in tobacco smoke, halogenated solvents, exposure to TCE or pesticides, and other xenobiotics. However, inconsistency in subgroup findings among studies, small numbers of exposed individuals, and the inability to replicate data suggest that further investigations are needed to clarify these associations.
Asbestos has been associated with elevated renal cancer mortality in two studies, one with insulators and the other with asbestos products workers [110, 113]. However, two extensive meta-analyses of occupational cohort studies of asbestos-exposed workers showed little relation to increased risk for renal cancer [114, 115]. An increased risk of renal cell carcinoma has also been linked to other industrial exposures, including chromium compounds, cadmium, lead, copper sulfate, solvents, benzene, vinyl chloride, pesticides, and herbicides [116–123]. Employment in certain occupations has also been associated with renal cell carcinoma risk, such as printers, aircraft mechanics, farmers, railroad workers, metal workers, mechanics, workers employed in vitamin A and E synthesis, and service station employees [56, 121, 122, 124, 125]. However, none of these occupations or exposures has been conclusively related to risk in epidemiologic studies. Other environmental exposures, such as arsenic, nitrate, and radon in drinking water, also have not been established as risk factors for developing RCC [126–130].
1.3.7 Dietary Factors
Geographic variations in incidence and mortality suggest a role for environmental and dietary factors in the development of RCC. There has not been convincing evidence for a protective role of a diet rich in fruits and vegetables in the development of RCC. A number of case–control studies reporting on associations between intake of fruits and vegetables and RCC risk have given inconclusive results. Although high fruit and vegetable consumption was associated with a decreased risk of RCC in a pooled analysis of several cohort studies, other large prospective cohort studies failed to demonstrate such an association [131–133]. Antioxidants such as vitamins A, C, and E and carotenoids that are common in fruits and vegetables also have not been consistently linked to renal cell carcinoma risk [134–136].
Dietary habits associated with a western lifestyle, including the consumption of red or processed meat, have been proposed as potential risk factors of RCC. In a meta-analysis of case–control studies, this was associated with increased risk of RCC; however, this association was not confirmed in a pooled analysis of cohort studies [137–139]. A recent report from a cohort study of Swedish women concluded that the risk of renal cell carcinoma was consistently reduced with increasing frequency of fatty fish consumption, but not with lean fish consumption [140].
A study conducted in Sweden detected high levels of acrylamide, a potential carcinogen, in commonly consumed fried and baked foods [141]. However, other epidemiological studies have yielded mixed results suggesting further studies in humans are important given the consumption of food items with elevated acrylamide levels [142, 143].
Moderate alcohol consumption has been inversely associated with renal cell carcinoma risk in a pooled analysis of prospective studies, with an estimated 28 % reduction in risk among those who drank ≥15 g/day, equivalent to slightly more than one alcoholic drink per day [144–146]. This inverse association was observed for all types of alcoholic drinks, including beer, wine, and liquor. In contrast, no association was found with coffee, tea, milk, juice, soda, and water [147]. A potential mechanism by which moderate consumption of alcohol may reduce renal cell carcinoma risk is through improvement in insulin sensitivity, thus lowering the risk of type 2 diabetes, production of insulin-like growth factor-I, and subsequent risk of renal cell carcinoma [148, 149].
1.4 Conclusion
Renal cell carcinoma incidence has continued to increase over several decades among all racial groups. This has been in the context of widespread use of diagnostic imaging and increasing prevalence of risk factors leading to the diagnosis of smaller tumors and localized disease. Cigarette smoking, excess body weight leading to increased BMI, and hypertension are established modifiable risk factors of RCC and have likely contributed to the increasing prevalence of RCC in both sexes. The variation in the prevalence of these factors across subpopulations may explain the racial and geographic variation in RCC incidence observed, not only in the United States but worldwide. These risk factors may contribute to as much as 50 % of all RCC cases and are targets for preventative strategies in reducing RCC incidence. The relative contribution of other risk factors such as occupational and environmental exposures, hormonal factors, and dietary considerations are not as clearly elucidated. While only a small proportion of renal cell carcinoma occur within the milieu of familial cancer syndromes, genetic susceptibility and its interplay with environmental exposures play an important role in the etiology and development of sporadic renal cell carcinoma. Genetic polymorphisms may modulate an effect on metabolic activation and detoxification enzymes, which will allow improved analysis and interpretation of exposure associations that are important in the initiation and progression of RCC. The multifactorial nature of RCC requires that further studies are conducted to explain underlying factors that may influence individual risk and to elucidate complex relationships between potential genetic, lifestyle, and environmental elements on cancer development.
Due to the advances in the molecular and genetic biology of renal cell carcinoma, a paradigm shift has occurred in the treatment of patients with advanced renal cell carcinoma. Advances in the molecular genetics of RCC syndromes have allowed earlier genetic testing leading to improvements in detection, surgical interventions, and therapeutic approaches. The development of targeted therapies involving the VEGF and mTOR pathway in renal cell carcinoma has drastically improved the survival and outcomes of patients afflicted with this malignancy.
Clinical Vignette
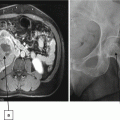
A 25-year-old Caucasian male with no past medical history presents with gross hematuria. Urinalysis confirms the finding of red blood cells in the urine. A cystoscopic evaluation was unrevealing. A bilateral renal sonogram demonstrated bilateral renal cysts with at least one of the cysts highly suspicious for malignancy due to complexity. The patient’s family history is significant for pheochromocytoma in his father and early death from kidney cancer in a paternal aunt. The patient asks his physician whether his clinical presentation is consistent with some form of familial cancer syndrome.
Q: What hereditary cancer syndrome is most likely to be associated with the patient’s presentation? What other possible consequences of this syndrome should the physician be aware of?
A: The most likely syndrome involved in this patient’s case is that of VHL syndrome (see chapter text for a full description).
References
1.
DeVita VT, Lawrence TS, Rosenberg SA (2011) Cancer: principles & practice of oncology: primer of the molecular biology of cancer. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia
2.
American Cancer Society (2011) Global cancer facts & figures, 2nd edn. American Cancer Society, Atlanta
3.
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, International Agency for Research on Cancer. Available from: http://globocan.iarc.fr. Accessed on 21 Sept 2014
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree