INTRODUCTION
SUMMARY
Eosinophils continue to be studied intensively, in large part, as a result of their potential role in the pathogenesis of asthma. The concept of the eosinophil as a cell that has protective effects against helminthic parasite infection, but can cause tissue damage when inappropriately activated, remains intact, although the evidence for both these roles is circumstantial. Eosinophil production and function are profoundly influenced by interleukin (IL)-5; and, thus, eosinophilia is associated with diseases characterized by T-helper (Th)2-mediated immune responses, including infections by helminthic parasites and extrinsic asthma. However, eosinophilia also occurs in diseases not obviously associated with Th2 dominance, such as intrinsic asthma, hypereosinophilic syndromes (HESs), and inflammatory bowel disease. Thus, IL-5 and other eosinophil mediators can be generated in various types of inflammatory response.
The eosinophil, like other leukocytes, can generate proinflammatory mediators. Eosinophil-specific granule proteins are toxic for a range of mammalian cells and parasitic larvae. Eosinophils, like mast cells, produce sulfidopeptide leukotrienes, as well as other lipid mediators, such as platelet-activating factor (PAF). Cytokine production by eosinophils broadens their potential functions, for example in wound healing through their generation of transforming growth factor (TGF)-α. Synthesis of TGF-β may explain the propensity of eosinophils to be associated with fibrotic reactions such as endomyocardial fibrosis, characteristic of HES, and fibrosing alveolitis.
Considerable effort has gone into trying to unravel the molecular basis of eosinophil tissue recruitment. The selective accumulation of eosinophils is the result of a concerted and integrated series of events involving their production in the marrow and egress therefrom, adhesion to endothelium, selective chemotaxis, and prolonged survival in tissues. These events are controlled, either directly or indirectly, by production of IL-4, IL-5, and IL-13.
The discovery that a proportion of patients with HES have either a clonal myeloid neoplasm resulting from an acquired mutation that generates a constitutively active, novel tyrosine kinase (FIP1L1-PDGFRα [F/P]) or a T-cell lymphoproliferative disease causing a reactive eosinophilia has offered the prospect of new and more effective treatments for these conditions, as well as giving new insights into the control of eosinophil production. There has long been a debate about the extent to which eosinophils cause tissue damage, are innocent bystanders, or even help to ameliorate the condition. This is now being resolved with data showing that specific reduction in eosinophils using anti–IL-5 monoclonal antibody is beneficial in eosinophilic airway disease and HES.
Acronyms and Abbreviations
AAV, ANCA-associated vasculitides; AHR, airway hyperresponsiveness; ANCA, antineutrophil cytoplasmic antibodies; BAL, bronchoalveolar lavage; BSA, bovine serum albumin; CCL, chemokine (C-C motif) ligand; CCR, chemokine receptor; CEL, chronic eosinophilic leukemia; CLC, Charcot-Leyden crystal; CLM-1, CMRF35-like molecule-1; CMPD, chronic myeloproliferative disease; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EGPA, eosinophilic granulomatosis with polyangiitis; EM, electron microscopic; EMR, mucin-like hormone receptor; FEV1, forced expiratory volume in 1 second; FISH, fluorescence in situ hybridization; GM-CSF, granulocyte-monocyte colony-stimulating growth factor; GPA, granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; HLA, human leukocyte antigen; ICAM, intercellular adhesion molecule; iHES, idiopathic hypereosinophilic syndrome; IL, interleukin; ILC, innate lymphoid cell; LAMP, lysosome-associated membrane protein; LIMP, lysosome integral membrane protein; LT, leukotriene; mAb, monoclonal antibody; MBP, major basic protein; MPA, microscopic polyangiitis; NADPH, nicotinamide adenine dinucleotide phosphate oxidase; NO, nitric oxide; ORMDL3, orosomucoid-like 3; PAF, platelet-activating factor; PIN1, peptidylprolyl isomerase; PSGL, P-selectin glycoprotein ligand; Siglec, sialic acid-recognizing animal lectin; SNARE, soluble N-ethylmaleimide–sensitive factor attachment protein receptor complex; TGF, transforming growth factor; Th, T-helper; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; TREG, T-regulatory cell; TSLP, thymic stromal lymphopoietin; TXB2, thromboxane B2; VCAM, vascular cell adhesion molecule; VIP, vasoactive intestinal peptide; VLA, very-late antigen; WHO, World Health Organization.
BIOLOGY OF EOSINOPHILS
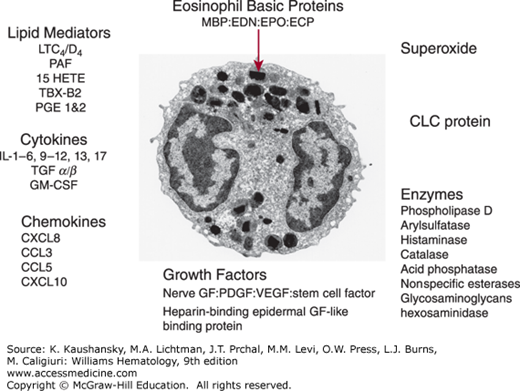
Eosinophils are spherical, end-stage, nondividing leukocytes, approximately 8 μm in diameter derived from the marrow.1 In vitro granulocyte-monocyte colony-stimulating factor (GM-CSF), interleukin (IL)-3 and IL-5 stimulate colony growth; additionally, IL-5 is a critical eosinopoietic factor in vivo involved in late differentiation.2 The electron microscopic (EM) morphology of the mature eosinophil has been well described (Fig. 62–1).3,4 The relatively specific features which distinguish the eosinophil from other leukocytes are the bilobed nucleus, the specific granules with an electron dense core, the paucity of mitochondria (approximately 20 per cell) and endoplasmic reticulum, and the dense network of cytoplasmic tubulovesicular structures or secretory vesicles that contain albumin and cytochrome b558 and are therefore thought to be involved in superoxide production. Eosinophils also contain lipid bodies, which are the major site of eicosanoid synthesis, primary granules, and small granules.5 Small granules are particularly prominent in tissue eosinophils and contain arylsulphatase B, acid phosphatase, and catalase. They may be derived from specific granules and act as a lysosomal compartment since specific granules express lysosome-associated membrane proteins (LAMP) 1 and 2, as well as lysosome integral membrane protein (LIMP, CD63) 1.6 Eosinophils also contain multilaminar bodies that contain transforming growth factor (TGF)–α. Eosinophil precursors derived from cord blood can be first identified morphologically when specific core containing granules appear, although expression of Charcot Leyden crystal (CLC) protein and the basic granules proteins can be detected by immunohistochemistry or mRNA expression at the promyelocyte stage where they are found in the endoplasmic reticulum, Golgi apparatus and large round coreless granules, most of which develop into specific granules. Electron microscopy can distinguish activated from resting blood eosinophils by the increased number of lipid bodies, primary and small granules, secretory vesicles, and endoplasmic reticulum. Cytoplasmic crystals of CLC protein may also be present. Eosinophils are relatively inefficient phagocytes, although they can ingest opsonized zymosan, which gets taken up into phagolysosomes formed in part by fusion with specific granules. The eosinophil also degranulates onto large opsonized surfaces such as a Sephadex bead or parasitic larvae in a process called frustrated phagocytosis.
Figure 62–1.
Transmission electron micrograph (×10,000) of an eosinophil showing the characteristic specific granules with their electron dense core and various mediators, receptors and granule proteins produced by eosinophils. CLC, Charcot-Leyden crystal; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPO, eosinophil peroxidase; GF, growth factor; GM-CSF, granulocyte-monocyte colony-stimulating growth factor; HETE, hydroxyeicosatetraenoic acid; LT, leukotriene; MBP, major basic protein; PAF, platelet-activating factor; PDGF, platelet-derived growth factor; PG, prostaglandin; PSGL, P-selectin glycoprotein ligand; TBX, thromboxane; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor. (Used with permission of Dr. A. Dewar, National Heart and Lung Institute.)
The ultrastructure of in vitro activated and tissue-infiltrating eosinophils has suggested three potential mechanisms of degranulation: necrosis or cytolytic degranulation, exocytosis or “classical degranulation,” and piecemeal degranulation.7 Cytolytic degranulation is associated with loss of eosinophil plasma membrane integrity and results in the release of clusters of free membrane-bound granules (termed Cfegs). This is commonly observed in eosinophilic inflammation and is particularly marked in severe disease, such as fatal attacks of asthma in which large quantities of basic proteins can be detected in the tissue by immunohistochemistry often with relatively few intact eosinophils.8 Exocytosis or classical degranulation occurs in mast cells and basophils after crosslinking of immunoglobulin (Ig) E receptors. It describes a process by which granules migrate to the plasma membrane and fuse with it leading to the extrusion of membrane free granule contents. This phenomenon has been described for eosinophils in the gut, but not the airway mucosa. Piecemeal degranulation has been described in cord blood derived eosinophils9 and refers to the appearance of empty or partially empty granules together with small vesicles in the cytoplasm which transport the granule proteins to the cell surface where they are released.10 These appearances are common in tissue eosinophils in asthma and other allergic diseases.
Many studies have used a mouse model involving ovalbumin challenge to generate a lung eosinophilia and increased airway hyperresponsiveness (AHR). A striking feature of this model is that lung eosinophils do not have the appearance of having undergone degranulation by either cytolysis or piecemeal degranulation.11 Immunostaining of the mouse lung in this model locates all the basic proteins within intact eosinophils and bronchoalveolar lavage (BAL) contains no free major basic protein (MBP).12 This is quite unlike human disease in which the cell free basic proteins can be readily detected in both tissue and BAL. Consistent with this observation, mice in which the gene for eosinophil peroxidase or MBP has been deleted had the same phenotype as wild-type mice.13 However some degranulation may be seen in the airway lumen.14 Mice were genetically modified using two approaches to completely delete eosinophils, one by inserting an eosinophil toxic gene into the lineage (PHIL) and one by deleting a high-affinity binding site in the GATA-1 promoter.15,16 The GATA mice still developed AHR and mucus secretion but not airway remodeling, which is consistent with evidence for their role in asthma. In contrast, the PHIL mouse did not develop AHR and mucus hypersecretion after airway challenge. Two other strains of mice, iPHIL and eoCRE, have been developed. iPHIL can induce eosinophil cell death at any point in the life of the mouse using diphtheria toxin and eoCRE can be used to selectively induce gene expression in eosinophils. These flexible “knock-in” strains of mice have revealed unexpected complexity in the role of the eosinophil in the allergic immune response.17 Apoptotic eosinophils are small cells with a shrunken nucleus and condensed chromatin but an intact plasma membrane. They are readily identifiable in aged cell populations in vitro and in cells from the airway lumen such as sputum, but are more difficult to identify in tissue. This has led some investigators to argue that the majority of airway eosinophils, at least in asthma and rhinitis, are removed through luminal entry rather than by undergoing apoptosis in tissue.7
Like all leukocytes, eosinophils express a large number of membrane receptors which allow them to interact with the extracellular environment (Tables 62–1 and 62–2). These include receptors required for locomotion, activation, growth and mediator release. Most of the receptors are shared to some extent with other leukocytes but some have a degree of specificity in terms of level of expression and function. An important feature of tissue eosinophils is that they express a different pattern of receptors, compared to blood eosinophils, consistent with a more activated phenotype. This includes induction of expression of CD69, intercellular adhesion molecule (ICAM)-1 and FcγR1 and increased expression of human leukocyte antigen (HLA)-DR and Mac-1. Changes in expression can be induced in vitro by culture with cytokines such as IL-5, but also occur to some extent as the result of transmigration through endothelium.18 A major difference between eosinophils and neutrophils that has been exploited to purify eosinophils by immunomagnetic selectin is the expression of CD16 by neutrophils but not eosinophils. Another important difference is the expression of VLA-4 by eosinophils, but not to any great extent in neutrophils. Sialic acid-recognizing animal lectin (Siglec) 8 has been identified as a receptor expressed only by eosinophils, mast cells, and basophils.19,20,21 Siglecs are of the immunoglobulin superfamily. Eosinophils, as well as monocytes and a subset of dendritic cells, also express Siglec 10.22 In contrast, neutrophils express Siglec 9.23 Siglec 8 is important in triggering eosinophil apoptosis.24,25 Epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1) is expressed exclusively on eosinophils and has the potential to be a therapeutic target for eosinophilic disorders.26 Eosinophils express both CD48 and its ligand CD244 (2B4), both members of the IgG superfamily. Crosslinking of CD48 causes eosinophil degranulation.27 Eosinophils also express a number of inhibitory receptors including CMRF35-like molecule-1 (CLM-1) that negatively regulate eotaxin-induced eosinophil responses.28
| Ligand | ||
|---|---|---|
| Receptor | Endothelial | Matrix Protein |
| INTEGRINS | ||
| α4β1 (VLA-4) | VCAM-1 | Fibronectin |
| α4β6 | Laminin | |
| α4β7 | MAdCAM-1 | Fibronectin |
| LFA-1 (αΛβ2) | ICAM-1-3 | |
| Mac-1 (αMβ2) | ICAM-1 | |
| P150,95 (αxβ2) | ||
| αdβ2 | VCAM-1 (ICAM-3?) | |
| SELECTINS AND LIGANDS | ||
| PSGL-1 | P-selectin (E-selectin) | |
| l-Selectin | Gly-CAM-1, CD34, Podocalyxin | |
| OTHER | ||
| CD44 | Hyaluronate | |
| ICAM-3 | ||
| PECAM | PECAM | |
| Immunoglobulin receptors: Fcγ R11 (CD32); Fcα R; |
| Receptors for mediators: CCR3*; CCR1; PAF-R; LTC4/D4/E4-R; LTB4-R; C5aR; C3aR; IL-5R*; IL-3R; IL-4R; IL-13R; CRTh2 |
| Receptors induced by cytokine stimulation: Fcγ RIII (CD16); Fcγ R1 (CD69); HLA-DR; ICAM-1; CD25; CD4 |
| Well-expressed miscellaneous receptors: CD9; CD45; CR1; CD154 (CD40 ligand); CD95 (Fas); Siglec 8*; ERM1*; CLM-1 |
Eosinophils are nondividing, end-stage cells that, like other leukocytes, differentiate from the hematopoietic stem cell in the marrow. They share a progenitor with basophils before further differentiation separates the lineages. GATA-1 is a particularly important transcription factor for eosinophil development with deletion of the high-affinity binding site in GATA-1 resulting in specific loss of the eosinophil lineage.29 The F/P receptor mutation which is responsible for a myeloproliferative form of hypereosinophilic syndrome (HES)/chronic eosinophilic leukemia (CEL) works through cEBPα and GATA-2, as well as GATA-1, showing that these transcription factors are also important in eosinophil development.30 Eosinophilopoiesis requires the combined expression of MBP and eosinophil peroxidase.31
Eosinophils migrate into the blood, where they circulate with a half-life of about 18 hours before entering the tissues. Eosinophils are primarily tissue-dwelling cells, and it has been estimated that there are approximately 100 tissue eosinophils for each eosinophil in the blood, although relatively few studies have been performed on eosinophil kinetics and even fewer have compared eosinophil turnover in health and disease. However, studies demonstrate that eosinophils can be tracked in vivo using radiolabeling and that the kinetics of migration through the lung, spleen, and marrow are distinct from that of neutrophils.32,33 Normal human adult marrow contains approximately 3 percent eosinophils of which one-third are mature and two-thirds are precursors.
Eosinophilia is often T-cell dependent. Characterization of T-cell–derived supernatants led to the characterization of IL-5 and awareness of the pivotal role that this cytokine plays in eosinophil development.34 IL-3 and GM-CSF are also important in eosinophil development. The three cytokines bind to receptors that share a common β chain but have distinct α chains. IL-5 seems to be a rate-limiting step for eosinophil production in that administration of IL-5 either exogenously or through transgenic manipulation in mice results in a marked eosinophilia35 and anti–IL-5 in humans dramatically diminishes the blood eosinophil count in asthma.36 Increased eosinophilopoiesis as a result of increased IL-5 synthesis appears to be a feature of a number of diseases, including parasitic and allergic diseases. For example, pulmonary eosinophilia caused by Necator americanus infection in mice is IL-5–dependent37 and both the eosinophilia and host defense to filariasis and Trichinella spiralis is markedly impaired in IL-5–deficient mice.38 In asthma, IL-5 mRNA can be detected in increased amounts in the airways in asthma.39 However, IL-5 gene-deleted mice have a baseline eosinophilia and can develop pulmonary eosinophilia after infection with paramyxovirus, demonstrating that other late differentiation factors such as chemokine (C-C motif) ligand (CCL)-3 may be involved.40,41 It is therefore an accepted paradigm that a blood and tissue eosinophilia in IgE-mediated diseases, such as atopic asthma and helminthic parasite infections, are a result of antigen-dependent activation of T-helper (Th)-2 cells leading to IL-5 production and increased eosinophilopoiesis and tissue recruitment of eosinophils. The control of development of Th2 and Th1 cells is beyond the scope of this chapter, but may relate to the cytokine milieu at the time of sensitization, genetically regulated transcriptional control of IL-4, or the route of sensitization and the way in which the antigen is presented (Chap. 76).42,43 The HLA haplotype of individuals responsive to certain allergens has also been investigated. A degree of restriction has been observed, particularly to simple allergens, with, for example, the phenotype DR2.2 being overrepresented in individuals atopic to the ragweed allergen Amb a V. However, with the majority of allergens, no clear pattern has emerged. Although HLA haplotypes may influence responses to individual allergens, it is unlikely to provide a universal explanation for Th2-type responsiveness.
Many eosinophilic diseases, including many cases of pulmonary eosinophilia, are not associated with atopy and IgE production and therefore do not entirely fit with the Th2-driven eosinophilic paradigm. Intrinsic asthma is generally assumed to be associated with IL-5-producing T cells; however, the evidence for this is limited. One model for non–IgE-associated eosinophilic disease is those cases of eosinophilic esophagitis caused by a defined food allergen in which there is no specific IgE.44 In some of these cases, the patients are patch-test–positive to the food allergen concerned, which raises the possibility of a Th2 type of type IV cell-mediated immunity, but this is still unexplored.
There is increasing interest in the role of T regulatory cells (TREG) in controlling inappropriate immune responses including those associated with Th2 cell activation.45 TREG were first identified as mediating some aspects of immune tolerance and were then found to play an important role in suppressing immune-mediated inflammatory bowel disease in mice. Three types of TREG cells have been identified: CD4+/CD25+ cells which require direct contact to mediate their immunosuppressive effects, TREG cells producing TGF-β and TREG cells producing IL-10.46,47,48 A current idea is that the increase in allergic disease that is also paralleled by an increase in autoimmune disease is not caused by a Th1 to Th2 switch, but by a failure to develop TREG responses, which leads to enhancement of both Th1 and Th2 immunity.49,50,51 IL-10 producing TREG is of particular interest in the context of pulmonary eosinophilia because of evidence that immunotherapy works by inducing expansion of an antigen specific IL-10 producing TREG cells.52 In addition, regulatory T cells were able to suppress ovalbumin-induced pulmonary eosinophilia in mice.53
Blood eosinophils from normal individuals are relatively dense cells that can be separated from other leukocytes by density-gradient centrifuge. For many years these differences were the basis for the standard method of purifying eosinophils. This method has now been largely superseded by negative immunomagnetic selection based on the expression of the low affinity (Fcγ RIII, CD16) IgG receptor by neutrophils but not eosinophils. This latter technique has the advantage of improved purity and cell yields as well as enabling purification of eosinophils from individuals with low eosinophil counts, A proportion of eosinophils from individuals with elevated eosinophil counts are less dense than eosinophils from normal subjects. So-called hypodense eosinophils appear to be vacuolated and contain smaller granules, although in equal numbers to normal-density eosinophils. The mechanism for this heterogeneity is unclear; although a correlation with eosinophil activation has been a favored hypothesis, the evidence to support this hypothesis is contradictory.54
Eosinophils are not normally found in tissues other than the gut and the appearance of increased numbers of these cells can be a notable feature of the pathology of a number of diseases. The normal pattern of gut homing of eosinophils is likely to be mediated by CCL-11 (eotaxin 1), which is constitutively expressed in the gut, and the integrin α4β7 binding to mucosal addressin cell adhesion molecule (MAdCAM)-1, which is selectively expressed in the intestine.55 Type 2 innate lymphoid cells are central to this process, constitutively producing IL-5, which generates an eosinophilia, and IL-13, which stimulates production of eotaxin. Activation of innate lymphoid cell (ILC)-2 was modulated by nutrient intake and circadian rhythms, which may explain the circadian cycling of blood eosinophils.56 Although an eosinophilia can accompany a general inflammatory response, as for example in idiopathic pulmonary fibrosis where increased numbers of eosinophils and neutrophils can be seen in the BAL fluid, it often occurs without a marked increase in other leukocytes raising the question of the mechanism behind the specific tissue accumulation of these picturesque leukocytes. Selective eosinophil accumulation occurs as a result of the coordinated effect of a number of adhesion, chemotactic and growth/survival orientated signals at each stage in the life cycle of the cell. Generally speaking, these events are controlled by mediators associated with Th2 cells, in particular the cytokines IL-4, IL-5, IL-13, and possibly IL-9.57,58 Another group of epithelial derived cytokines—IL-25, IL-33, and thymic stromal lymphopoietin (TSLP)—play a key role in the development of eosinophilic inflammation through the activation of a newly described class of innate immune cells called ILC2.59
As well as being crucial for differentiation, IL-5 is also important in promoting emigration from the marrow. In particular, it acts as a priming factor for specific chemoattractants such as eotaxin.60 The observation that eotaxin decreased adhesion to vascular cell adhesion molecule (VCAM)-1 while increasing adhesion to the CD18 ligand bovine serum albumin (BSA) may be a mechanism for promoting egress from the marrow.61 Localized inflammatory responses can cause systemic effects after allergen challenge in mice in which IL-5–producing cells (both T cells and non–T cells), increase in the marrow.62
Accumulation of leukocytes in tissue is a highly regulated process with the aim of being able to respond effectively to noxious insults without causing an inappropriate inflammatory response. An obligate step in the migration of all leukocytes from the systemic circulation into tissue is their capture by endothelium as they flow at high shear rates through the postcapillary endothelium. A key receptor mediating eosinophil capture is P-selectin, whose low-level surface expression is selectively induced on endothelium by IL-4 and IL-13. Eosinophils express higher levels of P-selectin glycoprotein ligand (PSGL)-1 (the primary receptor for P-selectin) than other leukocytes and this results in increased avidity for P-selectin compared to neutrophils, especially at the low levels of expression induced by Th2 cytokines.63 Increased expression of PSGL-1 leading to enhanced recruitment has also been reported in allergic disease.64 IL-4 and IL-13 can also induce low levels of VCAM-1 expression which can bind eosinophils through very-late antigen (VLA)-4 and also capture flowing cells albeit at lower shear stresses. VLA-4/VCAM-1 and PSGL-1/P-selectin cooperate as a major endothelial control point for selective eosinophil migration.65 Once captured, eosinophils roll along the surface of the blood vessel until they are activated, which allows the CD18 integrins binding to ICAM-1 and ICAM-2 to nonselectively promote transmigration. VLA-4/VCAM-1 can also exert selective pressure at this stage.66 In mice, this process is dependent on the intracellular RAC-binding protein SWAP-70.67 The activation step mediated by chemoattractants expressed on the endothelial surface is another potential point of eosinophil selection, as shown by the effect of exogenously added chemoattractants such as eotaxin, but the identity of the endogenous chemoattractant involved and the extent to which it is selectively expressed in eosinophilic inflammation remains to be conclusively resolved. Orosomucoid-like 3 (ORMDL3), a molecule that has been identified by genetic epidemiology to be associated with asthma, is expressed by eosinophils and gene deletion in a mouse model resulted in reduced adhesion and recruitment into the lung.68
Once the eosinophil has transmigrated through the endothelium it has to migrate through the basement membrane and into the tissue. Chemokines as well as other eosinophil chemoattractants are likely to be central to this process (Table 62–3). Many eosinophil-active chemokines bind to the chemokine receptor (CCR)-3 and deletion of this gene severely impairs eosinophil migration into the lung in the mouse asthma model. The three specific eosinophil chemokines, eotaxins 1 to 3, appear to play overlapping roles in eosinophil migration into the lung in mice.69 However, a potent CCR3 antagonist had no effect on eosinophil migration into the airways in human asthma, questioning the physiologic relevance of the mouse data in human disease.70
Apoptosis is the universal mechanism by which cells undergo cell senescence in a manner that allows them to be efficiently removed by macrophages without inducing an inflammatory response. Morphologic observations have indicated that eosinophil apoptosis is an unusual event in tissue and that most eosinophils either die by cytolysis or migrate into the lumen where they do become apoptotic.7 A slow rate of apoptosis in tissues is consistent with the survival signals delivered to eosinophils by the extracellular matrix as part of normal homeostasis as well as increased production of eosinophil growth factors during Th2-mediated inflammation.71,72 The importance of prolonged survival of eosinophils in tissue as a mechanism for selective accumulation has been emphasized by studies using anti–IL-5, which effectively inhibits blood and sputum eosinophil numbers but has a much-less-marked effect on tissue eosinophils.73 Unlike neutrophils, where they prolong survival, glucocorticoids directly enhance the rate of eosinophil apoptosis, an effect inhibited by IL-5.74 Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), another family of survival modulating mediators related to tumor necrosis factor (TNF)-α, prolongs eosinophil survival, both in vitro and ex vivo, after allergen challenge.75
The biochemical mechanism by which growth factors mediate eosinophil survival is dependent on both new protein synthesis and phosphorylation events. The survival effects of IL-5 are dependent on activation of the Ras-Raf-MEC pathway and the Jak-2 Stat 1 and Stat 5 pathways, and involve LYN kinase, which binds to the IL-5Rα chain.76 The roles of p38 and phosphatidylinositide (PI) 3 kinase are less clear, and wortmannin, which blocks PI3 kinase, had no effect on eosinophil apoptosis, although it did inhibit IL-5 enhancement of adhesion to fibrinogen. Eosinophils express significant amounts of the proapoptotic BAX and the antiapoptotic BCL-xl, but very little Bad or BCL-2. As in other cell types, both spontaneous and FAS-induced eosinophil apoptosis is associated with the migration of BAX into the mitochondria. This event led to loss of mitochondrial membrane potential, cytochrome c release, and activation of downstream caspases. These events are all inhibited by IL-5, demonstrating that IL-5 works by blocking BAX translocation.77,78 Inhibition of BAX activation prevents eosinophil apoptosis even in the absence of cytokine. Treatment of eosinophils with dexamethasone also leads to loss of mitochondrial permeability.79 GM-CSF–activated ERK1/2, which phosphorylates BAX at Thr167, facilitates interaction with peptidylprolyl isomerase (PIN1). If interaction with PIN1 is prevented, BAX is activated and translocated to the mitochondria, resulting in apoptosis. It appears, therefore, that the eosinophil growth factors exert their antiapoptotic effects through fostering the PIN1–BAX interaction.80 IL-5–mediated eosinophil survival is also regulated by the balance between the signaling through two paired immunoglobulin-like receptors, PIR-A and PIR-B, with PIR-B counteracting the proapoptotic effect of PIR-A.81
Another potential mechanism involved in eosinophil tissue accumulation is in situ differentiation from eosinophil precursors. Eosinophil precursors can be identified in an IL-5Rα+CD34+ population in blood, increased after allergen challenge and in atopic disease. These cells have also been found in asthmatic airways.82
Of equal importance as endothelial interactions to the kinetics of eosinophil migration are the factors controlling the fate of the eosinophil once it enters the tissue. There are three possible outcomes. The eosinophil can remain in the tissue interacting with matrix proteins, other leukocytes, or structural cells such as, in the bronchial mucosa, the epithelium, airway smooth muscle, mucus glands, and nerves; alternatively, the cell can migrate into the lumen of the gut or airway where it is likely to undergo apoptosis and be removed; or it can return to the circulation via the lymphatics. The length of time that eosinophils remain in tissue before migrating into the lumen is unclear as there are virtually no studies of the kinetics of eosinophil migration in vivo in humans. An anti–IL-5 completely inhibited migration into the lumen, which suggests that transepithelial migration is IL-5–dependent. However, it only inhibited tissue numbers by at best 50 percent, emphasizing that different compartments are controlled by different mechanisms.73 In the mouse model of asthma, eosinophil migration into the lumen does not occur in the MMP-2 gene-deleted mouse, and the lack of migration causes the mouse to asphyxiate.83 As with senescent neutrophils, when tissue eosinophils become senescent they start to alter their receptor phenotype in a way that inhibits tissue retention and promotes migration into the lumen.84 The factors controlling the retention and survival of eosinophils in tissue are likely to involve the integration of chemoattractant, adhesive, and survival signals delivered by interactions with matrix proteins and structural cells. Studies modeling eosinophil migration in a tissue context using collagen gels have shown a different pattern to standard Boyden chamber assays with a much greater, albeit random, migratory response to growth factors than to chemoattractants.85 This observation suggests that migration into the lumen requires both a growth factor and a chemotactic stimulus.
Animal models, particularly the mouse model of ovalbumin challenge, which results in a selective and marked pulmonary eosinophilia, have been used extensively to analyze the molecular basis of eosinophil trafficking and the pathologic consequences of this movement.86 The majority of studies have focused on the role of eosinophilic inflammation in asthma.87 The combination of transgenic, gene-deletion, and antibody-based manipulations in the mouse make this a powerful tool for analyzing the biology of eosinophil migration, although the relevance of the findings to human disease should be treated with caution. Generally speaking, these studies support the concept of eosinophil migration as being caused by a series of interlinked and obligate steps with IL-5 necessary for providing a pool of circulating eosinophils, priming eosinophils for chemotactic responsiveness, and prolonging eosinophil survival. IL-4 and IL-13 control adhesion-related events in the endothelium and enhance the release of eosinophil chemoattractants, particularly CCR3-binding chemokines from mesenchymal cells within the airway.58 However, there are a number of other studies that look at other aspects of the immune response and as a result challenge this neat concept, in particular showing potential roles for innate immunity as well as other inflammatory mediators.88,89,90 Our understanding of the role of eosinophils in health and disease, using mouse models of disease, has been summarized.17
The eosinophil exerts its effects largely through its mediators (see Fig. 62–1). These chemicals are either newly generated, as is the case with leukotrienes and other lipid mediators, or stored preformed in various compartments within the cytoplasm and released when the eosinophil receives a degranulating stimulus. The eosinophil is relatively biosynthetically inactive and, although new protein synthesis does occur, the majority of its protein mediators are stored. Although the eosinophil can phagocytose particles its interactions with larval forms of helminthic parasites have formed the model by which eosinophil function has been described. In this situation, the eosinophil adheres tightly to the organism and releases its granule contents in local, high concentrations onto the surface in a process described as frustrated phagocytosis. The paradigm of eosinophil effector function in host defense was developed from the observation that the basic granule proteins, in particular, were highly toxic for larval parasites. This observation was extended to include a proinflammatory role in which they were also shown to be toxic for bronchial epithelium and, therefore, associated with the epithelial desquamation which is a well-established feature of severe asthma. Eosinophils are not thought to play a major role in bacterial host defense and, indeed, bacterial sepsis causes an eosinopenia; however, eosinophils can release mitochondrial DNA which has antibacterial properties.91 Eosinophils can also release a plethora of cytokines and chemokines although many of these are generated in low amounts compared to other cells and the extent to which they are important in eosinophil function is not clear.92
Most research into the role of eosinophils has focused on host defense against helminthic parasites and as effector cells in asthma. However, since 2010 there has been groundbreaking research emphasizing a potential homeostatic role for eosinophils in a number of areas, including antigen presentation, tissue repair, adipogenesis and glucose homeostasis, and B-cell development.93 There has been evidence for many years that eosinophils can present antigen to T cells, although the physiologic relevance of this function in the context of an intact dendritic cell antigen-presenting capacity remains uncertain.94 Type 2 immunity is necessary for the regeneration of skeletal muscle after injury. Muscle damage resulted in the rapid recruitment of eosinophils, which secretes IL-4 and activates muscle resident stem cells.95 Similarly, eosinophil-derived IL-4 was necessary for liver regeneration.96 A role in adipose tissue and glucose homeostasis was first suggested in 2011 when it was demonstrated that eosinophils, through the production of IL-4, were necessary for the maintenance of adipose tissue associated alternatively activated macrophages, which, in turn, were necessary to control glucose metabolism and body fat.97 This same pathway in concert with ILC2 cells is involved in the development of cold-induced, beige fat, which provides a defense against cold and obesity.98,99 Long-lived plasma cells, which survive in specialized niches in the marrow, do so as a result of growth factors (APRIL and IL-6) supplied by colocalized eosinophils.100 Eosinophils are also important in gut immune homeostasis, with eosinophil deficiency resulting in a reduction in IgA+ plasma cells and secreted IgA, as well as defects in the intestinal mucous shield, alteration in the gut microbiota, and the formation of CD103+ Tr and dendritic cells.101 B-cell development was also shown to be regulated by eosinophils in mice and humans.102 These studies were almost exclusively undertaken in mice, but they are potentially clinically relevant as a number of biologic therapies targeted at the Th2 pathway, including specific antieosinophil therapies, are in the late stages of clinical development for asthma and other eosinophilic diseases. So far, these drugs appear relatively free of serious adverse effects, so it is possible that the relatively modest effects of these drugs on the tissue eosinophilia means that the homeostatic role of eosinophils will not be perturbed.
Eosinophils can release a number of lipid mediators and are one of the relatively few sources of suliphidopeptide leukotrienes, although per cell they release approximately 10-fold less than mast cells and basophils.103 This is in contrast to neutrophils that produce large amounts of leukotriene (LT)B4, but little, if any, LTC4. LTC4 generation by human eosinophils also occurs after stimulation with opsonized zymosan and beads coated with IgG. Eosinophils can also generate substantial quantities of 15-HETE (hydroxyeicosatetraenoic acid) via 15-lipoxygenase. Eosinophils also generate platelet-activating factor (PAF) after stimulation with either calcium ionophore or IgG-coated beads. Eosinophils can also generate mediators of the cyclooxygenase pathway, including prostaglandins E1 and E2, thromboxane B2 (TXB2), and prostaglandin D2.104 The principal sites of eicosanoid formation in eosinophils are the lipid bodies, which contain large amounts of arachidonic acid and enzymes required for eicosanoid synthesis, including 5-lipoxygenase, LTC4 synthase, and cyclooxygenase.105 Eosinophils release significant amounts of TGF–β and TGF–α and this has stimulated interest in a potential role in causing airway remodeling. There is evidence that TGF–β released by eosinophils can promote the generation of fibromyocytes and anti–IL-5 reduced the amount of tenascin in the reticular subepithelial membrane.106,107 Thickening of this membrane is closely associated with eosinophilic airway inflammation, although not with AHR or airflow obstruction.108 Vasoactive intestinal peptide (VIP) has been detected in eosinophils in granulomas from mice infected with schistosomes and the eosinophil contains a number of granule-stored enzymes, whose roles in eosinophil function are not clear. These enzymes include acid phosphatase, collagenase, arylsulfatase B, histaminase, phospholipase D, catalase, nonspecific esterases, vitamin B12–binding proteins, and glycosaminoglycans. Eosinophils can undergo a respiratory burst with release of superoxide ion and H2O2 in response to stimulation, with both particulate stimuli, such as opsonized zymosan, and soluble mediators, such as leukotriene and phorbolmyrisate acetate. Eosinophils are twice as chemoluminescent as neutrophils.
A specific and important feature of eosinophils is the large amounts of basic proteins they contain within their specific granules. These are MBP, eosinophil cationic protein (ECP), eosinophil peroxidase, and eosinophil derived neurotoxin (EDN).109 MBP has a molecular mass of 13.8 kDa and an isoelectric point (pI) of 10.9. Its 17 arginine residues account for its alkalinity. It is initially synthesized as an acidic proprotein that is stored in the eosinophil granule. MBP becomes toxic only after it is released and processed into its final form. Purified MBP is cytotoxic for the schistosomula of Schistosoma mansoni, and adherence of eosinophils to IgG-coated schistosomula results in the secretion of MBP onto the tegument of the larvae, resulting in loss of viability.110 MBP at concentrations as low as 10 mg/mL is toxic for both guinea pig and human respiratory epithelial cells, as well as for rat and human pneumocytes. The mechanism of action of MBP on epithelial cells is mediated through inhibition of adenosine triphosphatase (ATPase) activity. MBP and eosinophil peroxidase are strong agonists for platelet activation as well as activation of mast cells, basophils, and neutrophils.111 The mechanisms of action of MBP is likely to be related to its hydrophobicity and strong negative charge. Basophils also contain MBP but only approximately 2 percent that of eosinophils.
Eosinophil peroxidase is a heme-containing protein that is synthesized as a single protein and then cleaved into 14- and 58-kDa subunits. The molecule shares a 68 percent identity in amino acid sequence with human neutrophil myeloperoxidase as well as other peroxidase enzymes. The protein is toxic for parasites, respiratory epithelium, and pneumocytes, either alone, or (more potently) when combined with H2O2 and halide, the preferred ion in vivo being bromide.
ECP is an arginine-rich protein. The complementary DNA (cDNA) encodes for a 27-amino-acid leader sequence and a 133-amino-acid mature polypeptide with a molecular mass of 15.6 kDa. ECP has 66 percent amino-acid-sequence homology with EDN and 31 percent homology with human pancreatic ribonuclease, but it has low ribonuclease activity compared to EDN. ECP is toxic for helminthic parasites, isolated myocardial cells, and guinea pig tracheal epithelium. ECP also inhibits lymphocyte proliferation in vitro. Both ECP and EDN produce neurotoxicity (the Gordon phenomenon) when injected into the cerebrospinal fluid of experimental animals. ECP may damage cells by a colloid osmotic process, as it can induce non–ion-selective pores in both cellular and synthetic membranes.
EDN, also called EPX, is a 16-kDa, glycosylated protein possessing marked ribonuclease activity. The cDNA predicts a 134-amino-acid, mature polypeptide that is identical to human urinary ribonuclease. Like ECP, it is a member of a ribonuclease multigene family. EDN expression is not restricted to eosinophils, as it is found in mononuclear cells and possibly neutrophils. It is also probably secreted by the liver. It does not appear to be toxic to parasites or mammalian cells, and its only known effect, other than its ribonuclease activity, is neurotoxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree