INTRODUCTION
SUMMARY
The neutrophil circulates in blood as a quiescent cell. Its main function as a phagocytic and bactericidal cell is performed outside the circulation in tissues where microbial invasion takes place. Neutrophil function is traditionally viewed as chemotaxis, phagocytosis, and bacterial killing. Although these conceptionally represent distinct entities, they are functionally related, and rely to a large extent on the same intracellular signal transduction mechanisms that result in localized rises in intracellular Ca2+, changes in organization of the cytoskeleton, assembly of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase from its cytosolic and membrane integrated subunits, and fusion of granules with the phagosome or neutrophil plasma membrane. Clinical disorders of the neutrophil may arise from impairment of these normal functions. The clinical presentation of a patient who has a qualitative neutrophil abnormality may be similar to that of one who has an antibody, complement, or toll-like receptor disorder. In general, evaluation for phagocyte cell disorders should be initiated among those patients who have at least one of the following clinical features: (1) two or more systemic bacterial infections in a relatively short time period; (2) frequent, serious respiratory infections, such as pneumonia or sinusitis, or otitis media, or lymphadenitis; (3) infections present at unusual sites (liver or brain abscess); and (4) infections associated with unusual pathogens (e.g., Aspergillus pneumonia, disseminated candidiasis, or infections with Serratia marcescens, Nocardia species, or Burkholderia cepacia).
Acronyms and Abbreviations
ADP, adenosine diphosphate; ARF, ADP-ribosylation factor; ASC, apoptosis-associated speck-like protein with a caspase recruitment domain; ATPase, adenosine triphosphatase; BPI, bacterial permeability-increasing protein; cAMP, cyclic adenosine monophosphate; cANCA, cytoplasmic antineutrophil cytoplasmic antibody; CARD, caspase activation and recruitment domain; c/EBP, CCAAT/enhancer binding protein; CGD, chronic granulomatous disease; CHS, Chédiak-Higashi syndrome; DAG, diacylglycerol; DOCK8, dedicator of cytokinesis 8; ESAM, endothelial cell-selective adhesion molecule; FAD, flavin adenine dinucleotide; FMF, familial Mediterranean fever; fMLP, formyl-methionyl-leucyl-phenylalanine; G6PD, glucose-6-phosphate dehydrogenase; GDP, glucose diphosphate; GPI, glycosylphosphatidylinositol; GTP, guanosine triphosphate; GTPase, guanosine triphosphatase; H2O2, hydrogen peroxide; HBP, heparin-binding protein; HETE, hydroxyeicosatetraenoic acid; HLA, human leukocyte antigen; HNP, human neutrophil peptide (synonym: defensin); ICAM, intercellular adhesion molecule; IFN, interferon; Ig, immunoglobulin; IL, interleukin; IP3, inositol triphosphate; ITAM, immunoreceptor tyrosine-based activation motif; JAMs, junctional adhesion molecule A, B, and C; LAD, leukocyte adhesion deficiency; LFA-1, leukocyte function-associated antigen-1; LPS, lipopolysaccharide; LSP-1, lymphocyte-specific protein-1; LTB4, leukotriene B4; Mal/TIRAP, MyD88-adaptor-like/toll/interleukin-1 receptor domain containing adaptor protein; MAPK, microtubule-associated protein kinase; MBL, mannose-binding lectin; MMP, matrix metalloproteinase; MPO, myeloperoxidase; MyD88, myeloid differentiation factor 88; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); NBT, nitroblue tetrazolium; NEM, N-ethylmaleimide; NET, neutrophil extracellular trap; NF-κB, nuclear factor-κB; NGAL, neutrophil gelatinase-associated lipocalin; NK, natural killer; NSF, N-ethylmaleimide–sensitive fusion protein; NSP4, neutrophil serine protease 4; PA, phosphatidic acid; PAF, platelet-activating factor; PECAM, platelet endothelial adhesion molecule; phox, phagocyte oxidase; PI3K, phosphatidylinositol 3′-kinase; PIP1, phosphatidylinositol-4-monophosphate; PIP2, phosphatidylinositol-4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; PLD, phospholipase D; PLS, Papillon-Lefèvre syndrome; PSGL, P-selectin glycoprotein ligand; SGD, specific granule deficiency; SH3, Src homology 3; sLex, sialyl Lewis X; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor; TIR, toll/interleukin-1 receptor; TLR, toll-like receptors; 7TMRs, seven trans-membrane–spanning domain proteins; TNF, tumor necrosis factor; TRAM, TRIF-related adaptor molecule; TRAPS, tumor necrosis factor receptor–associated periodic syndrome; TRIF, TIR domain-containing adaptor inducing interferon (IFN)-β; VAMP-2, vesicle-associated membrane protein-2.
NEUTROPHIL STRUCTURE AND FUNCTION
The similarity between neutrophil locomotion and that of amebas was noted long ago.1 Neutrophils respond to spatial gradients of chemotaxins with differences in concentration of chemotaxin of as little as 1 percent across the cell,2 although there has been contention as to whether chemotaxis also requires temporal, as well as spatial, sensing.3 Even with populations of cells as “homogenous” as neutrophils, a broad range of responsiveness is found.4 During locomotion toward a chemotactic source, neutrophils acquire a characteristic asymmetric shape (Fig. 66–1). In the front of the cell is a pseudopodium, referred to as the lamellipodium, that advances before the body of the cell containing the nucleus and the cytoplasmic granules. At the rear of the moving cell is a knob-like tail, the uropod. The lamellipodium undulates or “ruffles” as the neutrophil moves, at a rate of up to 50 μm/min. The membrane lipids also flow during locomotion,5 and enhanced cytosolic Ca2+ is observed along the membrane margin.6 The lamellipodium, which is very thin, forms immediately when the cell encounters a gradient of chemotactic factor. As the cell moves, the cytoplasm behind the lamellipodium streams forward, almost obliterating it. At this point, some granules appear to contact the cell periphery and release granule contents in response to chemotactic agents. The lamellipodium extends again and the process repeats itself. A flow of cortical materials, composed particularly of actin filaments, has been proposed to account for chemotaxis as well as other cellular movements.7 This may also account for changes in cell viscosity. Polarity and movement is orchestrated by the cytoskeleton through signals generated from receptor associated G-proteins in an intricate network that regulates both direction and intensity of movement.8,9,10,11
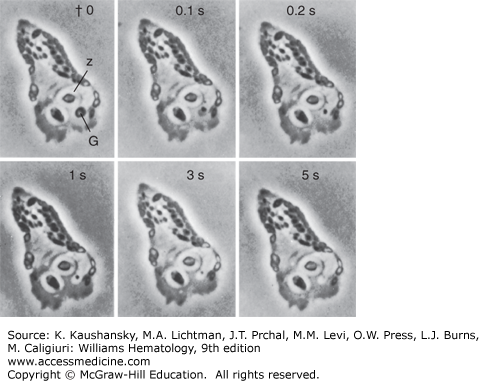
Figure 66–1.
Cinemicrophotographic observation of granule lysis of a chicken neutrophil following phagocytosis of zymosan particles. Note the lysis of the cytoplasmic granule (G) against one of two ingested zymosan particles (Z). The dense body of the granule disappears from view in the interval of 5 s (original magnification ×1200). (Reproduced with permission from Hirsch JG: Cinemicrophotographic observations on granule lysis in polymorphonuclear leucocytes during phagocytosis. J Exp Med 116:827–834,1962.)
When a neutrophil comes in contact with a particle, the pseudopodium flows around the particle, its extensions fuse, and it thereby encompasses the particle within the phagosome.1 The ingestion phase can be said to extend from recognition to the end of pseudopodium fusion. The particle thus becomes enclosed within a phagosome into which granules are rapidly discharged, as illustrated in Fig. 66–1. As with locomotion, phagocytosis results in Ca2+ being released in the vicinity of the active membranes.6 The number of ingested particles may be eventually limited by the availability of plasma membrane.7 Locomotion is not a prerequisite for ingestion: If neutrophils collide with a particle not secreting a chemotactic substance, pseudopodia form abruptly at the contact point and envelop the particle.12
The formation of a lamellipodium is essential for neutrophil locomotion and is also required for ingestion. When dissolution of the lamellipodium occurs, the interior contents of the cell are allowed to contact the cell membrane. Granule discharge may occur. Fusion of membranes is a common feature of (1) ingestion, where pseudopodia fuse; (2) degranulation, where granules fuse with the phagosome; and possibly (3) locomotion, where some granules may fuse with the plasma membrane. Pseudopodia form whether neutrophils are suspended in liquid medium or are attached to a surface, but the cell can only move translationally when fixed to a surface; thus it crawls but does not swim. Such “stickiness” is also a phase of ingestion.7 The neutrophil membrane adheres firmly to particles they ingest, presumably to provide the frictional force needed to move pseudopodia around the particles. Thus, the formation of pseudopodia, membrane fusion, and membrane adhesiveness are all characteristics associated with the functional responses of neutrophils.
The dual neutrophil functions of immune surveillance and in situ elimination of microorganisms or cellular debris require rapid transition between a circulating nonadherent state to an adherent state, allowing the cells to migrate into tissues when necessary. Initially neutrophils appear at sites on the endothelium adjacent to the site of inflammation. Adhesion molecules on endothelium are induced by the inflammatory mediators tumor necrosis factor (TNF)-α and interleukin (IL)-1. Lipopolysaccharide (LPS), released by local activated macrophages and microorganisms, results in local extravasation of the neutrophil. In postcapillary venules or in pulmonary capillaries the slow rate of blood flow, further reduced by vessel dilatation at sites of inflammation, permits a loose and somewhat transient adhesion referred to as “tethering,” and results in the rolling of the neutrophil along the endothelium.13 Extensions from the rear of the neutrophil wraps around the rolling neutrophils as so called slings and provide “crawler tracks” at their front that assists in adhesion to the endothelium.14 During this tethering step, neutrophils respond to ligands, primarily chemokines dispatched on the endothelial surface by a signaling event that acts to reorganize the neutrophil surface membrane, thereby exposing adhesion molecules, which, in turn, lead to sustained adhesion and spreading (Chap. 19).
Circulating neutrophils contain surface microvilli of a diameter of 0.3 μm.15 Moesin, ezrin, and p205 radixin are actin-binding proteins associated with neutrophil plasma membranes and are important for organization of microvilli on the surface of the cell.16,17 These actin binding proteins tether the primary adhesion proteins exposed on the microvilli, L-selectin and P-selectin glycoprotein ligand 1 (PSGL-1).18 L-selectin and PSGL-1 are filamentous glycosylated proteins protruding from the tips of the microvilli. E-selectin ligand 1 (ESL-1) located in the side of microvilli,19 and CD44, located on the cell body, both serve a ligands for E-selectin.20 L-selection, like the other selectins, including P-selectin, which is expressed on platelets and endothelial Weibel-Palade bodies, and E-selectin, which is expressed in endothelial cells, bind with a variable affinity to sialyl fucosylated oligosaccharides including sialyl Lewis X (sLex), which is present on multiple specific glycolipids and glycoproteins on leukocytes and inflamed endothelial cells.21 When binding to their ligands, L-selectin, PSGL-1, ESL-1, and CD44 recruit Syk (spleen tyrosine kinase), a tyrosine kinase, which binds to the immunoreceptor tyrosine-based activation motif (ITAM). ITAMs are present in the cytoplasmic domains of surface membrane proteins, and Syk then orchestrates the further signaling to initiate cell activation.22,23,24,25
P-selectin is mobilized rapidly to the endothelial cell surface following stimulation by thrombin, histamine, or oxygen radicals and interacts with neutrophil PSGL-1 to initiate neutrophil rolling.21 Rolling subsequently involves newly expressed E-selectin, which appears on endothelial cells 1 to 2 hours after cell stimulation by IL-1, TNF-α, or LPS. E-selectin counterreceptors include PSGL-1, ESL-1, and CD44.19,24 Both P- and L-selectin contribute sequentially to leukocyte rolling, but L-selectin is involved in the prolonged neutrophil sequestration on inflamed microvasculature. L-selectin is constitutively present on neutrophils and its binding capacity is rapid and transiently increased after neutrophil activation, possibly via receptor oligomerization. Activation of ADAM17, a matrix metalloproteinase expressed at the neutrophil surface, severs L-selectin from the surface of neutrophils and impairs their recruitment to endothelium.26,27 Thus far, only one inducible L-selectin counterreceptor has been identified on inflamed endothelium.28 In addition to its binding to endothelial ligands, neutrophil PSGL-1 is a counterreceptor for L-selectin, which permits previously adherent neutrophils to recruit other neutrophils to inflamed endothelium (Chap. 19).13,21
Figure 66–2 shows a sequence of molecular and biophysical events leading to neutrophil activation and increased adherence during acute inflammatory response in vivo. The inflamed endothelium produces chemoattractants such as platelet-activating factor (PAF), leukotriene B4 (LTB4), and various chemokines, immobilized by proteoglycans on the luminal surface of endothelial cells.29 Among these chemokines, IL-8 specifically attracts neutrophils. IL-8 is synthesized by endothelial cells in response to IL-1, TNF-α, or LPS, and is stored in Weibel-Palade bodies; IL-8 can be released by histamine or thrombin.30 Additionally, IL-8 can be internalized by endothelial cells and transcytosed from the abluminal surface via vesicular caveolae, and presented to the tips of microvilli of the endothelial cell luminal surface.31 The binding of signaling molecules such as PAF and IL-8 to surface receptors on the leukocytes activates them in a juxtacrine fashion and triggers changes in affinity or avidity of β2 integrins, leukocyte function-associated antigen (LFA)-1 (CD11a/CD18), which is constitutively expressed on neutrophil plasma membranes, and Mac-1 (CD11b/CD18), which becomes incorporated in the neutrophil plasma membrane from secretory vesicles.13,21,32 β2 Integrins are recognized by counterligands on endothelial cells, including members of the intercellular adhesion molecule (ICAM) family such as ICAM-1 and ICAM-2. The ICAM glycoproteins are induced by cytokines that include TNF and IL-1. The relative affinity of the β2 integrins for ICAM is increased by exposure of neutrophils to numerous stimuli, including C5a, N-formylated bacterial peptides, IL-8, and LTB4. The extracellular domains of unactivated integrins are in a bent position and not able to bind ligands. Intracellular signals, such as those generated via Syk as discussed above, can transform the integrins into an extended but not fully open conformation, capable of ligand binding with weak affinity permitting the integrin (LFA-1) to participate in rolling33 and an extended and fully open form capable of ligand binding with strong affinity, mediating firm adhesion. The molecular mechanisms have been worked out in great detail. In essence, tailins and kindlins are recruited and bound to membrane near phosphotyrosine (NPxY and NxxY) motifs present on the integrin β chains and twist the cytoplasmic domains of the α and β chains. This changes the conformation of the extracellular domains from the bent to the open state, thereby permitting binding to ligands, and in so doing transmit signals from outside to inside.21,34,35,36 Neutrophils integrate the signals of integrin engagement and those delivered simultaneously by inflammatory cytokines or chemoattractants to activate a cascade of intracellular events resulting in cell spreading (Fig. 66–2). The CD11b/CD18 integrin (MAC-1) is known to interact in cis fashion with glycosylphosphatidylinositol (GPI)-anchored membrane proteins such as FcγRIIIB (CD16), the LPS receptor CD14, and the urokinase plasminogen activator receptor (uPAR; CD87). Integrins behave as transducers mediating signals transferred by these GPI-linked receptors.37 For instance, FcγRIIIB interaction with CD11b/CD18 promotes antibody-dependent phagocytosis, whereas CD14 interaction with CD11b/CD18 occurs in the presence of LPS and LPS-binding protein to generate proinflammatory mediators, and uPAR interaction with CD11b/CD18 mediates neutrophil migration by recruiting and activating the urokinase-type plasminogen activator.29
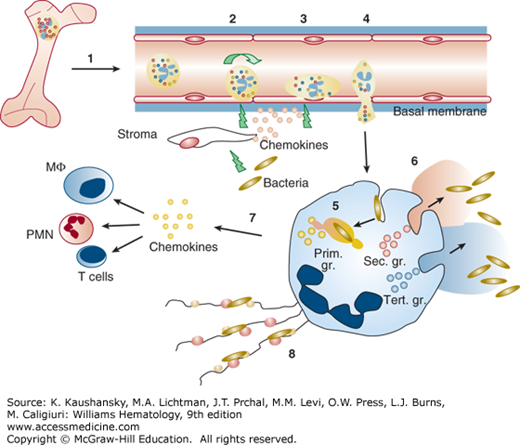
Figure 66–2.
Neutrophil-mediated inflammatory response. (1) Egress of mature neutrophils from marrow to circulation. (2) Initial tethering and rolling are dominantly mediated by selectins present both on neutrophils and endothelial cells and their ligands. Invasion by bacteria stimulates tissues macrophages to secrete inflammatory cytokines, interleukin (IL)-1 and tumor necrosis factor, which, in turn, activate endothelial cells to express E- and P-selectin and IL-8. E- and P-selectin serve as counterreceptors for the neutrophil P-selectin glycoprotein ligand-1. (3) Activated endothelial cells express intercellular adhesion molecule (ICAM)-1 and ICAM-2, which serve as ligands for the neutrophil β2 integrins. The β2 integrins mediate tight adhesion and arrest of the leukocytes in cooperation with the selectins. Localized activation of neutrophils by juxtacrine signaling molecules or chemoattractants that bind to surface receptors is critical for inside-out signaling of β2 integrins, making them adhesive for the ICAM ligands on the endothelium. (4) Neutrophil invasion through the vascular basement membrane with release of proteases and reactive oxidative intermediates that cause local destruction of the extracellular matrix which allows for migration of the neutrophils into tissues. (5, 6) Uptake of microorganisms into the phagocytic vacuole with concomitant degranulation both into the phagocytic vacuole (azurophil granules and specific granules) and to the exterior (specific granules and gelatinase granules). (7) A burst of transcriptional activity is initiated during diapedesis of neutrophils and during phagocytosis, which results in generation of chemokines such as IL-8, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and IL-1β that may recruit additional cells of the immune system.58 (8) Formation of neutrophil extracellular traps by extrusion of chromatin and cationic bactericidal granule proteins.262 MΦ, macrophages; PMN, polymorphonuclear neutrophil.
Fully extended and open integrins bind ICAM-1 firmly and thus mediate attachment of neutrophils to endothelial cells.38 ICAM-1 and -2 direct the motion of neutrophils to points of egress from the vascular lining. The majority are guided to points where three or more endothelial cells join. Intracellular signals from ICAMs loosen the binding between endothelial cell junctions provided by homotypic interaction of VE-cadherins.39
Platelet endothelial cell adhesion molecule 1 (PECAM-1), endothelial cell-selective adhesion molecule (ESAM), junctional adhesion molecule A, B, and C (JAMs), and CD99 also form homotypic interactions between endothelial cells; however, neutrophils also express these adhesion proteins and may displace the interendothelial cell homotypic binding with neutrophil–endothelial cell binding mediated by the same proteins. In this way neutrophils can “zipper” through40,41,42 and exit by this paracellular route. A minority of neutrophils exit by a transcellular route through so-called endothelial cups.42
Pericytes are perivascular contractile cells that interact with endothelial cells and regulate vascular permeability. Neutrophils exit the vascular wall through gaps between pericytes.43 Pericytes adopt different morphologies and distributions in different tissues. Such may explain differences in neutrophil recruitment to viscera.44
Once out in tissues the forefront neutrophils generate IL-8 and LTB4 in order to recruit an additional swarm of neutrophils to the area and recruit later incoming monocytes and macrophages.45
Several proteins associated with the surface of the neutrophil function in the normal housekeeping activities such as Na+/K+ adenosine triphosphatase (ATPase), but others serve specific functions such as L-selectin, PSGL-1, and integrins. The surface of neutrophils is highly dynamic as a result of the incorporation of membrane from intracellular vesicles and granules, a process that is known to add significantly to the total cell surface measured by an increase in electric capacitance.46 A number of membrane-bound receptors are localized to secretory vesicles and incorporated into the surface membrane when secretory vesicles fuse with the plasma membrane, as occurs during diapedesis. This enhances the ability of neutrophils to respond to the signals presented by endothelial cells or present in the extravascular tissue.
Neutrophils and other cells of the innate immune system recognize microbes through germline-encoded receptors, which recognize molecular patterns that are relative unique to pathogens and shared among groups of pathogens, so-called pathogen-associated molecular patterns (PAMPs). These pattern-recognition receptors (PRRs) include the membrane-bound toll-like receptors (TLRs) and C-type lectin receptors (CLRs), and the cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and RIG-like receptors (RLRs).47,48,49,50 Although PRRs are highly expressed in myeloid cells, they are also widely expressed in cells that are regularly exposed to microorganisms, particularly in epithelial cells.
TLRs are type 1 transmembrane signaling receptors that are activated by dimerization induced by ligand binding.51 The TLRs may dimerize both as homodimers and heterodimers. TLRs that recognize microbial membrane components are largely present on the cell surface, and include TLR2 that recognizes lipoproteins and lipopeptides in association with either TLR1 or TLR6. CD14 is known as an LPS-binding protein but is not itself able to signal and presents LPS to TLR4.51 TLR5 binds flagellin, and TLR11 binds profilin-like proteins of protozoa.52 TLRs that recognize viral components are largely expressed on intracellular vesicles that may fuse with phagosomes and include TLR3 (not present in neutrophils) that recognizes double-stranded RNA, TLR7/8 that binds viral single-stranded RNA,53 and TLR9 that binds unmethylated GpC regions on DNA.54
Ligand binding, that is, dimerization of TLRs leads to recruitment of one of four intracellular adaptor proteins to the TIR (toll/IL-1 receptor) domain of the TLR. These proteins include MyD88 (myeloid differentiation factor 88), Mal/TIRAP (MyD88-adaptor-like/toll-IL 1 receptor domain containing adaptor protein), TRAM (TRIF-related adaptor molecule), and TRIF (TIR domain-containing adaptor inducing IFN-β). While many TLRs (5, 7, 8, and 9) exclusively use MyD88, TLR2 requires both Mal and MyD88 and TLR4 can use either Mal (MyD88-adaptor-like) and MyD88 or TRAM and TRIF to signal to NF-κB (nuclear factor-κB) or interferon regulatory factor (IRF)-3.55,56,47
CLRs comprise a heterogeneous group of trans-membrane receptors that bind carbohydrates such as mannose, fucose, and β-glucans present on a variety of microbes, fungi in particular. They signal largely via their cytosolic ITAMs and Syk to activate NF-κB, nuclear factor of activated T cell (NFAT), and microtubule-associated protein kinases (MAPKs) resulting in production of proinflammatory cytokines.48
NLR proteins are cytosolic proteins that are divided into five subfamilies, NLRA, NLRB, NLRC, NLRP, and NLRX.50 Their N terminus contains either a caspase activation and recruitment domain (CARD) or a pyrin domain (PYD). The NLRC members NOD1 and NOD2 recognize peptidoglycans of both Gram-positive and Gram-negative bacteria and signal to activate the NF-κB pathway. Other members of the NLRC and NLRP subfamily are essential in organizing the inflammasome. The NLRs multimerize through their CARDs into inflammasomes,50 cytoplasmic structures that activate caspase-1, which, in turn, convert pro–IL-1 and pro–IL-18 to the mature proinflammatory cytokines that are secreted.57
A variety of chemokine receptors are found on the surface of the neutrophil. These are in general G-protein–coupled receptors. Other G-protein–coupled receptors on neutrophils are the purine receptors for adenosine diphosphate (ADP) and ATP, the PAF receptor C5a, and formyl-methionyl-leucyl-phenylalanine (fMLP) receptors. Receptors not belonging to the G-protein–coupled receptor family include receptors for IL-1, IL-10, and TNF-α, and the growth factors receptors for granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Both growth factor receptors are important for myeloid development, and play an important role in enhancing neutrophil function and gene transcription in mature neutrophils. A burst of transcriptional activity is associated with the diapedesis of neutrophils into tissues, which results in downregulation of proapoptotic genes and upregulation of genes coding for antiapoptotic proteins, upregulation of genes encoding chemokines and cytokines that may recruit macrophages, T cells and additional neutrophils, and downregulation of genes encoding chemokine receptors (see Fig. 66–2).58
Neutrophils express the Fc α receptor (CD89) for immunoglobulin (Ig) A and IgG receptors, FcγRIIA (CD32), and FcγRIII (CD16). Neutrophils also express receptors for the complement components, including CD1qR, CR1 (CD35), CR3 (CD11/CD18), and CR4. CR1 binds CD3b, C4b, and C3bi with decreasing affinity. CR3 recognizes C3bi (a proteolytic fragment of C3b). Of particular importance is that both Fcγ receptors and GPI-coupled receptors appear to be localized to lipid rafts. Lipid rafts are important, but elusive structures that facilitate signal transduction leading to phagocytosis by promoting several membrane protein interactions. Initially the rafts were conceptionally associated with caveolae, which are structures identified on endothelial cells and thought to be important for transendothelial cell traffic. The caveolae were identified by their high content of cholesterol lipids and the presence of the structural protein, caveolin. Rafts were subsequently identified on neutrophils, but these cells are devoid of caveolin.59 Rafts are perhaps best viewed as patches of surface membrane that attract many hydrophobic proteins including signaling molecules such as tyrosine kinases and phosphatases. Other membrane protein receptors that are not normally associated with rafts may change their conformation and subsequently associate with rafts upon binding their ligands. This is particularly true for the Fcγ and GPI-coupled receptors.
Secretory vesicles are small intracellular vesicles that were discovered during the search for the structural basis for upregulation of a variety of surface molecules on neutrophils in response to nanomolar concentrations of fMLP and other chemotactic stimuli. They were initially identified by “latent” alkaline phosphatase.60 Secretory vesicles of neutrophils should not be confused with the vesicles that carry cargo from endoplasmic reticulum and Golgi in the constitutive secretory pathway of other cells and that are sometimes also named secretory vesicles. Secretory vesicles of neutrophils are specialized endocytosis vesicles that are formed in the final stages of neutrophil maturation in the marrow. They contain plasma proteins, seemingly without any selectivity. Albumin thus serves as a marker for secretory vesicles and has allowed the identification of these as small intracellular vesicles that are scattered throughout the cytoplasm of neutrophils as is true for neutrophil granules. The plasma proteins inside secretory vesicles show no sign of degradation, thus no fusion takes place with lysosomal structures.61 Secretory vesicles behave like the traditional neutrophil granules. They require a specific signal for mobilization.62 Secretory vesicles are not important for their cargo (plasma proteins), but for their membrane which becomes fully incorporated into the plasma membrane of the neutrophil upon stimulation.61,63,64,65,66 Secretory vesicles host most of the neutrophil chemotactic and GPI-coupled receptors, TLRs, and one of the early acting downstream effectors, phospholipase D.67 They enrich the plasma membrane with receptors for adhesion and signaling, and can be seen as the structural basis for transition of neutrophils from circulating quiescent cells that do not respond well to stimuli such as chemoattractants and objects to be phagocytosed, to highly responsive cells capable of establishing firm contact with endothelium. The signals generated by tethering of selectins or PSGL-1 to the endothelium are sufficient to mobilize secretory vesicles. Secretory vesicles are completely mobilized in vivo during neutrophil diapedesis.21,66
The first identified marker of secretory vesicles, latent alkaline phosphatase, is known to be elevated in chronic myeloproliferative disorders except for chronic myelogenous leukemia (CML), but the content of secretory vesicles in neutrophils from patients with chronic myeloproliferative disorders is not different from normal neutrophils.68,69,70 The best marker for secretory vesicles is CD35, a transmembrane protein of 160 to 250 kDa that binds complement components C3b and C4b, because CD35, in contrast to alkaline phosphatase, is absent from the plasma membrane of unstimulated neutrophils, and because it is absent from granules (in contrast to αMβ2).32,65,71 It is not known whether secretory vesicles contain lipid rafts, but most GPI-linked proteins are raft-associated72 and are localized to secretory vesicles in neutrophils.
The neutrophil is known for its granules. When Paul Ehrlich introduced aniline dyes in histochemistry and discovered the different subsets of leukocytes, the neutrophil granules were divided into those that took up the azure dye, the azurophilic granules, and the others, the specific granules.73,74 When the peroxidase reaction was introduced, the azurophil granules were found to be peroxidase-positive as a result of the presence of the major myeloid cell protein, myeloperoxidase (MPO), and the specific granules were thus named peroxidase-negative granules.75,76 Because the azurophil granules are formed first, in the promyelocyte, and the specific granules later, in the myelocyte, these are also termed primary and secondary granules, respectively. A tertiary granule subset was identified in human neutrophils and shown to contain gelatinase,77 but the ultrastructure was not determined until the issue of the neutrophil gelatinase (matrix metalloproteinase [MMP]-9) as a possible complex with neutrophil gelatinase-associated lipocalin (NGAL) was identified.78,79
Granules were initially viewed of as small bags that emptied their content of bactericidal substances onto the ingested microorganisms when granules fuse with the phagocytic vacuole during phagocytosis, but it later became clear that granules are not only important for their cargo, but also for their membranes, as they contain proteins that become incorporated into the membrane of the phagocytic vacuole and into the surface membrane when the granules are mobilized.80,81 If granules were classified by their content, both of matrix proteins and membrane proteins, the number of different granule subsets that exists in neutrophils would be meaninglessly high. Yet nature has provided a beautiful setting that allows the neutrophil to fine tune its response to a specific task. A priori, there would be two reasons for having different subsets of granules: One would be to ensure that proteins, which cannot coexist, are segregated; that is, protease-sensitive proteins are separated from proteases. The other reason would be to have proteins whose service is needed at one time separated from proteins whose service is needed at a different time.
Among the peroxidase-positive granules, subsets can be identified that are rich in defensins as well as some that are not.82,83 Functionally, no difference has been identified in terms of the regulation of exocytosis of these peroxidase-positive granule subsets.84 Other constituents include the serine proteases elastase, cathepsin G, proteinase 3, and neutrophil serine protease 4 (NSP4), and the inactive serine protease azurocidin (aka CAP 37), the antimicrobial proteins BPI (bacterial permeability-increasing protein), lysozyme, and the α defensins, which are the dominating species.80 Defensins are also named HNPs (human neutrophil peptides). The membrane of the azurophil granules contains CD63 (granulophysin) and CD68, but their role in neutrophil function remains unclear.85,86 Many of the proteins present in peroxidase granules are proteolytically processed both at the N-terminus and the C-terminus to the active mature forms, which are stored in the granule matrix.
Peroxidase-negative granules can be divided into three subsets based on the distribution of the two marker proteins lactoferrin and gelatinase: granules that contain lactoferrin, but no gelatinase (15 percent of peroxidase-negative granules), granules that contain both proteins (60 percent), and granules that are rich in gelatinase, but low (or absent) in lactoferrin (25 percent).87 The latter are named gelatinase granules or tertiary granules, whereas those that contain lactoferrin are called specific or secondary granules. It is a characteristic of peroxidase-negative granules that the proteins present in their matrix are not proteolytically processed. The MMPs of peroxidase-negative granules are stored as a proform,88 as is the major bactericidal protein hCAP-18.89,90 No major differences have been identified in the content of membrane proteins of the peroxidase-negative granule subsets. All contain the flavocytochrome p22phox/gp91phox complex that is part of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and all contain the major β2-integrin αMβ2—and these are even shared with the membrane of secretory vesicles.32,91,92 The divalent cation transporter Nramp1 is localized only to gelatinase granules,93 and the membrane MMP leukolysin (MMP-25)94 is shared between gelatinase granules and secretory vesicles. However, the subsets differ markedly in their propensity for exocytosis. Following neutrophil stimulation, gelatinase granules are exocytosed to a larger extent than granules containing both lactoferrin and gelatinase, and these are more readily mobilized than granules containing lactoferrin but lacking gelatinase. These, in turn, are mobilized more readily than peroxidase-positive granules.62,66,79,87,95 This organization of granule subsets with different content and different set points to trigger exocytosis allows the neutrophil to mobilize MMPs and integrins necessary for movement through the basal membrane and tissue before the bactericidal peptides and serine protease are called to play, but it puts an enormous task on the organization of the biosynthetic apparatus to secure that the right granule proteins are targeted to the granules with a given trigger for exocytosis.
The content of isolated granules has been mapped by proteome analysis.96 High-resolution mass spectrometry has identified 1300 proteins associated with neutrophil granules, plasma membranes and secretory vesicles and confirmed that localization is largely determined by time of biosynthesis.97
The extreme heterogeneity of neutrophil granules and their individual control of exocytosis can be explained simply by timing of their biosynthesis (Fig. 66–3). Granule proteins are synthesized during myelopoiesis from myeloblasts to band cells and segmented neutrophils in the marrow.75,76,98 The window of biosynthesis of each granule protein is highly controlled by combinations of transcription factors that change as the cells differentiate and mature.99,100 If all granule proteins are targeted to granules during synthesis, the content of newly formed granules would change as the cell matures because the profile of biosynthesis changes. A global view of the change in transcriptional activity of neutrophil precursors during maturation in the marrow confirmed the association between granule localization and transcriptional activity.101 This simple mechanism largely explains the heterogeneity of granules102 and their contents, but it does not account for the differences in exocytotic rates among individualized subsets. By timing the biosynthesis of the proteins essential for fusion103,104 to granule membranes during maturation, it is possible to regulate the rates of exocytosis. Indeed, the v-SNARE (SNAP receptor), vesicle-associated membrane protein (VAMP)-2 is present in a higher density on gelatinase granules than on specific granules and is most highly expressed on secretory vesicles,105,106 which correlates with the ease of releasing granule subsets from the neutrophil following activation.
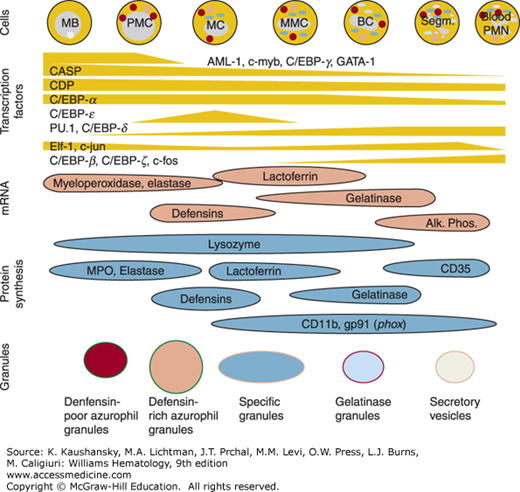
Figure 66–3.
Formation of granule subsets during myelopoiesis and regulation of granule protein transcription. Difference in the appearance and disappearance of transcription factors regulate the individual window of granule protein gene transcription and translation into protein that is targeted to forming granules, explaining the heterogeneity of neutrophil granules.
Although the sorting by timing can explain the granule heterogeneity of neutrophil granules, it does not provide any clues to the mechanisms responsible for diverting newly synthesized proteins to granules as opposed to immediate (constitutive) secretion. Not all granule proteins are equally efficiently directed to granules. Lysozyme is poorly retained during biosynthesis.107 This explains the high concentration of lysozyme in plasma.108 MPO is efficiently retained and the plasma level of MPO is consequently very low. A particularly interesting observation pertains to α defensins. These are localized exclusively to azurophil granules, but their window of biosynthesis is very similar to that of lactoferrin,107,100 and defensins and lactoferrin are both controlled by the transcription factor C/EBPε (CCAAT/enhancer binding protein ε), which is absolutely required for biosynthesis of specific granule proteins.109,110 The absence of defensins from specific granules, despite an active biosynthesis when other specific granule proteins are formed, is explained by a complete lack of sorting of defensins to granules in myelocytes.99,100,107 Only defensins synthesized at the late promyelocytic stage are routed to granules, whereas defensins synthesized at the myelocyte stage are secreted from cells after biosynthesis107 and is present in relatively high concentrations in plasma.111 The defensins that are targeted to granules are processed to mature defensins, whereas the defensins that are secreted remain unprocessed. Processing of defensins removes a charge neutralizing propiece, sorting of defensins and other granule proteins to granules may depend on their ability to interact with negatively charged proteoglycans that are present in the matrix of granules.112,113,114 Serglycin, an intracellular proteoglycan is present in Golgi and immature granules of promyelocytes and disappears as the cells mature.115 Serglycin is absolutely critical for confining a variety of mast cell proteins to the mast cell granules.116 Granulocytes from mice with a targeted disruption of the serglycin gene are morphologically normal and contain normal levels of granule proteins except elastase.117 CD63 was demonstrated to be involved in sorting of elastase to azurophil granules,118 but this may be indirectly via serglycin. An N-terminal sorting domain has been identified in serglycin and was shown to be essential for routing of serglycin to mast cell granules.119 No common denominator has been identified that can fully explain why neutrophil proteins are sorted to granules. Perhaps the lack of efficient sorting to granules may not solely be taken as inefficiency, but may be a way to secure a desirable level of antibiotic protein such as lysozyme108 and hCAP-18120 in plasma which renders the myeloid cells of the marrow a major secretory organ.
The biosynthesis of neutrophil granule proteins is controlled at the transcriptional and not the translational level (see Fig. 66–3).98,99,100 Not all transcription factors that are responsible for biosynthesis of granule protein have been identified, and the role of an individual transcription factor may be difficult to identify from gene knockout studies as transcription factors may work at multiple stages during myelopoiesis. The transcription factor PU.1 is essential for myelopoiesis because knockout mice do not form myeloid progenitors beyond myeloblasts121,122; but this does not preclude PU.1 from regulating transcription of individual granule proteins at a later stage of development.123,124,125,126 Figure 66–3 shows the profile of important myeloid transcription factors during maturation of normal myeloid cells in the marrow in vivo. RUNX1 (AML-1), c-MYB, CASP, C/EBPα, C/EBPγ, GATA-1, and ELF-1 gene products are all strongly expressed in the myeloblast and promyelocyte, and some of these are required for azurophil granule protein expression. Then c-MYB, AML-1, GATA-1, and ELF-1 gene products are downregulated as the cells enter the myelocyte stage, heralded by a brisk and transient upregulation of C/EBPε to initiate expression of peroxidase-negative granule proteins99 in agreement with the lack of specific granules in C/EBPε –/– mice and with the observation of a C/EBPε mutation in patients with a rare specific granule deficiency.109,110,127,128 PU.1, C/EBPβ, and C/EBPδ also appear at the promyelocyte myelocyte transition, but in contrast to C/EBPε, continue to increase as the cells mature to neutrophils. ELF-1 reappears at the metamyelocyte stage followed by C/EBPξ, c-Jun, and c-fos that are expressed at the band cell stage and increase in content as the cells mature.99
MicroRNAs (miRNAs) are important regulators of protein synthesis. In general, they bind to the 3′-end of mRNA and inhibit translation. Just like genes for granule proteins, mRNAs are expressed during maturation of neutrophils in the marrow depending on the stage of neutrophil maturation and can be classified into six groups, each with its characteristic expression profile.129 So far, miRNAs have been shown to regulate proteins of importance for proliferation but not (yet) expression of individual granule proteins. Expression of the myeloid-specific miRNA-223 increases during maturation of neutrophils in the marrow, and after their release into the circulating. One of the targets of miRNA-223 is the Mef2c transcription factor. Mice that lack miRNA-223 expand granulopoiesis and the mature neutrophils mount an enhanced respiratory burst in response to phorbol myristate acetate (PMA), indicating that miRNA-223 acts as a negative regulator of granulopoiesis and neutrophil activation.130 miRNA-130a is highly expressed in myeloblasts and promyelocytes and targets SMAD4, which, despite a high level of mRNA in promyelocytes, is not expressed at the protein level, and the cells are consequently insensitive to growth inhibition by transforming growth factor β (TGF-β)–induced growth inhibition. miRNA-130a also inhibits C/EBPε which induces exit from cell cycle and growth arrest at the myelocyte stage. Hence, miRNA-130a seems important for the expansion of myeloblasts, promyelocytes and early myelocytes.131,132
FUNCTION OF INDIVIDUAL GRANULE PROTEINS AND THEIR ROLE IN OXIDATIVE AND NONOXIDATIVE MICROBIAL KILLING
Table 66–1 lists the physical-chemical and functional properties of neutrophil granules.
| Granule Protein | Localization | Physicochemical Properties | Function |
|---|---|---|---|
| Myeloperoxidase | Azurophil granule (AG) | Heme protein, 90-kDa proform with an internal disulphide bond between the 57- and the 13.5-kDa subunits, generated by proteolytic processing that takes place during routing to granules | The MPO–halide–H2O2 system generates hypochlorous acid (HOCl), other chlorination products, tyrosine radicals, and reactive nitrogen intermediates, each of which can attack the surface membrane of microorganisms |
| Bacterial permeability-increasing (BPI) protein | AG | 55-kDa protein with high homology to the LPS-binding protein of plasma | BPI is organized into two largely symmetrical subdomains one of which is responsible for the binding of lipopolysaccharide (LPS) and for the antimicrobial activity against Gram-negative microorganisms |
| Defensins: three α-defensins; human neutrophil peptides (HNPs) 1–3 | AG | 7-kDa proforms processed by proteolytic cleavage to mature 3-kDa defensins that share a characteristic three disulfide bond motif: 1–6, 2–4, 3–5 | Defensins are small, amphipathic, pore-forming, antibacterial cationic peptides with a broad spectrum of antibacterial activity |
| Serine proteases of azurophil granules: elastase, cathepsin G, proteinase 3, and neutrophil serine protease 4 (NSP4); azurocidin (CAP37 or heparin-binding protein [HBP]) is enzymatically inactive | AG | 28-kDa proforms, processed to active proteases en route to azurophil granules | Serine proteases, but both elastase and cathepsin G have direct antibacterial activities that are not dependent on their enzymatic activity; proteinase 3 liberates the antibacterial peptide LL37 from hCAP-18; HBP is chemotactic for monocytes; HBP may open endothelial cell tight junctions |
| Lysozyme | AG ~30%; specific granules (SG) ~50%; gelatinase granules (GG) ~20% | Cationic antimicrobial peptide of 14 kDa; in contrast to many neutrophil granule proteins, lysozyme is inefficiently targeted to granules and circulates free in plasma in a substantial quantity that reflects the normal myelopoietic activity | Lysozyme cleaves peptidoglycan-polymers of bacterial cell walls and displays bactericidal activity toward the nonpathogenic Gram-positive bacteria Bacillus subtilis; a particular high serum level is characteristic for the myelomonocytic leukemias |
| Lactoferrin | SG | 78-kDa iron chelator; member of the transferrin protein family with a high affinity for iron and similar iron-binding characteristics as ferritin | The antibacterial activity of lactoferrin does not depend exclusively on its ability to sequester iron. Proteolytic fragments, some of which are known as lactoferricin, are directly bactericidal |
| Neutrophil gelatinase-associated lipocalin (NGAL) or siderochelin | SG | 25-kDa N-glycosylated member of the lipocalin protein family | NGAL is the first known siderophore-binding eukaryotic protein; NGAL binds enterochelin/enterobactin with high affinity, and blocks growth of Escherichia coli by sequestering siderophore–iron complexes |
| hCAP-18 | SG | 18 kDa; only human member of the cathelicidin protein family | Stored and released intact; binds endotoxin; C-terminal antibactericidal peptide, LL-37 released by proteinase 3; active mainly against Gram-positive bacteria, is chemotactic for T cells, monocytes, and neutrophils, and has angiogenetic properties |
| Neutrophil collagenase | SG | 75-kDa matrix metalloproteinase 8 (MMP-8); like other MMPs, MMP-8 is stored inactive and must be N-terminally trimmed to remove the inhibitory peptide | Active against types I, II, and III collagen |
| Olfactomedin 4 (OLFM4) | SG | 65-kDa protein that forms multimers by disulfide bonding | Function is unknown, but OLFM4 is present only in a subset (25%) of neutrophils |
| Gelatinase | GG | 92-kDa MMP-9; stored inactive | Active against type IV collagen |
| Leukolysin, which is distributed among of resting neutrophils | SG ~10%; GG ~40%; secretory vesicles (SV) ~30%; plasma membranes (PM) ~20% | Leukolysin is a 56-kDa glycosylphosphatidylinositol (GPI)-anchored membrane-bound MMP (MT6-MMP/MMP-25) | Active against fibronectin, chondroitin sulfate proteoglycan, dermatan sulfate proteoglycan |
| Cytochrome b558, (gp91phox, p22phox) | SG ~60%; GG ~25%; SV ~15% | Heterodimeric flavoheme protein; 91-kDa glycoprotein subunit (heme-flavin binding); 22-kDa protein subunit, possibly heme binding | Together with p47phox, p67phox, and p40phox, cytochrome b558 constitutes the superoxide generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of phagocytes |
| CD11b/CD18 (Mac-1, Mo1, CR3, αMβ2) | SG ~60%; GG ~25%; SV ~15% | Most prominent β2-integrin in neutrophils; CD11B = αM and is a glycoprotein of 170 kDa; CD18 = β2 and is a glycoprotein of 95 kDa | Multifunctional integrin that functions as an adhesion receptor binding to members of the immunoglobulin family intercellular adhesion molecule (ICAM)-1, to fibronectin, collagen; is important in mediating firm adhesion to vascular endothelial cells; functions as a phagocytosis receptor for C3bi-coated particles |
| Pentraxin-3 | SG | Pentamer of 47-kDa subunits | Binds complement C1q, selected microorganisms |
| Ficolin-1 | GG | Multimer of 32-kDa subunits | Binds acetylated carbohydrates on microorganisms; may activate mannose-binding lectin-associated serine proteases |
| Arginase 1 | GG | 37-kDa glycoprotein | Degrades arginine, the substrate for nitric oxide (NO) synthase |
The protein MPO is a marker of azurophil granules. It is formed as a 90-kDa precursor with an internal disulphide bridge that forms a link between the 57- and 13.5-kDa subunits that are generated by the proteolytic processing that takes place during routing to granules. The heme group, which is necessary for the reduction-oxidation (redox) functions of MPO, associates with the 90-kDa subunit.133 This seems to be a necessary prerequisite for subsequent processing.134 MPO reacts with H2O2, formed by the NADPH oxidase, and increases the toxic potential of this oxidant. Through oxidation of chloride, tyrosine, and nitrite, the hydrogen peroxide (H2O2)-MPO system induces formation of hypochlorous acid (HOCl), other chlorination products, tyrosine radicals, and reactive nitrogen intermediates, each of which can attack the surface membrane of microorganisms.135,136 MPO may be found on endothelial cells during inflammation and can inactivate nitric oxide (NO).137 In addition to the activities of MPO itself, MPO is known for the anti-MPO autoantibodies that are characteristic of the pANCAs (perinuclear antineutrophil cytoplasmic antibodies) that are found in vasculitides, in particular those that primarily affect kidneys.138,139
BPI is a 55-kDa protein with high homology to the LPS-binding protein of plasma. It is organized into two largely symmetrical subdomains, one of which is responsible for the binding of LPS and for the antimicrobial activity against Gram-negative microorganisms. In contrast to LPS-binding protein, which presents endotoxin to CD14 and elicits a proinflammatory response, BPI binds LPS independent of CD14 and neutralizes the effects of LPS.140 A transgene expressing high levels of BPI has enhanced resistance against endotoxin.141 Important effects not related to LPS have been described, such as chemotaxis, opsonization, and dendritic cell function, as reviewed in Ref. 142.
Defensins are small antibacterial cationic peptides with a broad spectrum of antibacterial activity.143 They share a characteristic three-disulfide-bond motif.82,144,145 Based on this, mammalian defensins are divided into α defensins, β defensins, and the cyclical θ defensins.146 Only α defensins are found in human neutrophils, and reside exclusively in azurophil granules. They are by far the dominating proteins of azurophil granules, yet are only expressed in a subset of granules that are formed late in the promyelocyte stage.83,100,107 Three defensins have been isolated from azurophil granules, HNP-1 to HNP-3.82 Large amounts of unprocessed defensing, that is, prodefensin, are secreted from late promyelocytes and myelocytes in the marrow and may serve as a marker of normal myelopoietic activity.111 In contrast to lysozyme and MPO, prodefensins are not expressed by acute leukemia cells. A rise of prodefensin in plasma precedes detectable neutrophils by 6 days and is a measure of normal granulopoiesis which may be of clinical use in the setting of myeloablative treatment for acute leukemia.111
The serine proteases of azurophil granules include elastase, cathepsin G, proteinase-3, and NSP4.147,148 Azurocidin, which is also named CAP37 or heparin-binding protein (HBP), is an enzymatically inactive serine protease.149,150,151,152,153 Both elastase and cathepsin G have direct antibacterial activities that are not dependent on their enzymatic activity. Proteinase 3 expression leads to autoantibodies against itself in Wegener granulomatosis, which is known as cANCA (cytoplasmic antineutrophil cytoplasmic antibody).154 Proteinase 3 is also bound to the surface of circulating neutrophils at levels that vary considerably amongst individuals but are highly constant throughout life in a given individual. The binding is mediated by the NB1 antigen (CD177).155,156 A secreted precursor of proteinase 3 has been suggested to inhibit normal myelopoiesis157 and to play a role in regulation of myelopoiesis. So far, the only specific substrate of proteinase 3 identified is the cathelicidin of specific granules hCAP-18. Proteinase 3 activates hCAP-18 by removing the cathelicidin part, and unleashing the antibacterial activity of the C-terminal LL-37 peptide.90 Cathepsin C, also known as dipeptidyl peptidase 1, removes two inhibitory N-terminal amino acids from the serine proteases prior to their storage in granules.158 Patients with the Papillon-Lefèvre syndrome lack cathepsin C activity and are not able to store serine proteases in their neutrophils.159 The condition is characterized by severe juvenile periodontitis and keratosis in hands and feet, but not by major systemic infections,160 arguing against the notion the serine proteases are important for immune defense.161
The membrane of azurophil granules contains CD63 (granulophysin), which is implicated in transmembrane signaling with the β2 integrins in the activated neutrophil.85,86,162 Also, the CD68 antigen100,163 and presenilin appear localized exclusively to the membrane of azurophil granules,164 whereas stromatin is found in the membrane of all granules165 and the vacuolar-type H+-ATPase is shared between azurophil, gelatinase granules and secretory vesicles.166 These membrane proteins will translocate to the phagocytic vacuole or to the plasma membrane when neutrophils are activated and engaged in phagocytosis.
For an overview, see Table 66–1.
Lactoferrin is the dominating protein of specific granules.167 It is a 78-kDa iron-chelator, member of the transferrin protein family with a high affinity for iron and similar binding characteristics as ferritin.168,169 The antibacterial activity of lactoferrin does not depend exclusively on its ability to sequester iron because proteolytic fragments of lactoferrin, some of which are known as lactoferricin, are directly bactericidal.170,171
NGAL, or lipocalin 2, is a 25-kDa N-glycosylated member of the lipocalin protein family.79 Lipocalins are transport proteins that bind small and often lipophilic substances in their canonical lipocalin pocket.172 Some NGAL is associated with gelatinase (MMP-9) in a subset of specific granules,173 but the majority is present either as a monomer or as a homodimer in specific granules. NGAL interferes with the activation and stability of MMPs,174 but the major function of NGAL is to bind and sequester siderophores. NGAL binds enterochelin/enterobactin with high affinity, and blocks growth of Escherichia coli by sequestering siderophore-iron complexes,175 which might not be only a neutrophil-specific antibacterial defense because NGAL can be induced in a variety of epithelial cells during inflammation by IL-1.176 NGAL has been demonstrated to play a protective role in infections against E. coli,177 Klebsiella pneumoniae,178 Salmonella typhimurium,179 and Mycobacterium tuberculosis.180 NGAL has effects not explained by sequestering bacterial siderophores and was shown to worsen the outcome of pneumococcal pneumonia by deactivating macrophages.181 It is possible that the ability of NGAL to bind endogenous siderophore-like structures and transport iron may explain some of these effects.182 Nramp1, the cation transporter, was initially identified first in macrophages as an essential resistance factor against mycobacterial infection. It is present in membranes of both specific and gelatinase-containing neutrophil granules.93,183
Lysozyme is a cationic antimicrobial peptide of 14 kDa.184 In agreement with its biosynthetic profile, lysozyme is present in all granule subsets, with peak concentrations in specific granules.100,108 Lysozyme cleaves peptidoglycan polymers of bacterial cell walls and displays bactericidal activity toward the nonpathogenic Gram-positive bacteria Bacillus subtilis.185 Lysozyme also binds LPS186 and reduces cytokine production and mortality caused by LPS in a murine model system of septic shock.187 In contrast to many neutrophil granule proteins, lysozyme is inefficiently targeted to granules and circulates free in plasma in a substantial quantity that reflects the granulopoietic activity.107,108 Lysozyme is also secreted from activated macrophages,188 and a particular elevated serum level is characteristic of the leukemias with a large proportion of monocytes.189
hCAP-18,89 also known as LL-37190 or CAMP, is the only human member of a family of antimicrobial peptides known as cathelicidins. Cathelicidins are typically found in peroxidase-negative granules of mammalian neutrophils.191 hCAP-18 is a prominent protein of neutrophil specific granules present in equimolar concentrations with lactoferrin.192 It is also present in plasma at a substantial concentration bound to lipoproteins.120 In general, cathelicidins are proantibiotic peptides that share a common and highly conserved 14-kDa N-terminal region known as the cathelin region, whereas the C-terminal regions vary extensively among the different cathelicidins. The C-terminal peptides must be liberated from the cathelin domain by proteolysis to become antibacterial. In most species this is carried out by elastase, but in human neutrophils this is done by proteinase 3 from azurophil granules. The liberated C-terminal peptide is known as LL-37.90,190 Like several other neutrophil proteins, hCAP-18 is formed by cells in other tissues, particularly epithelial cells.90,193,194,195 It is constitutively expressed in the testis and present in semen. Here, the activating protease is gastricin, a prostate protease that is active at low pH. This cleaves hCAP-18 to ALL-38, which has the same antibacterial spectrum as LL-37.196 The cathelin part, which is released has some protease inhibitory activity by itself.197 The LL-37 stimulates neutrophil, monocyte, and T-cell chemotaxis via the formyl peptide receptor-like-1.198 In addition, hCAP-18/LL-37 has angiogenic199 and endotoxin-neutralizing properties.200
Three MMPs have been identified in neutrophils: neutrophil collagenase (MMP-8,75 kDa), which is localized to specific granules,201 gelatinase (MMP-9,92 kDa), which resides predominantly in gelatinase granules,78,202 and leukolysin (MT6-MMP/MMP-25,56 kDa), which is distributed among specific granules (approximately 10 percent), gelatinase granules (approximately 40 percent), secretory vesicles (approximately 30 percent), and the plasma membrane (approximately 20 percent) of resting neutrophils.94,203 The MMPs are stored as inactive proforms that are proteolytically activated following exocytosis. Together, the MMPs are capable of degrading major structural components of the extracellular matrix, including collagens, fibronectin, proteoglycans, and laminin, and they are believed to be of central importance for the degradation of vascular basal membranes and interstitial structures during neutrophil extravasation and migration.
Two pattern-recognition molecules, pentraxin 3 and ficolin1, are found in specific granules and gelatinase granules, respectively. Pentraxin 3, a member of the long pentraxins family, is synthesized in myelocytes and metamyelocytes and stored in specific granules of neutrophils. Pentraxin-3 binds the complement component C1q and mediates activation of the classical complement cascade. In addition, pentraxin-3 binds K. pneumoniae outer membrane protein A (KpOmpA) from Gram-negative bacteria, especially the Enterobacteriaceae species, and binds Aspergillus fumigatus conidia. Pentraxin-3 was shown to play a major role in uptake and killing of A. fumigatus conidia by neutrophils in a mouse model.204,205
Ficolin-1 is present in gelatinase granules. Ficolin-1 binds acetylated carbohydrate structures on Gram-positive bacteria and can recruit mannose-binding lectin-associated serine proteases (MASPs) and activate the lectin complement cascade.206
Arginase-1 is a constituent of gelatinase granules207 and may participate in regulation of T-cell activities by removing arginine, the essential substrate for inducible nitrous oxide synthase. The product, proline, is essential for collagen synthesis and arginase-1 from neutrophils may thus support wound healing.
Olfactomedin 4, a 65-kDa specific granule protein, forms huge multimers, but only in approximately 25 percent of neutrophils, ranging from 5 to 40 percent between individuals and constant in each individual. The functional consequence is unknown.208,209
Membrane proteins of peroxidase-negative granules are shared among the subsets of peroxidase-negative granules that can be distinguished based on their matrix proteins; that is, specific and azurophil granules. Two exceptions are Nramp1 and MMP-25, which are both present, predominantly in the membrane of gelatinase granules and secretory vesicles.93,94 Cytochrome b558, which is comprised of gp91phox and p22phox, forms the membrane component of the NADPH oxidase and is a prominent membrane protein of peroxidase-negative granules.81,210 It codistributes with the major β2-integrin of neutrophils CD11b/CD18, with the major segment in specific granules, some in gelatinase granules, and some in secretory vesicles. Secretory vesicles are rapidly mobilized, and even though only 15 percent of the total cytochrome b588 and CD11b localizes to secretory vesicles, this is the fraction that is primarily translocated to the plasma membrane during neutrophil diapesis.66,32 Hv1is a voltage-gated proton channel situated in the plasma membrane and membranes of peroxidase-negative granules.211 Hv1 associates with nascent phagosomes, and neutralizes the negative charge induced by transport of electrons by the NADPH oxidase.212,213 The CD66 antigens found in the membrane of specific granules may play a role as bacterial receptors (galectin receptors) and generate signals to activate the NADPH oxidase.214,215
Stimulus-response coupling by neutrophils has been the subject of intense research for many years. This work has been fruitful in illuminating some of the underlying causes of defects in cell activation. Studies of neutrophil degranulation and oxidative metabolism have also revealed transduction mechanisms common to a wide variety of other important secretory cell types, thereby greatly expanding the relevance of this work. This chapter considers next our current understanding of the activation process, which is shown schematically in Fig. 66–4.
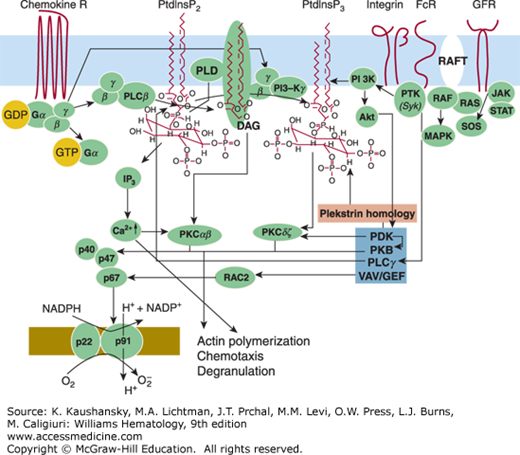
Figure 66–4.
Signal transduction in neutrophils. G-protein–coupled receptors are seven transmembrane receptors that couple to heterotrimeric guanosine triphosphate (GTP)-binding (G) proteins. Agonist binding to the receptor triggers exchange of guanine diphosphate (GDP) for guanine triphosphate (GTP) on the Gα subunit of the G-protein, and consequently, the disassociation of the α subunit for the βg-dimer. Both subunits can regulate the activity of multiple effectors such as phospholipase Cβ (PLCβ). PLCβ cleaves an endogenous lipid, namely phosphatidylinositol bisphosphate (PtdInsP2), yielding diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 is known to liberate calcium from bound intracellular stores leading to a rise in intracellular-free calcium (Ca2+)i. The increase in intracellular Ca2+ is augmented by an influx of Ca2+ from the extracellular space. Increased DAG, in concert with elevated Ca2+ can activate protein kinase isozymes α and β (PKCαβ) leading to their translocation to membranous sites. Phospholipase D (PLD) can be activated by PKC converting phosphatidylcholine to phosphatidic (PA) acid. Elevations in PA can mobilize the cytosolic proteins, p47, p67phox, and p40phox to bind to the membrane-bound proteins gp91phox and p22phox, which then reduces O2 to O2– in the presence of NADPH.
Neutrophil responses can be evoked by a variety of particulate and soluble stimuli. Opsonized particles, immune complexes, and chemokine and chemotactic factors produced during the inflammatory process activate neutrophils by binding to specific cell-surface receptors. Of the neutrophil chemotactic receptors, the N-formyl peptide receptor is the best characterized. N-formyl peptide, the synthetic analogues of bacterial N-formyl peptide products, induces a variety of neutrophil responses and has been extensively employed as activating stimuli. Specific receptors for the chemotactic peptide, fMLP, have been identified on the neutrophil surface,216 and binding of the formyl peptide to its receptor correlates with its ability to induce chemotaxis and degranulation.217 The formyl peptide receptor,218 like the receptors for C5a, IL-8, LTB4, and PAF, belongs to a family of seven trans-membrane–spanning domain proteins (7TMRs) that are coupled to heterotrimeric G-proteins (containing G α and β,γ subunits).219,213 Upon ligand binding, guanine diphosphate (GDP) bound to the Gα subunit is exchanged with guanine triphosphate (GTP) and the β,γ subunits dissociate from the receptor and mediate downstream signaling. Phosphorylation of the receptors then augments their affinity for β arrestins. The association with β arrestins block association with β,γ subunits and mediate internalization of the receptors, but may also induce additional signals via β arrestins.220,221 The formyl peptide receptor has been studied in most detail in neutrophils. The receptor is highly glycosylated and has a relative molecular mass (Mr) of 50 to 70 kDa. It has been identified on the membranes of gelatinase granules and secretory vesicles, and shown to be mobilized to the cell surface following stimulation.222
Activation of the complement system generates C5a, a derivative of C5 and the most potent of the chemotactic proteins. C5a induces neutrophil chemotaxis, degranulation, and superoxide generation.222,223 Responses to C5a result from interactions with specific receptors on the cell surface.222,224 The receptor was identified as a single polypeptide in the plasma membrane with an apparent mass of 40 to 48 kDa.222,225 Binding studies show that there are 50,000 to 113,000 receptor sites per cell with a dissociation constant (Kd) of 2 × 10–9 M. The C5a receptor has been isolated and cloned, and is a member of the seven transmembrane-spanning class of G-protein–coupled receptors.226
Three other important G-protein–coupled receptors are for PAF, IL-8, and LTB4. PAF and IL-8 receptors have been cloned.227,228 Their intracellular stores and signal transduction mechanisms are largely similar to those used by other G-protein–coupled receptors (e.g., fMLP).227 IL-8 has two related receptors, for which slightly different signal transduction pathways have been detected.229
Neutrophils also express receptors for the complement-derived chemotactic factors C3b and C3bi. Receptors for C3b and C3bi (also known as CR1 and CR3, respectively) are sparse on resting neutrophils, but increase significantly in numbers following activation with several stimuli because of incorporation from secretory vesicles (CR1 and CR3) and gelatinase and specific granules (CR3, which is the integrin Mac-1).32,71 The C3b receptor (CR1) is a glycoprotein with a molecular weight of 205 kDa and is located in secretory vesicules.65,71
CD11/CD18 integrins also play an important role in cell signaling. The adhesion of cells to surfaces or to other cells can either activate neutrophils directly or “prime” them for an enhanced response to other stimuli. For example, the oxidative burst of neutrophils is very different in cells that are suspended versus those that are adherent to surfaces.230 H2O2 production in response to chemotaxins is influenced by monoclonal antibodies to CD11b, but not CD11a.231
Neutrophils possess three different receptors for immunoglobulins. Unstimulated cells express FcγRIIA and FcγRIII, also known as CD32 and CD16, respectively. Functionally, the most important of the two is the FcγRIII for clearing immune complexes,232 and it is attached to the membrane by a GPI linkage.232 The linkage is relatively labile, so the amount of FcγRIII on the membrane reflects a balance between shedding and mobilization from intracellular stores. FcγRIIA is a protein that spans the plasma membrane.233 The signal transduction pathways initiated by FcγRIII can crosstalk with the formyl peptide receptor, with CR3, and even with each other. A direct physical linkage between CD11b and FcγRIIIB has been demonstrated by experiments in which capping of one receptor results in co-capping a substantial fraction of the other receptor. CD11b can also interact with the transmembrane FcγRII, and both of these molecules can modify each other’s signals.234
The tyrosine kinase Syk, plays a critical role in the phagocytic pathway mediated by FcγRIIA.235 A cytoplasmic amino acid motif, known as ITAM, is present on FcγRIIA and FcγRI/γ (a receptor found on IFN-γ–stimulated myeloid cells) and is essential for the phagocytic response during crosslinking of these two Fc receptors. Binding of Src family protein tyrosine kinases to the ITAM leads to activation of Src family protein tyrosine kinases and ITAM tyrosine phosphorylation. This serves to recruit phosphatidylinositol 3′-kinase (PI3K) and Syk, which when activated phosphorylates multiple substrates, including neighboring ITAMs. Syk is recruited from the cytosolic pool. The essential role for Syk-affecting signal transduction is reflected by ITAM-dependent activation of actin assembly. Other tyrosine kinases, including Src kinases, especially Lyn, facilitate the formation of the phagosome.236 Once active microfilaments are formed, they enhance the activity of phospholipase D (PLD) to generate phosphatidic acid (PA), a necessary phospholipid for phagocytosis to ensue.237,238
The next step in signal transduction can be attributed to interactions of receptor-activated G proteins or through FcγRIIA and tyrosine kinases with phospholipases.239,240 For instance, a membrane-associated phosphoinositide-specific phospholipase is activated upon stimulation with chemotactic stimuli. In particular, phospholipase C (PLC) hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) and phosphatidyl inositol-4-monophosphate (PIP1) to the putative second-messenger products inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG) (see Fig. 66–4).241 In neutrophils, IP3 interacts with a specific intracellular receptor and stimulates the release of Ca2+, as well as opens Ca2+ channels on the plasma membrane, resulting in rises in intracellular Ca2+.242 Activation of the small GTP-binding proteins of the Rac, Rho, and Cdc42 families regulates actin-dependent processes such as membrane ruffling, formation of pseudopodia, and stress fibers leading to cell adhesion and motility, and appears critical in neutrophil function,243,244 while working in concert with the phospholipases.
Even in the absence of PLC metabolism, there is a significant increase in DAG and Ca2+ intracellularly that accompanies phagocytosis.245 Ca2+ is necessary for granule phagosome fusion and DAG has been linked to both particle ingestion and degranulation.246 Both can be formed by the activation of PLD, which hydrolyzes phosphatidylcholine to produce PA and choline. Activation of PLD is mediated by Rho and/or ADP-ribosylation factor (ARF).247 Diacylglycerol is then generated by PA phosphohydrolase, which catalyzes the dephosphorylation of PA. The hallmark of the phosphatidylcholine-derived DAG is the presence of 1-O-alkyl linkages. During PA formation by the action of PLD on phosphatidylcholine, PA can act as a Ca2+ ionophore, thereby initiating fusogenic activity.248 Thus, the phosphatidylcholine acid generated during phagocytosis may promote fusion of neutrophil granules with newly formed phagosomes.
Another downstream target of DAG in phagocytosis is the activation of protein kinase C (PKC), particularly PKCδ, a Ca2+-independent isozyme of PKC found in neutrophils.237 PKCδ is one of four PKC isozymes that translocate to the plasma membrane during phagocytosis. During phagocytosis, PKCδ is translocated from the cytosol to the plasma membrane. Accompanying the translocation of PKCδ to the membrane, RAF-1 translocation is promoted. Following translocation of these two key components, mitogen-activated extracellular signal-regulated kinase (MEK) activation occurs, which is followed by activation of mitogen-activated protein (MAP) kinase/extracellular signal-related kinase (ERK)-2 and then myosin light-chain kinase.240 Following phosphorylation of myosin, reorganization of the actin cytoskeletal occurs leading to phagocytosis. Concomitant with the activation of PLD, ceramide is generated by a neutral sphingomyelinase activity found in the plasma membrane of neutrophils and it is most likely important in attenuating the activity of the cells through inhibition of PLD.240 Following engagement of the Fc receptors and Syk activation in the neutrophil, PI3K is also activated. Inhibition of PI3K activity impedes phagocytosis.237
Arachidonate Metabolism In addition to their participation as putative second-messenger products in the stimulus–response coupling pathway, many lipid metabolites may be released from stimulated neutrophils, and, in turn, modulate cell function by interacting with receptors on other neutrophils. Phospholipase A2, present on both the granules and plasma membranes of neutrophils,249 as well as the cytosol,250 is activated during neutrophil stimulation, yielding arachidonic acid as one of the major end products. Arachidonic acid is not only released from stimulated neutrophils, but also serves as a regulator of phospholipase A2 (PLA2) activity and as a stimulus for these cells.251 Sensitivity of the cells to other stimuli can be enhanced with arachidonic acid and other long-chain fatty acids.252
Arachidonic acid can also be metabolized by the lipoxygenase pathway to produce hydroxyeicosatetraenoic acids (HETEs), including 5-HETE, 12-HETE, and 5,12-diHETE.253 These compounds have also been shown to induce several neutrophil responses.254 Stimulated neutrophils also produce the diHETE LTB4 through the lipoxygenase pathway. LTB4 and other leukotrienes can be released in response to a variety of stimuli.255 Receptors for LTB4 have been partially purified, and their activation serves as a potent stimulus for chemotaxis and adherence.256
Another potent mediator of inflammation produced by stimulated neutrophils is 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphoryl choline, also known as PAF.21 Not only is PAF synthesized by neutrophils and activated endothelial cells, but it induces degranulation, aggregation, and superoxide generation.29 Inflamed endothelium generates PAF, which serves to immobilize neutrophils on the luminal surface of the endothelial cells, thereby facilitating the interaction of the neutrophil integrin receptors with the ICAM ligands on the endothelial cells.
In stimulated cells the signal transduction cascade activates G proteins, followed by enhanced intracellular Ca2+, lipid remodeling, and protein kinase activation. These events culminate in secretion. This ultimate event—the fusion of granule membranes with phagosomes or the plasma membrane—occurs rapidly and is highly efficient.
Fusion Proteins Over the past 20 years the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) hypothesis has become the reigning paradigm for fusion of biomembranes.104 The hypothesis is centered around the protein that is sensitive to N-ethylmaleimide (designated NEM-sensitive fusion protein or NSF) and several SNAREs on the participating membranes. The SNAREs are divided into the v-SNAREs, being found on vesicles or granules, and t-SNAREs, being found on the target plasma membranes. The SNARE hypothesis has proven to have great predictive value as the constellation of fusion proteins and their interactions appears in almost all species and tissues. Initial docking of granules with the membrane to which they fuse is likely mediated by Rab-GTPases. Once docking is obtained, SNAREs are recruited to both membranes and interact and mediate actual fusion assisted by SNARE-interacting proteins such as sSec1/Munc18 proteins and a local rise in Ca2+. Disassembly of the fusion complex is mediated by NSF in an ATP-dependent process.257 The t-SNARE VAMP-2 is localized to the membranes of specific and gelatinase granule and secretory vesicles in resting human neutrophils,105,106 and the t-SNARE syntaxin 4 is associated with the plasma membrane as shown by immunoelectron microscopy. Munc18–3 may interact with syntaxin 4 and regulate fusion of secondary and gelatinase granules. VAMP-7 is associated with azurophil granule fusion and Munc18–2 may interact with syntaxin 3 and regulate azurophil granule fusion.258,259
What previously was considered pus was identified as a highly bactericidal structure composed of strands of chromatin and bactericidal neutrophil granule proteins attached.260,261 These NETs (see Fig. 66–2) are extruded from neutrophils in a process called netosis and represent one of three death programs of neutrophils: apoptosis, necrosis, and netosis. Neutrophils only undergo netosis if they have mounted a respiratory burst.262 Elastase and MPO are also required for netosis.263 The NADPH oxidase activity of stimulated neutrophils thus serves two purposes, namely to generate reactive oxygen species for microbial killing and to induce formation of the bactericidal NETs after the intact neutrophil has ceased to function. This, in turn, means that patients with defective NADPH oxidase assembly (patients with chronic granulomatous disease [CGD]) lack both the ability to generate microbicidal oxygen species and the ability to form the NETs. Patients with the Papillon-Lefèvre syndrome (PLS) lack elastase and are incapable of generating NETs. PLS patients do not have a major immune defect and their symptoms are largely related to periodontal infections, in contrast to CGD patients. Negative effects of NETs have been noted as NETs may induce thrombosis.264 Neutrophils are able to generate NETs and maintain their structural integrity as an anucleate cell still capable of migration and phagocytosis.265
CLINICAL DISORDERS OF NEUTROPHIL FUNCTION
Neutrophil dysfunction may arise from (1) the absence of antibodies or complement components required to opsonize microorganisms, an interaction that provides a chemotactic signal; (2) the abnormalities of cytoplasmic and granule movement that alter the chemotactic response or that result in abnormalities of the plasma membrane affecting the cells in terms of capability to modulate movement; and (3) defective microbicidal capability. Comprehensive reviews of these syndromes are available to the interested reader.266,267,268
ABNORMALITIES OF THE SIGNAL MECHANISM AS A RESULT OF ANTIBODY AND COMPLEMENT DEFECTS OR IMPAIRMENT OF PATTERN RECEPTOR RECOGNITION
Because the synergistic action of immunoglobulins and complement proteins creates the opsonins that coat microorganisms and stimulate the development of chemotactic factors, a deficiency of either one may result in impaired neutrophil function. The most profound disturbances arise from abnormalities in C3, because this protein is the focal point for generation of opsonins and chemotactic factors (Chap. 19).269,270,271 Opsonins such as C3b, generated from cleavage of C3, serve to coat bacteria. Opsonization in general refers to the coating of pathogens by serum proteins such that they are more likely to be ingested. Activation of C3 can occur in the absence of an antibody or the classical complement components C1, C2, and C4; thus, disorders of these latter molecules result in less-severe clinical conditions. C3 deficiency is inherited as an autosomal recessive disorder. Homozygotes have undetectable serum levels of C3 and suffer from recurrent severe pyrogenic infections, whereas asymptomatic heterozygotes have half the normal values.
A functional deficiency of C3 protease resulting in severe pyrogenic infections also is seen in patients with a deficiency in C3b inactivator, a protein inhibitor of the alternative complement pathway. Unchecked activation of this pathway leads to hypercatabolism of C3 and factor B.272 Properidin deficiency also results in a functional deficiency in C3.273 Properidin is a serum protein that belongs to the alternative complement pathway; it is involved in the stabilization of the enzyme complex C3bBb. The protein is a multimeric glycoprotein with a subunit Mr of 56,000, the gene of which has been cloned.274 Absence of properidin is associated with severe, often fatal, pyrogenic infections, often with meningococci.
Approximately 5 percent of the population have low serum levels of mannose-binding lectin (MBL),275 a serum lectin secreted by the liver that binds mannose sugars present and on the surface of bacteria, fungi, and some viruses. MBL is one of the soluble collectin effector proteins that contribute to the basic armamentarium of innate immunity. MBL can function as an opsonin when bound to the surfaces by activating the complement cascade. A deficiency of MBL has been reported in infants with frequent unexplained infection, chronic diarrhea, and otitis media.275 Other studies have identified an increased susceptibility to infection by specific pathogens in MBL-deficient individuals, including human immunodeficiency virus, Plasmodium falciparum, Cryptosporidium parvum, and Neisseria meningitidis.276 The deficiency in MBL largely results from three relatively common single-point mutations in exon 1 of the gene, which leads to the failure of MBL to activate complement.277 In addition, the protein also modulates disease severity, at least in part through complex, dose-dependent influences on cytokine production.
Phagocytes, including neutrophils, express a large number of cell surface proteins that play crucial functional roles in their biology. Microbial PRRs are an essential component of innate immunity, in which they recognize and detect PAMPs, resulting in activation of neutrophils and other phagocytes. The mammalian TLR family comprises an important class of PRRs, which recognize a wide range of microbial pathogens and pathogen-related products. At least 12 different TLRs that can be found on mononuclear phagocytes have been described.278 TLRs signal via MyD88, an adapter protein. MyD88 deficiency in humans can lead to recurrent infections with both Gram-positive and Gram-negative infections, thereby indicating a role for both mononuclear cells and neutrophils in host defense in the MyD88-deficient state.279
Because a large number of chemoattractants are generated during inflammation, it is difficult to establish the relative significance of a given individual component. Furthermore, chemotactive factors and opsonins are involved in the activity of both neutrophils and mononuclear phagocytes. Therefore, it is not clear whether the clinical consequences of disorders involving these substances are unique to one or the other of these phagocytic cells. Patients with antibody- or complement-deficient syndromes suffer mainly from infections with encapsulated pathogens such as Haemophilus influenzae, pneumococci, streptococci, and meningococci.280 Furthermore, splenectomized individuals deprived of an organ rich in mononuclear phagocytes have a small, but finite risk of sepsis because of the same microorganisms. Encapsulated pathogens characteristically are not associated with neutropenic states. Antibody coating of encapsulated organisms facilitates their ingestion by mononuclear phagocytes, but may be less important for their ingestion by neutrophils.
Definition and History This rare autosomal recessive disease was initially recognized as one in which neutrophils, monocytes, and lymphocytes contained giant cytoplasmic granules.281 Chédiak-Higashi syndrome (CHS) is now recognized as a disorder of generalized cellular dysfunction characterized by increased fusion of cytoplasmic granules.282 Pigmentary dilution affecting the hair, skin, and ocular fundi results from pathologic aggregation of melanosomes and is associated with failure of decussation of the optic and auditory nerves (Table 66–2).283 Patients with this syndrome exhibit an increased susceptibility to infection, which begins in infancy. Infections most commonly involve the skin and respiratory systems. The susceptibility to infection can be explained in part through defects in neutrophil chemotaxis, degranulation, and bactericidal activity.281 The presence of giant granules in the neutrophil interferes with their ability to traverse narrow passages between endothelial cells. Other features of the disease include neutropenia, thrombocytopathy,284 natural killer cell abnormalities,281,285 and peripheral neuropathies.286 Similar genetic syndromes have been described in mice, mink, cats, rats, cattle, and killer whales.286
| Disorder | Etiology | Impaired Function | Clinical Consequence |
|---|---|---|---|
| DEGRANULATION ABNORMALITIES | |||
| Chédiak-Higashi syndrome | Autosomal recessive; disordered coalescence of lysosomal granules; responsible gene is CHSI/LYST which encodes a protein hypothesized to regulate granule fusion | Decreased neutrophil chemotaxis; degranulation and bactericidal activity; platelet storage pool defect; impaired NK function, failure to disperse melanosomes | |