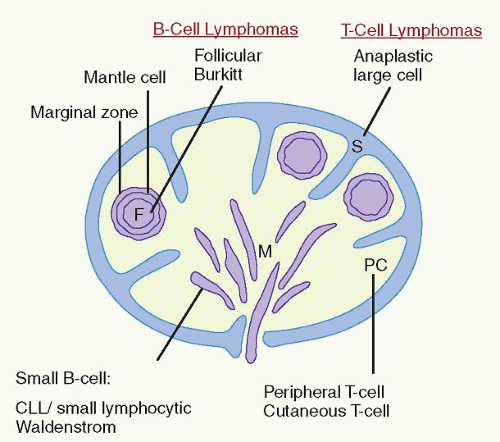
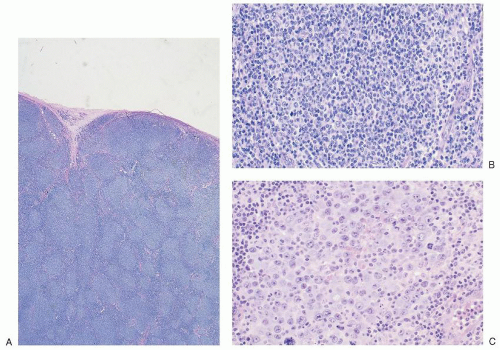
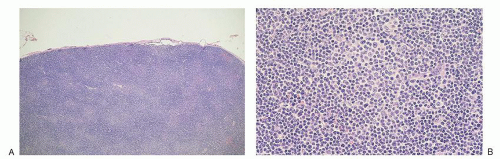
lymphomas (e.g., mantle cell lymphoma vs. small lymphocytic lymphoma [SLL]) (Table 86.2), can identify important nonlineagerelated markers (e.g., CD15, CD30, and CD56), and can determine the proliferative rate of lymphomas. Immunoglobulin (Ig) light chain restriction is evidence of B-cell clonality, whereas aberrant B-cell or T-cell phenotypes infer clonality.1,2 As small monotypic (light chain-restricted) B-cell or genotypically clonal B-cell or T-cell populations may be seen in reactive processes, correlation of these studies with the morphologic features is essential to prevent misdiagnosis and clinical confusion.3, 4 and 5
TABLE 86.1 IMMUNOPHENOTYPIC MARKERS USED IN DIAGNOSIS OF MALIGNANT LYMPHOMAS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 86.2 PATHOLOGIC FEATURES IN THE DIFFERENTIAL DIAGNOSIS OF SMALL B-CELL LYMPHOMAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
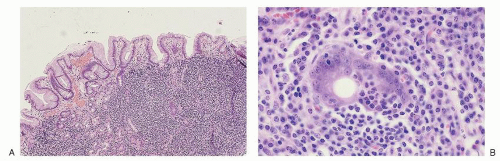
may occur at any age, the majority of these rare lymphomas have been described in children and young adults. These neoplasms present frequently in extranodal sites such as bone and soft tissue or as skin tumors of the scalp and face.31,32,33,34 They almost never present as mediastinal masses.
TABLE 86.3 WORLD HEALTH ORGANIZATION CLASSIFICATION OF MALIGNANT LYMPHOMAS | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
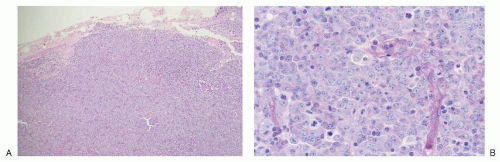
lymphomas of large centrocytes exhibit a predominantly diffuse growth pattern. Several studies suggest that these lymphomas have a course similar to that of low-grade follicular lymphomas composed predominantly of small centrocytes.64
and 17 in almost two-thirds of patients. Deletion of 13q is the most frequent abnormality and correlates with a stable clinical course in older patients. Trisomy 12, ATM deletions (11q), and TP53 deletions (17p) are associated with progressive disease.109,110 Molecular genetic studies divide CLL/SLL into two major groups based on the presence or absence of somatic mutation. The absence of somatic mutation is associated with a more aggressive clinical course and correlates with an increased expression of CD38 and the tyrosine kinase, ZAP-70.111,112,113,114,115 Risk stratification in CLL/SLL has therefore become an increasingly complex and controversial exercise with no clear consensus as to the relative roles of cytogenetics, molecular genetics for somatic mutation status, and use of surrogate markers such as CD38 and ZAP-70.116,117
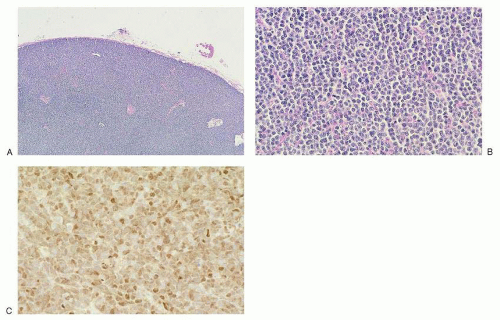
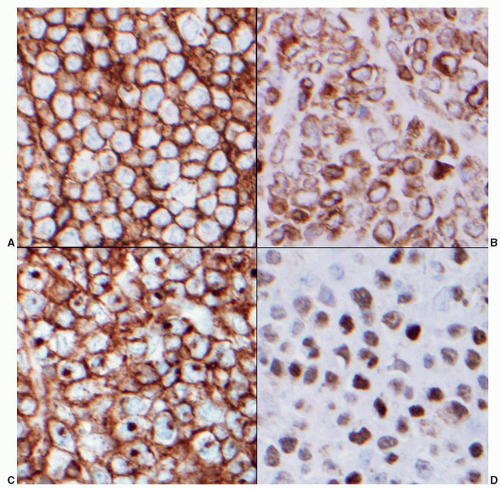
antibodies to pan-B-cell antigens CD19, CD20, and CD22. CD23 is negative or sometimes partially expressed, and FMC7 is positive in contrast to the tumor cells of CLL/SLL.107,142 Overexpression of cyclin D1 is almost universal in mantle cell lymphoma143 (Fig. 86.4C), is not seen in follicular lymphoid hyperplasia, and is uncommon in other small B-cell malignancies, with the exception of plasmacytic neoplasms and hairy cell leukemia.144 Recent gene expression studies have identified SOX11 expression as another specific marker for mantle cell lymphoma.145 Rare “in situ” mantle cell lymphomas have been recognized in which cyclin D1-positive cells fill unexpanded mantle zones without distortion of nodal architecture.146 The clinical significance of these cases is unclear at this time. High Ki-67 expression is associated with a poor prognosis.147,148
TABLE 86.4 RISK FACTORS FOR EXTRANODAL MARGINAL ZONE B-CELL LYMPHOMAS OF MUCOSA-ASSOCIATED LYMPHOID TISSUE (MALT LYMPHOMAS) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
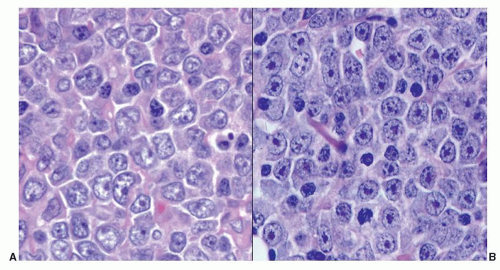
for practical purposes, several immunohistochemistry algorithms, Choi,204 Hans,205 Muris,206 Nyman,207 and Talley,208 have been proposed by which GCB and non-GCB are assigned. All have been shown to predict prognosis of R-CHOP-treated DLBCL patients in some, but not all studies. Therefore, phenotyping to assign a DLBCL to the GCB vs. non-GCB subtype for the purpose of selecting optimal therapy has been inconsistently applied to routine practice. Approximately 5% of DLBCL cases express CD5.200,209 These cases are distinguished from the blastoid variant of mantle cell lymphoma because they are cyclin D1–. They tend to occur in older patients, are more frequent in women than in men, and tend to be disseminated at the time of diagnosis.209 They are biologically distinct by genetic and gene expression profiling studies,210,211 but are not recognized as a separate entity in the WHO classification. One other marker of interest in DLBCL is Ki-67, a marker of cells in cycle. The fraction of Ki-67+ cells in a tumor is a general indicator of its proliferative rate. Ki-67 positivity ranges from 30% to nearly 100% in DLBCL cases, and it too, if expressed in greater than 90% of the cells, has been suggested as an adverse prognostic indicator in DLBCL.212
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree