- In the Wisconsin study [1], proliferative diabetic retinopathy (PDR) occurred in 67% of persons with type 1 diabetes mellitus (T1DM) for 35 or more years. One would therefore expect that two-thirds of people with T1DM would need laser treatment for PDR during their lifetime.
- In the same study, the 4-year incidence of panretinal photocoagulation was 2.5 times higher than the rate of macular laser.
- In patients with type 2 diabetes mellitus (T2DM), the rate of PDR is not as high but it is estimated that 1 in 3 patients with T2DM will develop sight-threatening diabetic retinopathy requiring laser during their lifetime.
- The prevalence of blindness is influenced by duration of diabetes, blood glucose and blood pressure control, and by the presence or absence of screening and preventive laser treatment.
- Achieving a high compliance as achieved in Iceland [2] can lower the risk of blindness to very low levels.
Introduction – a historical perspective
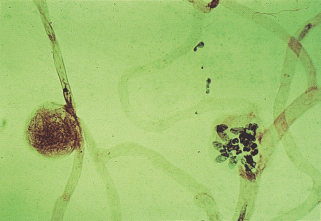
In 1877, Mackenzie and Nettleship [3], in one of the first pathologic reports on diabetic retinopathy (DR), observed capillary aneurysms. In 1900, the average life expectancy for men was 48.5 years and for women was 52.4 years and this rose over the next century to 76.0 years for men and 80.6 years for women. In 1921, Banting and Best discovered insulin in the laboratory of Dr. J. MacLeod. In 1923, Banting and MacLeod were awarded the Nobel Prize for Medicine. In 1943, Ballantyne and Loewenstein [4] examining flat unstained retinas, noted many capillary aneurysms and they first coined the phrase “diabetic retinopathy.” In 1953, Ashton [5] described changes in the arterioles in DR, studied in retinas removed postmortem. Examples of the Indian ink preparations published in his article [5] written in 1953 are shown in Figure 36.1.
Figure 36.1 Diabetic retinopathy showing an artery on the left and vein on the right. Hemorrhages and microaneurysms are present; in the center of the picture they are seen on an arteriole. The arteriolar side of the circulation is extensively atrophied. Injected Indian ink. Periodic acid–Schiff stain ×44. Reproduced from Ashton N. Arteriolar involvement in diabetic retinopathy. Br J Ophthalmol 1953; 37:282–292.
The normal eye
The eye is an approximate globe with the innermost surface of the eye, the retina, containing specialized photoreceptor cells (Figure 36.2).
Figure 36.2 (a) Cross-section diagram of the eye and major structures. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing. (b) Normal human fovea. This shows the inner and outer nuclear cell layers at the edge of the fovea and the concentration of cones centrally. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing. (c) Color photograph of the left central retina, including the macula and disc. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing.
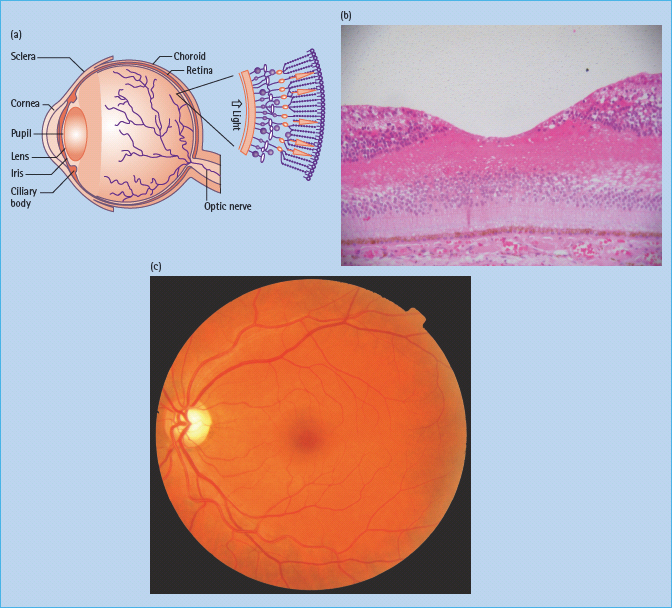
The central foveal region is thinner and is devoid of retinal blood supply; essential supply is provided through diffusion from the capillaries of the innermost vascular layer of underlying choroid through contact with the retinal pigment epithelium. The majority of the retina is provided with oxygen by means of the retinal vascular circulation (Figure 36.3).
Figure 36.3 (a) This figure shows a retinal structure cross-section diagram. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing. (b) A histologic section showing the ganglion cell layer, the bipolar layer, the nuclei of the cones and rods, the pigment epithelium, Bruchs membrane and choroids vessels. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing.
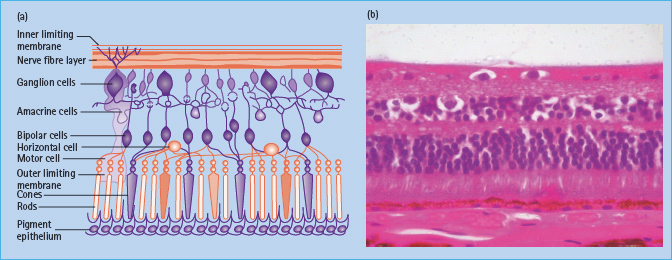
The blood vessels in the retina exhibit tight junctions between adjacent cells maintaining the blood–retinal barrier. The normal structure of retinal vessels is shown in Figure 36.4.
Figure 36.4 An inner layer of endothelial cells is enclosed by a tube of basement membrane, which in turn is surrounded by pericytes.
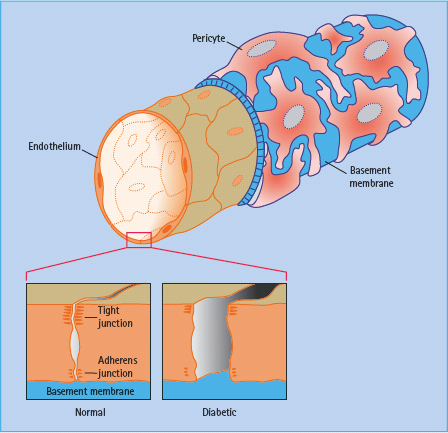
The cells of the choroidal circulation have small gaps (fenestrations) between the cells in the walls of smaller choroidal vessels allowing transport of essential nutrients and other small molecules and hence support the nutrition and oxygenation of the foveal region through the retinal pigment epithelium.
Risk factors for diabetic retinopathy
Major international epidemiologic trials have established that the development of DR is related to the following modifiable and non-modifiable risk factors.
Modifiable risk factors
Blood glucose
Evidence for the link between poor glucose control and greater progression of DR was provided by numerous early studies.
The study that confirmed that intensive blood glucose control reduces the risk of new onset DR and slows the progression of existing DR for patients with type 1 diabetes mellitus (T1DM) was the Diabetes Control and Complications Trial (DCCT) [6]. In the DCCT [7], early worsening of DR was reported at the 6-and/or 12-month visit in 13.1% of patients assigned to intensive treatment; however, the long-term benefits of intensive insulin treatment greatly outweighed the risks of early worsening.
Similarly, for type 2 diabetes (T2DM) the UK Prospective Diabetes Study (UKPDS) [8] demonstrated that intensive blood glucose control reduces the risk of new onset DR and slows the progression of existing DR for patients with T2DM.
Blood pressure
Control of systemic hypertension has been shown to reduce the risk of new onset DR and slow the progression of existing DR [9,10].
Lipid levels
There is evidence that elevated serum lipids are associated with macular exudates and moderate visual loss; partial regression of hard exudates may be possible by reducing elevated lipid levels [11,12].
Smoking
There is some evidence that smoking may be a risk factor in the progression of DR in T1DM as described by Muhlhauser et al. [13] and Karamanos et al. [14]; however, in T2DM the evidence is controversial.
Non-modifiable risk factors
Duration
The major non-modifiable determinant of progression of DR is duration of diabetes [15,16].
Age
There is a rather complex link with age, with the Wisconsin Epidemiological Study [1,17,18] demonstrating that in those whose age of diagnosis was less than 30 years and who had diabetes of 10 years’ duration or less, the severity of retinopathy was related to older age at examination, whereas when the age at diagnosis was 30 years or more, the severity of retinopathy was related to younger age at diagnosis. In the UKPDS [19], in those who already had retinopathy, progression was associated with older age.
Genetic predisposition
Early studies of identical twins with diabetes suggest familial clustering of DR. An association between severity of DR and human leukocyte antigens has been suggested in a number of studies [20,21], although this has not been uniformly accepted [22]. The majority of candidate genes studied exhibit weak or no association with retinopathy status, and where associations have been detected these results have not been replicated in multiple populations.
Ethnicity
Emanuele et al. [23] reported a higher prevalence of DR scores >40 in Latin Americans (36%) and African-Americans (29%) than for whites of Northern European ancestry (22%). Simmons et al. [24] compared ethnic differences in the prevalence of DR in European, Maori and Pacific peoples with diabetes in Auckland, New Zealand. They demonstrated that moderate or more severe DR is more common in Polynesians than Europeans. In neither of these two studies could the differences be accounted for by an imbalance in traditional risk factors such as age, duration of diagnosed diabetes, HbA1c and blood pressure.
Pathophysiologic events in diabetic retinopathy
Basement membrane thickening
An early histopathologic sign of DR is thickening of the basement membrane (Figure 36.5) [25].
Figure 36.5 (a) Basement membrane thickening (arrow) in a retinal arteriole. Periodic acid–Schiff stain, original magnification ×250. (b) Pericyte loss, in a flat-mounted tryptic digest of retina. Pericytes (small, dark nuclei) normally occur in a 1 : 1 ratio with the endothelial cells (pale, elongated nuclei). Original magnification ×400.
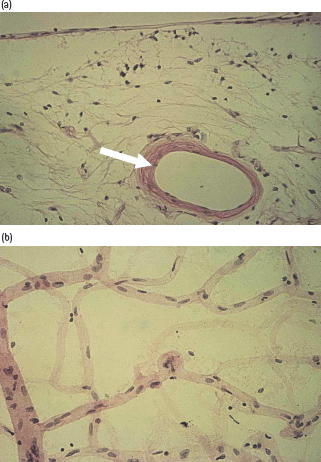
Pericyte loss
Fallout of pericytes, which are sensitive to high glucose concentrations and undergo apoptosis [26], is an early and crucial event in DR (Figure 36.5b). Loss of retinal capillary endothelial cells follows soon after pericyte loss with the formation of acellular capillaries.
Increased capillary permeability
Endothelial cells form a continuous sheet with each cell being fused to neighbors by tight junctions that maintain the inner blood–retinal barrier, which may break down in DR leading to leakage of plasma proteins. Focal leakage is also observed around microaneurysms but this is likely to be caused by local endothelial cell damage caused by inflammatory mediators released from adherent leukocytes [27].
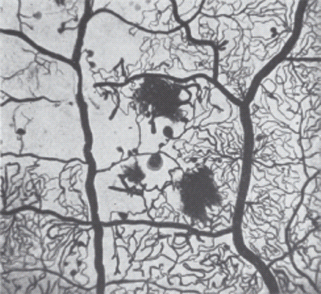
Microaneurysms
The microaneurysm is the hallmark of retinal microvascular disease in patients with diabetes (Figure 36.6). It has been suggested that microaneurysms may be asymmetric dilatations of the capillary wall where it is weakened or damaged, following the loss of the supporting pericytes (Figure 36.5b) and localized increases in hydrostatic pressure [28]. Early stage microaneurysms may be extensively infiltrated with large numbers of monocyte and polymorphonuclear cells (Figure 36.6) [28].
Figure 36.6 Tryptic digest of diabetic retina, showing two large microaneurysms in an area of acellular capillaries. One aneurysm (left) appears to be filled with clotted blood, while the second is hypercellular. Hematoxylin and eosin stain, original magnification ×400.
Smooth muscle death
Progressive death of vascular smooth muscle cells in the retinal arteries and arterioles has been established in the diabetic human retina [29].
Capillary weakening
Increasing closure of capillaries may be linked with the occurrence of intraretinal microvascular abnormalities (IRMA). These structures contain large numbers of endothelial-like cells and occur in association with acellular capillaries close to the arterial side of the circulation [29].
Retinal blood flow
Most clinical hemodynamic studies in diabetes conclude that increased blood flow and impaired autoregulation are features of DR [30]. Persistent dilatation of retinal arterioles is a well-known phenomenon in diabetes [31]. As DR progresses, increasingly large and widespread areas of retinal ischemia develop, caused by capillary occlusion and intravascular coagulation, which is considered to be enhanced by increased platelet stickiness in some studies [32], but not others [33].
The consultation
A carefully taken history and high quality clinical examination is a vital component of the care of any patient with DR.
Practical assessment
- History, which includes past ocular, diabetes, medical, family, drug and psychosocial history.
- Eye examination, which includes assessment of visual acuity and, where appropriate, color vision, inspection of external structures, visual fields to confrontation, ocular movement, pupillary reactions to light and accommodation, red reflex with an ophthalmoscope and slit-lamp biomicroscopy of the anterior eye.
- Both pupils are dilated with 1% tropicamide and in many patients 2.5% phenylephrine as well.
- Direct ophthalmoscopy has a limited two-dimensional field of view and has been shown to have a limited sensitivity and specificity for the detection of sight-threatening DR but is useful for ad hoc detection of DR.
- Slit-lamp biomicroscopy of the retina is the most common method employed by ophthalmologists to diagnose and monitor retinal disease using condensing lenses or fundus contact lenses (contact lens biomicroscopy).
- Binocular indirect ophthalmoscopy is useful for evaluating the posterior segment and retinal periphery. A larger area can be viewed than with slit-lamp biomicroscopy but this view is less magnified.
Multidisciplinary management
It is very important for an ophthalmologist to be aware of the control of risk factors in the individual patient and to have good communication with the diabetic physician or general practitioner who is looking after this aspect of the patient’s management.
Investigative techniques to assess diabetic retinopathy
Retinal photography
Digital color retinal photography is increasingly being used as a record of retinal lesions.
Fundus fluorescein angiography
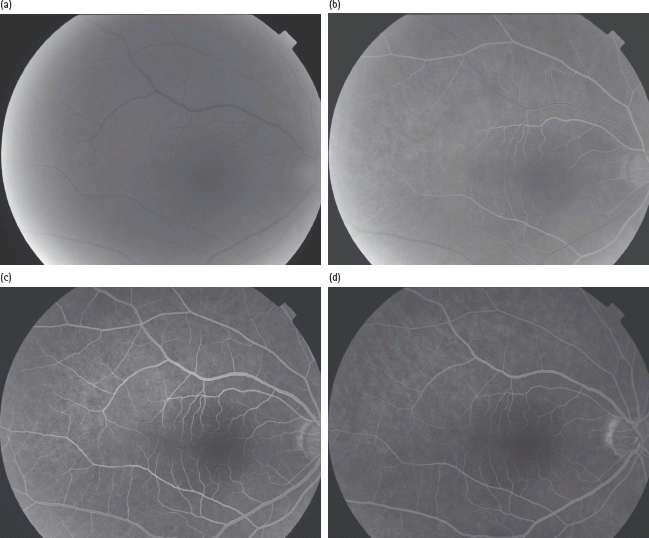
This is a diagnostic procedure, when sodium fluorescein injection is given rapidly into the arm and the fluorescence of the dye in blue light enables photographs to be taken as it appears in the retina (Figure 36.7). The fluorescein dye causes a few side effects such as nausea, vomiting, occasional syncope, skin rashes and itching. More serious side effects of bronchospasm, anaphylactic shock and cardiac arrest are extremely rare.
Figure 36.7 Fundus fluorescein angiography. (a) Choroidal flush. (b) Retinal arterial stage with very early signs of fluorescein in veins. (c) Arteriovenous phase. (d) Venous phase.
Optical coherence tomography
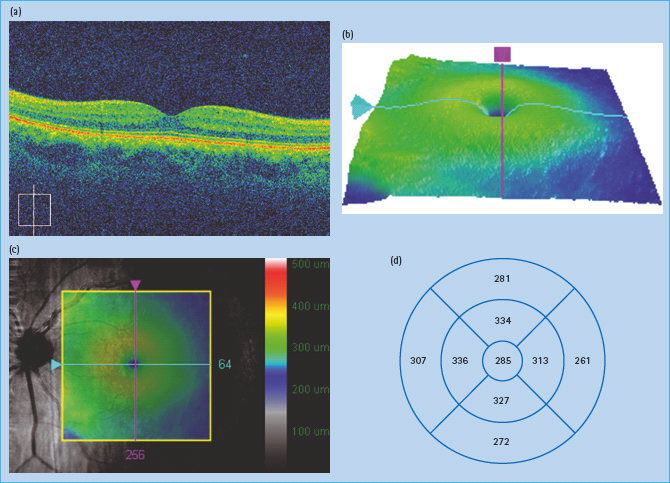
Optical coherence tomography (OCT) is an imaging technique that interprets reflected optical waves using interferometry. OCT images can be presented as either cross-sectional images or as topographic maps (Figure 36.8).
Figure 36.8 (a) Normal cross-sectional optical coherence tomography (OCT) image. (b) Topographic map of macular area. (c) Topographic map superimposed on red free image of macular area. (d) Measurements of thickness in macular area.
Ultrasound B scan examination
Ultrasound B scan examination uses high frequency ultrasound to examine the density and extent of a vitreous hemorrhage and the presence or absence of a retinal detachment where the retinal view is obscured.
Perimetry
Perimetry is the systematic measurement of differential light sensitivity in the visual field by the detection of the presence of test targets on a defined background in order to map and quantify the visual field, normally testing each eye independently but binocularly for driving field assessment.
Screening for diabetic retinopathy
The definition of screening that was adapted by the World Health Organization (WHO) [34] in 1968 was “the presumptive identification of unrecognized disease or defect by the application of tests, examinations or other procedures which can be applied rapidly. Screening tests sort out apparently well persons who probably have a disease from those who probably do not. A screening test is not intended to be diagnostic. Persons with positive or suspicious findings must be referred to their physicians for diagnosis and necessary treatment.”
Applying this definition to DR demonstrates that it is a very suitable condition for screening:
- Sight-threatening DR is an important public health problem [35,36].
- The incidence of sight-threatening DR is going to become an even greater public health problem; the International Diabetes Federation (IDF) has predicted a worldwide increase in diabetes and DR.
- Sight-threatening DR has a recognizable latent stage [19,37].
- Laser treatment for sight-threatening DR is effective [38,39] and agreed universally. Favorable long-term visual results have been reported [40–42] and there is also evidence that intensive control of blood glucose control [6,19] and systemic hypertension [10] reduces the risk of new onset DR and slows the progression of existing DR.
- A suitable and reliable screening test is available (digital photography) for sight-threatening DR [43–46], and is acceptable to both health care professionals and to the public.
- The costs of screening and effective treatment of sight-threatening DR balance economically in relation to total expenditure on health care [47–50], including the consequences of leaving the disease untreated.
Lesions and classifications of diabetic retinopathy
Microaneurysms and retinal hemorrhages
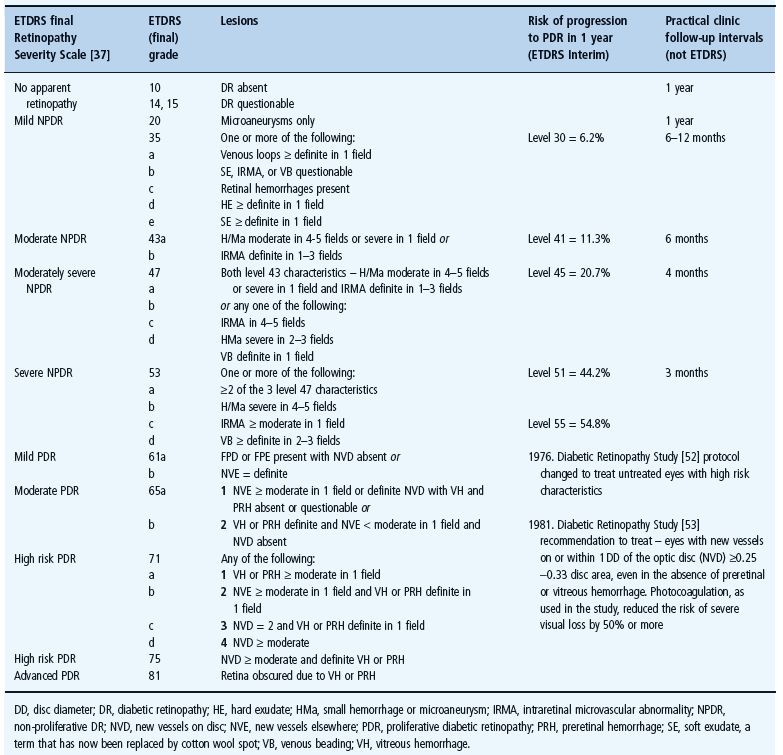
The lesions that the Early Treatment Diabetic Retinopathy Study (ETDRS) (Table 36.1) [51] described as critical to the stages of progression of DR were:
Table 36.1 Early Treatment Diabetic Retinopathy Study (ETDRS) Research Group diabetic retinopathy classification of progression to proliferative diabetic retinopathy based on 7 × 30° field stereo photographs of each eye.
- Microaneurysms – a microaneurysm is defined as a red spot <125µm (approximate width of vein at disc margin) and sharp margins.
- Small retinal hemorrhages – a hemorrhage is defined as a red spot, which has irregular margins and/or uneven density, particularly when surrounding a smaller central lesion considered to be a microaneurysm.
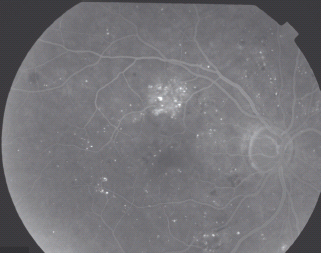
- Hemorrhage/microaneurysm (HMa) – because the ETDRS recognized that it was very difficult to differentiate between microaneurysms and small hemorrhages, the concept of HMa was introduced, which is a small hemorrhage or microaneurysm (Figure 36.9).
Figure 36.9 An example of the venous phase of a fluorescein angiogram showing small hemorrhages (dark), microaneurysms fluorescing, some of which are leaking shown by the fluffy edge.
Other larger retinal hemorrhages
- Flame hemorrhages – superficial hemorrhages just under the nerve fiber layer (Figure 36.10).
Figure 36.10 An example of more elongated lighter red flame hemorrhages and more oval and darker red blot hemorrhages.
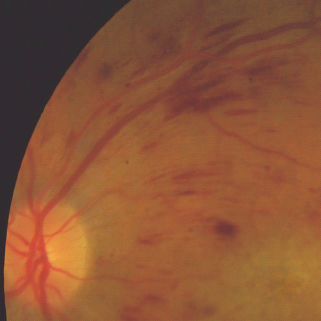
- Blot hemorrhages – deeper hemorrhages, which are a sign of retinal ischemia in the area of the retina in which they occur.
Hard exudates
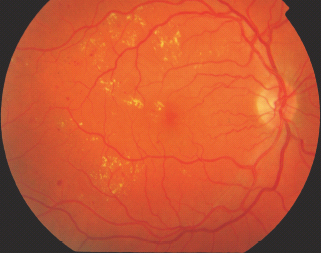
Hard exudates (sometimes now just referred to as exudates) are defined as small white or yellowish-white deposits with sharp margins, located typically in the outer layers of the retina, but they may be more superficial, particularly when retinal edema is present (Figure 36.11).
Cotton wool spots
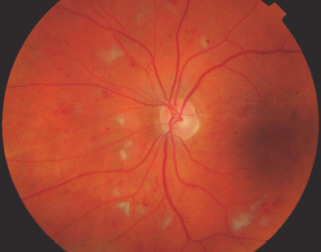
Cotton wool spots (referred to as soft exudates in the ETDRS, but this term is now rarely used) are fluffy white opaque areas caused by an accumulation of axoplasm in the nerve fiber layer of the retina, which is caused by an arteriolar occlusion in that area of retina that is apparent on a fluorescein angiogram (Figure 36.12).
Figure 36.12 An example of cotton wool spots. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing
Intraretinal microvascular abnormalities
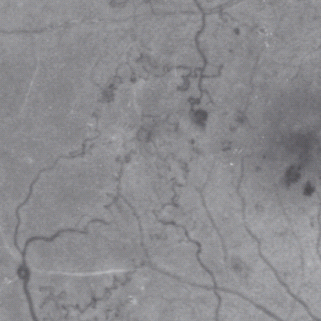
IRMA are defined as tortuous intraretinal vascular segments, varying in caliber, derived from remodeling of the retinal capillaries and small collateral vessels in areas of microvascular occlusion and are therefore a sign of retinal ischemia (Figure 36.13).
Figure 36.13 An example of intraretinal microvascular abnormality (IRMA) in the temporal retina of the right eye (the right macula is seen in the right side of this red free photograph).
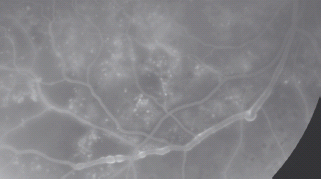
Venous abnormalities
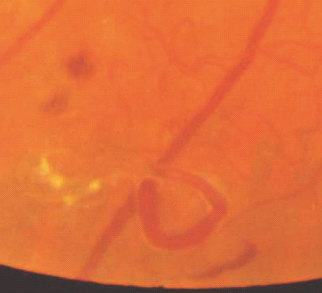
- Venous loops – abrupt curving deviations of a vein from its normal path (Figure 36.14).
- Venous beading – in the ETDRS, venous beading is described as a localized increase in caliber of the vein and the severity is dependent on the increase in caliber and the length of vein involved. It is associated with retinal ischemia (Figure 36.15).
Figure 36.15 A fluorescein angiogram showing venous beading adjacent to an ischemic area of retina. Reproduced from Scanlon PH, Wilkinson CP, Aldington SJ, Matthews DR. A Practical Manual of Diabetic Retinopathy Management. Published 2009 by Blackwell Publishing.
Other venous changes that occur in DR are as follow:
- Venous dilatation;
- Venous narrowing;
- Opacification of the venous wall; and
- Perivenous exudate.
Arteriolar abnormalities
Other arteriolar changes that occur in DR are as follow:
- Arteriolar narrowing;
- Opacification of arteriolar walls; and
- Arteriovenous nipping.
Fibrous proliferation at the disc
Fibrous proliferation at the disc (FPD) usually occurs when new vessels at the disc start to regress and fibrosis occurs.
Fibrous proliferation elsewhere
Fibrous proliferation elsewhere (FPE) usually occurs when new vessels elsewhere start to regress and fibrosis occurs.
New vessels on and/or within 1 disc diameter of the disc
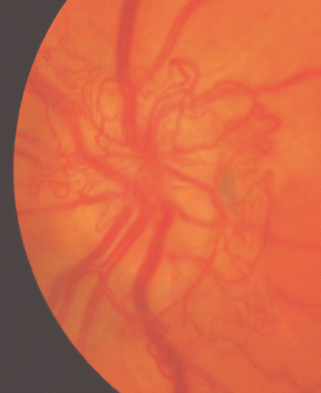
For new vessels on and/or within 1 disc diameter (DD) of the disc (NVD), see Figure 36.16.
New vessels elsewhere
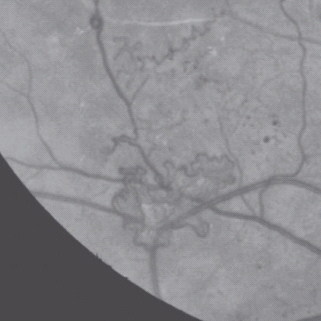
For new vessels elsewhere (NVE), see Figure 36.17.
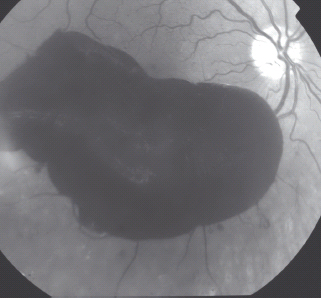
Vitreous hemorrhage
Vitreous hemorrhage (VH) is a hemorrhage that is in the vitreous gel.
Preretinal hemorrhage
Preretinal hemorrhages (PRH) are boat-shaped hemorrhages and roughly round, confluent or linear patches of hemorrhage just anterior to the retina or under the internal limiting membrane (Figure 36.18).
Post laser treatment
Photocoagulation laser scars may be seen both after macular or panretinal laser treatment (Figure 36.19; Tables 36.1–36.4).
Figure 36.19 (a,b) Right macular and nasal views following panretinal photocoagulation to show laser treatment scars in the superior, inferior, temporal and nasal retina.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree