Diabetes Mellitus and Heart Disease
Michael T. Johnstone
Richard Nesto
Heart disease was thought to be associated with diabetes as early as 1883, when Vergeley recommended testing the urine of patients with angina for glucose (1). However, as more patients with diabetes survived following the discovery of insulin and improvements in treatments for renal failure and infection, there was a marked increase in morbidity and mortality from cardiovascular disease. Diabetes is the seventh leading cause of death in the United States, with much of that mortality a result of cardiovascular disease (2). However, because these statistics are based on the underlying cause of death, they underestimate the true impact of diabetes on mortality.
Ultimately, atherosclerosis accounts for 65% to 80% of all deaths among North American patients with diabetes, compared with one third of all deaths in the general North American population (3,4,5). A two- to fourfold excess in mortality due to coronary artery disease (CAD) among individuals with diabetes has been noted in a number of prospective studies encompassing
a variety of ethnic and racial groups (6). Diabetes also increases the likelihood of severe carotid atherosclerosis (7,8), and mortality from stroke is increased almost threefold in patients with diabetes (9). Both type 1 and type 2 diabetes are therefore powerful and independent risk factors for CAD, stroke, and peripheral arterial disease (3,9,10). Furthermore, when patients with diabetes develop clinical events, they sustain a worse prognosis than patients without diabetes (11). Coupled with these macrovascular complications are such microvascular complications as retinopathy, neuropathy, and nephropathy, all of which account for most of the morbidity and mortality associated with diabetes mellitus. Although diabetes may be a problem of glucose metabolism, the American Heart Association (AHA) has recently stated that “diabetes is a cardiovascular disease” (3).
a variety of ethnic and racial groups (6). Diabetes also increases the likelihood of severe carotid atherosclerosis (7,8), and mortality from stroke is increased almost threefold in patients with diabetes (9). Both type 1 and type 2 diabetes are therefore powerful and independent risk factors for CAD, stroke, and peripheral arterial disease (3,9,10). Furthermore, when patients with diabetes develop clinical events, they sustain a worse prognosis than patients without diabetes (11). Coupled with these macrovascular complications are such microvascular complications as retinopathy, neuropathy, and nephropathy, all of which account for most of the morbidity and mortality associated with diabetes mellitus. Although diabetes may be a problem of glucose metabolism, the American Heart Association (AHA) has recently stated that “diabetes is a cardiovascular disease” (3).
EPIDEMIOLOGY
More than 10 million Americans carry the diagnosis of diabetes mellitus, and another 5 million are estimated to have undiagnosed diabetes (3). The prevalence of type 2 diabetes, which accounts for 90% of all cases of diabetes, is increasing in the United States and around the world because of the advancing age of the population, improved screening and detection, and the increase in risk factors such as obesity and physical inactivity. A growing ethnic diversity in the United States, including ethnic groups particularly susceptible to type 2 diabetes such as Hispanics, blacks, and South Asians, also contribute to the increasing prevalence of type 2 diabetes (3,12). The obesity epidemic will result in an increasing number of patients with diabetes. In 1998, obesity affected 18% of the U.S. population (13). The problem of obesity is anticipated to grow with the increasing weight of the U.S. population. Between 1991 and 1998, the body weight of the American male increased by 3% and that of the American female increased by 5%.
Diabetes and Cardiovascular Mortality
A meta-analysis of several studies estimated the risk of death from CAD in patients with diabetes at 2.58 in men and 1.85 in women (14). These values are in contrast with those from the Rancho Bernardo Study (15), which followed subjects aged 40 to 79 for 14 years and found that while death rates were also increased in subjects with diabetes, the risk factor-adjusted relative odds were 3.3 in women and 1.9 in men. Factors associated with an increase in mortality rates among those with diabetes include male gender, black race, longer duration of diabetes, and insulin use (16). Overall, cardiovascular disease, which includes coronary artery and cerebrovascular disease, accounts for 65% of all deaths among persons with diabetes. Although much of these data are based on findings in patients with type 2 diabetes, patients with type 1 diabetes have similar causes of death, including CAD and renal failure (17,18).
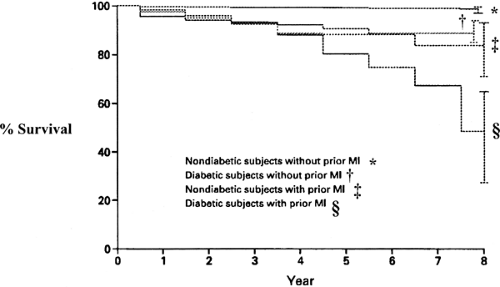
Life expectancy is shortened, with diabetic males living, on average, 9.1 years less and diabetic females living 6.7 years less than their nondiabetic counterparts (19). Haffner and colleagues examined the mortality among 1,000 persons with type 2 diabetes and 1,300 subjects without diabetes and found that the mortality of those with diabetes was similar to that for those without diabetes who had a myocardial infarction (MI) (20) (Fig. 58.1). These data suggest that caregivers should treat individuals with type 2 diabetes as if they had experienced an MI. Mukamal et al. (21) studied 1,935 patients hospitalized with an acute MI and found that the mortality among those with diabetes in the short-term period was similar to that of the patients without diabetes who had an MI previously and twice that of patients without diabetes who had suffered their first acute coronary event. Malmberg et al. (22) evaluated the findings of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) registry and found that patients with diabetes hospitalized for unstable angina or non–Q-wave MI had the same long-term morbidity and mortality as patients without diabetes with established cardiovascular disease.
Over the past three decades, there have been significant decreases in cardiovascular mortality in the United States. However, the effect on mortality in patients with diabetes has lagged well behind that in the general population (23). The death rate among nondiabetic men with CAD decreased by 36.4% as compared to a decrease of 13.1% for diabetic men, and the death rate among nondiabetic women decreased by 27% as compared to an increase of 23% among diabetic women (23).
Prevalence and Risk Factors for Coronary Artery Disease in Type 1 Diabetes
Long-term follow-up of patients with type 1 diabetes has demonstrated that the first cases of clinically manifest CAD occur late in the third decade or in the fourth decade of life regardless of whether diabetes developed early in childhood or during late adolescence. CAD risk increases rapidly after the age of 40, and by the age of 55 years, 35% of men and women with type 1 diabetes die of CAD (18) compared with 8% of those without diabetes. Women with type 1 diabetes lose most of the inherent protection from CAD observed in women without diabetes (18,24,25). The occurrence of severe coronary atherosclerosis before the age of 55 in a subset of patients with type 1 diabetes regardless of whether diabetes developed in childhood or adolescence suggests that diabetes mainly accelerates the progression of early atherosclerotic lesions that commonly occur, even in the absence of diabetes, at a young age in the general population (18).
Diabetic nephropathy, which develops in approximately 30% to 40% of patients with type 1 diabetes, dramatically increases the prevalence of CAD (18,26). Patients with persistent proteinuria who were followed in the Steno Memorial Hospital had a 37-fold increased mortality from cardiovascular disease relative to that of the general population, while patients without proteinuria had a cardiovascular mortality that was only 4.2 times higher (26). Patients with type 1 diabetes followed from the onset of microalbuminuria developed CAD eight times more frequently than patients without microalbuminuria (27). Krolewski et al. (17) reported that the risk of development of CAD in patients with persistent proteinuria was 15 times higher than the risk among those without proteinuria. Angiographic studies have shown that almost all patients with diabetic nephropathy older than age 45 have one or more clinically significant coronary stenoses (28). Microalbuminuria in type 1 diabetes is therefore not only a marker for renal disease but also a potent marker of CAD risk.
Several mechanisms contribute to the atherosclerotic process in the presence of diabetic nephropathy, including hypertension, lipid abnormalities, fibrinolysis, and coagulation alterations, all of which are detectable in the early stages of diabetic nephropathy when renal function is still normal (29). Hypertension is frequently present in patients with diabetic nephropa- thy even when the creatinine concentrations remain normal and can intensify CAD in patients with type 1 diabetes. Diabetic nephropathy is associated with an atherogenic lipoprotein profile that includes elevated levels of low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL), decreased levels of high-density lipoprotein (HDL), and elevated [Lp(a)] levels of lipoprotein a (30,31,32). Furthermore, a hypercoagulable state characterized by increased levels of plasminogen-activator inhibitor-1 (PAI-1), factor VII, and (plasma) fibrinogen, has been described in microalbuminuric patients with type 1 diabetes (33). Finally, reduced renal function leads to the accumulation of advanced glycosylation end products (AGEs) in the circulation and tissue (34,35).
The risk for the development of diabetic nephropathy is only partially determined by glycemic control and is highly influenced by genetic susceptibility (26,27). Several studies have established that a genetic susceptibility contributes to the high prevalence of CAD among patients with type 1 diabetes with nephropathy. CAD is twice as common a cause of death among parents of diabetic patients with nephropathy than among parents of diabetic patients without nephropathy. Among patients with diabetes with nephropathy, those who had a cardiovascular event are six times more likely than patients who did not have such an event to have a familial history of cardiovascular disease. A history of cardiovascular disease in both parents or in the father of a patient with type 1 diabetes increases the risk of nephropathy in the offspring tenfold and threefold, respectively (36). Parents of diabetic offspring with nephropathy also have higher blood pressure than parents whose diabetic offspring do not have diabetic nephropathy (37).
Interestingly, recent studies have shown that an association between the angiotensin-converting enzyme insertion/deletion (ACE I/D) polymorphism, potentially affecting the level of angiotensins and kinins in the kidney, can affect the development of renal disease in patients with type 1 diabetes (38). The same polymorphism has been linked to MI in patients without diabetes (39), as well as in patients with type 1 diabetes (40,41) and type 2 diabetes (42).
Prevalence and Risk Factors for Coronary Artery Disease in Type 2 Diabetes
Type 2 diabetes increases relative risk of cardiovascular disease two- to fourfold compared with the risk in the general population (43,44,45,46). The increase in cardiovascular risk is particularly high in women. The protection against atherosclerosis in premenopausal women is almost completely lost in women with diabetes (47,48).
While traditional risk factors play an important role in the development of atherosclerosis in subjects with diabetes, the rate of cardiovascular mortality and morbidity in persons with diabetes exceeds by 50% the rate predicted by these risk factors. Several other risk factors may account for this discrepancy. Possible nontraditional risk factors include insulin resistance, insulin levels, and hyperglycemia.
Many of these patients with type 2 diabetes have several of these risk factors for CAD. The term metabolic syndrome was first used by Gerald Reaven in 1988 (49) to describe this clustering of risk factors including hypertension, dyslipidemia, hyperglycemia, and insulin resistance. The National Cholesterol Education Program Adult Treatment Panel III (ATPIII) guidelines for cholesterol management in 2001 recognized that the metabolic syndrome is a collection of the risk factors mentioned above, as well as abdominal obesity (50).
PATHOPHYSIOLOGY OF DIABETIC CARDIOVASCULAR COMPLICATIONS
The increased risk of cardiovascular disease in individuals with diabetes is explained in part by the clustering of risk factors, including dyslipidemia, hypertension, hyperglycemia, hyperinsulinemia, and prothrombotic factors. Some of these risk factors are described in detail subsequently.
Insulin Levels, Insulin Resistance, and Hyperglycemia
Insulin resistance that is present many years or more before the clinical onset of overt diabetes resistance is associated with other atherogenic risk factors, such as hypertension, lipid abnormalities, and a procoagulant state (51,52,53,54,55,56,57) (Table 58.1) that promotes atherosclerosis many years before overt hyperglycemia ensues (58,59). Indeed, several studies have shown an inverse correlation between insulin sensitivity and atherosclerosis (60,61,62). Investigators using the Bruneck Study database suggest (63) that these risk factors are present in 84% of patients with type 2 diabetes. Thus, an increased prevalence of CAD is apparent in patients with impaired glucose tolerance (44,46,64)
and in those with newly diagnosed type 2 diabetes (65,66). The duration of insulin resistance among hyperglycemic and diabetic individuals probably contributes to the development of atherosclerosis. However, no obvious association between the extent or severity of macrovascular complications and the duration or severity of type 2 diabetes (24,67) has been found, most likely because the duration of insulin resistance is often unknown.
and in those with newly diagnosed type 2 diabetes (65,66). The duration of insulin resistance among hyperglycemic and diabetic individuals probably contributes to the development of atherosclerosis. However, no obvious association between the extent or severity of macrovascular complications and the duration or severity of type 2 diabetes (24,67) has been found, most likely because the duration of insulin resistance is often unknown.
TABLE 58.1. Cardiovascular Risk Factors Associated with Insulin Resistance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Another possibility is that the serum insulin level and not insulin resistance has direct cardiovascular effects. Despres and colleagues (68) followed 2,000 diabetic men without clinically overt CAD for 5 years and found that those who had a cardiovascular event had serum insulin levels that were 18% higher than those in controls.
Serum glucose levels may be an important risk factor for cardiovascular disease. Andersson and Svardsudd (69) demonstrated that fasting serum glucose levels are independently related to all-cause and cardiovascular mortality. The San Antonio Heart Study (70) showed similar findings for subjects in the highest quartile of fasting glucose levels, who had a 4.7 times greater risk of cardiovascular disease than did those in the first two quartile levels combined.
The direct relationship between glucose levels and cardiovascular disease also is seen in patients with type 1 diabetes. A 1% increase in levels of glycosylated hemoglobin doubled the increase in cardiovascular disease (71). Several studies have shown a direct relationship with the serum glucose levels on clinical events, including MI and strokes, with glucose levels ranging from an abnormal glucose tolerance test to frank diabetes (72,73,74). This graded effect of serum glucose levels on clinical events may be due in part to a direct effect on the vasculature, as evidenced by a similar direct relationship of serum glucose levels to the intima-media thickness of the carotid (as a marker for the presence and degree of atherosclerosis). The Atherosclerosis Risk in Communities (ARIC) study demonstrated that fasting glucose tolerance was directly related to carotid wall thickness in individuals free of symptomatic cardiovascular disease (8).
The level of chronic hyperglycemia, as determined by measurements of glycosylated hemoglobin, may also be an independent risk factor for coronary heart disease, particularly in women (75,76). Recent prospective studies demonstrated that microalbuminuria in patients with type 2 diabetes is also an independent predictor of increased cardiovascular mortality (77,78). Insulin resistance may play an important role as a risk factor in the development of diabetic cardiovascular disease. Hyperinsulinemia may be the mechanism by which the effect of hyperglycemia results in atherosclerosis. Insulin level is elevated in patients with the metabolic syndrome. The possibility that insulin resistance could result in an increase in cardiovascular disease was first demonstrated in population studies that showed an association between fasting insulin levels and cardiovascular mortality (60,79,80,81). In the Insulin Resistance Atherosclerosis Study, subjects were evenly divided among patients with normal serum glucose, hyperglycemia with normal glucose tolerance, and diabetes. The relationship of insulin levels and cardiovascular disease is further strengthened by basic research studies that showed the effect of insulin on various possible mediators for the development of atherosclerosis, specifically the increase in PAI-1 and the mitogenic effect on smooth muscle cells in vitro (82).
Dyslipidemia
An important mechanism for the development of diabetic atherosclerosis is dyslipidemia. The central feature of diabetic dyslipidemia is increased levels of VLDL due both to increased production of VLDL and to decreased catabolism of triglyceride-rich lipoproteins, including chylomicrons. The increase in hepatic production of VLDL occurs in response to increased delivery of fatty acids from (a) decreased free fatty acid uptake from the striated muscle and (b) increased delivery of the free fatty acids from the increased adipose tissue associated with central obesity.
The increase in triglyceride-rich lipoproteins accumulates not only because of increased VLDL production but also because of decreased catabolism of triglyceride lipoproteins. Lipoprotein lipase, which plays an important role in the metabolism of triglyceride-rich lipoproteins and in particular chylomicrons, is decreased in uncontrolled type 2 diabetes.
The increased level of triglyceride-rich lipoproteins provides an increase in substrate for the cholesterol ester transfer protein. This promotes the flux of cholesterol from HDL particles, which results in decreased HDL levels, a common finding in type 2 diabetes. Yet other mechanisms must be involved, because low HDL levels can occur in the absence of hypertriglyceridemia. The degree of HDL reduction is not related to the degree of control of diabetes or to the mode of treatment in type 2 diabetes. One mechanism of the protective effect of HDL against atherosclerosis may be its ability to prevent oxidation of LDL. There may be qualitative differences in HDL from patients with poorly controlled diabetes that may make it a less effective antioxidant than HDL from normal individuals (83).
Although the dyslipidemia of diabetes is not characterized by marked elevations of LDL, there are differences in the LDL type found in patients with type 2 diabetes. Specifically, the LDL is smaller and denser than typical LDL particles (84). These smaller, denser LDL particles have a greater tendency to undergo oxidation, which accelerates the atherosclerotic process.
Increased Oxidative Stress in Diabetes
There is recent evidence that increased oxidative stress in diabetes contributes to the development of diabetic complications (85). This increased stress may be due in part to the decreased availability of antioxidants such as ascorbic acid, vitamin E, uric acid, and glutathionine. In addition, there may be an increase in lipid peroxidation products and superoxide anion products, which may lead to altered vascular function (85,86,87).
The increase in oxidative stress may be the result of several pathways, including advanced glycation end product (AGE) production; small, dense LDL formation; altered polyol activity; or imbalance in the redox state (88). The activation of this polyol pathway is due to the conversion of glucose to sorbitol via aldolase reductase, which has been associated with microvascular complications (89,90).
The recent data from the Heart Outcomes Prevention Evaluation (HOPE) study have shown that treatment with the antioxidant vitamin E at 400 IU per day for a mean of 4.5 years had no apparent effect on cardiovascular morbidity or mortality in both diabetics and nondiabetics (91). King’s group has had rather intriguing results demonstrating that therapy with high doses of vitamin E (1800 IU per day) normalizes retinal hemodynamic abnormalities and improves renal function without improving glycemic control in patients with type 1 diabetes of short duration (92). Whether this effect is via antioxidant-dependent or -independent pathways remains to be elucidated.
Oxidative stress also precedes the formation of some AGEs, including pentosidine and N-ε-carboxymethyllysine (CML), and the activation of the diacylglycerol-protein kinase C (DAG-PKC) pathway.
Advanced Glycation End Products in Diabetes
AGEs occur as a result of the nonenzymatic glycation of both lipids and proteins. Initially, a labile covalent bond develops between the aldehyde of the glucose molecule and the amino acid side chain on both sugars and lipids. Specifically, glucose is covalently bound mainly to lysine residues in proteins, forming fructose-lysine residues. This reaction results in the development of a Schiff base, which, in turn, undergoes another chemical reaction to form a ketoamine, termed an Amadori product. These products result in cumulative oxidative damage to proteins. These products include CML (93) and pentosidine (94). The increased levels of pentosidine and CML correlate with the severity of diabetic complications, including nephropathy, retinopathy, and vascular disease. One such Amadori product is glycated (or glycosylated) hemoglobin A1c(HbA1c), which is commonly used to monitor glycemic control in diabetic patients. Since both free-radical oxidation and glycation are involved, these substances are also called glyoxidation products.
AGEs cross-link to the proteins composing the extracellular matrix and vascular basement membrane, which results in reduced solubility and decreased enzymatic digestion (95,96). AGE formation also prevents proper assembly of basement proteins, thereby altering their function. This in turn may alter the ability of cells to bind to their substrates.
AGEs are derived from oxidation of lipids (97,98). The side chains of unsaturated fatty acids undergo oxidation, which yields reactive carbonyl-containing fragments [malondialdehyde (MDA), glyoal 4-hydroxynonenal (4-HNE)] and then react with amino groups, mainly lysine residues.
Enhanced glycation, oxidation, and glyoxidation of lipoproteins have been postulated as a possible cause for the development of diabetic macrovascular disease. Certainly there are increased levels of AGE-modified LDL-apoprotein and LDL-lipid in persons with diabetes relative to levels in persons without diabetes (99). This would suggest that even in the face of similar glycemic control and other cardiovascular risk factors, the development of diabetic vascular complications would depend on differences of oxidative stress as well as on the tissue level of antioxidants.
The evidence for this possible role of these altered lipoproteins includes the presence of oxidized lipoproteins in the vessel wall (100,101) and the demonstration of lesion regression with antioxidants (102). One study (103) showed that the susceptibility of LDL to oxidation was correlated with the degree of atherosclerosis in 35 male survivors of an MI.
Vlassara and colleagues (104) identified a specific receptor for AGEs on monocyte/macrophages, termed RAGE (receptor for AGEs). The subsequent interaction with the AGE and its receptor may induce the release of the cytokines tumor necrosis factor (TNF) and interleukin-1 (105). Other cytokines that have been demonstrated include the synthesis and release of procoagulant activity and platelet-activating factor (PAF) by endothelial cells (106,107), as well as the induction of platelet-derived growth factor (PDGF-AA), which can be indirectly responsible for fibroblast and smooth muscle proliferation (108). Furthermore, increased AGE-receptor interaction has been shown to result in the enhanced expression of vascular cell adhesion molecule (VCAM) (109,110,111), which in turn results in increased atherogenesis.
The important role of the AGE receptor in the development of atherosclerosis was further strengthened by the demonstration that atherosclerosis was less severe in the usually atherosclerotic apolipoprotein E-knockout mice when they were administered an antibody-fragment that neutralized RAGE (112). This effect was seen without any effect on glycemic control or lipoprotein profile.
Thrombosis and Fibrinolysis in Diabetes
Plaque disruption with overlying thrombosis is a major cause of acute coronary syndromes, including MI, sudden death, and stroke. Because patients with type 1 and type 2 diabetes, particularly those with type 2 diabetes, have higher rates of acute coronary syndromes than the population without diabetes, heightened arterial prothrombotic reactivity may play a pivotal role in the development of these macrovascular complications.
There are three underlying mechanisms for this prothrombosis: heightened platelet reactivity, increased procoagulant activity, and decreased antithrombotic and fibrinolytic activity. The principal components of a thrombus are platelets and fibrin. The coagulation is initiated by the exposure of tissue factor within the arterial plaque at the time of plaque disruption. This results in the activation of factor VII/VIIa, which forms the “tenase complex” with factors X and V, resulting in the activation of thrombin. Thrombin stimulates platelet reactivity and the conversion of fibrinogen to fibrin, producing a thrombus.
The platelets of diabetic individuals appear to have an increased adherence to the vessel wall and increased circulating platelet mass (113). Platelet aggregometry studies that measure in vitro platelet reactivity have demonstrated increased aggregation of platelets in response to the agonists ADP, collagen, and thrombin and even spontaneous aggregation of platelets without any agonist (114,115,116,117,118). Assessment of platelet reactivity in vivo by measurement of blood or urine metabolites released from activated platelets such as thromboxane B2 has shown increased reactivity relative to that of normal healthy controls (114,115).
Patients with diabetes have increased concentrations of fibrinogen, von Willebrand factor, and factor VII (119,120,121). Although the mechanisms of the increased concentrations of these factors have yet to be elucidated, the level of serum fi-brinogen correlates with the levels of proinsulin and insulin in the blood (122). However, neither the plasma level of fibrinogen control nor the level of the plasma prothrombin fragment 1+2, a cleavage product of prothrombin, is reduced with improved metabolic control.
Several reports indicate that the activity of antithrombotic factors, including protein C and antithrombins, are decreased in subjects with diabetes, which further potentiates the hypercoagulable state (123,124,125,126).
Fibrinolysis is also impaired in individuals with diabetes, particularly those with type 2 diabetes (127,128). This
impairment may be due to the increased activity of PAI-1 in the blood, which counteracts the action of native tissue plasminogen activator (t-PA) to induce fibrinolysis. PAI-1 is elevated not only in resting states but also in response to physiologic stimuli. The serum level of PAI-1 may be elevated as a result of several factors, including elevated serum levels of insulin, lipids, and glucose (129). The impairment of the fibrinolytic system can potentially exacerbate the development and persistence of thrombi, resulting in an increased risk of vascular occlusion.
impairment may be due to the increased activity of PAI-1 in the blood, which counteracts the action of native tissue plasminogen activator (t-PA) to induce fibrinolysis. PAI-1 is elevated not only in resting states but also in response to physiologic stimuli. The serum level of PAI-1 may be elevated as a result of several factors, including elevated serum levels of insulin, lipids, and glucose (129). The impairment of the fibrinolytic system can potentially exacerbate the development and persistence of thrombi, resulting in an increased risk of vascular occlusion.
Endothelial Function and Diabetes
Alterations in endothelial function may play an important role in the development of diabetic complications. Decreased blood flow in many organs has been reported, including the kidney, retina, and peripheral retinal nerves. Patients with recent diabetes have decreased retinal blood flow, as indicated by increased vascular resistance. The mechanism of this increased vascular resistance is probably partly due to the increase in the intercellular signal transduction kinase, protein kinase C (PKC) (130,131,132,133). This increase in PKC may result in an increase in endothelin-1. It has been documented that abnormalities in hemodynamic profiles precede diabetic nephropathy. This increase in glomerular filtration is probably due to the effect of hyperglycemia on arteriolar resistance.
The vascular endothelium has been shown to be important in modulating blood cell-vessel wall interaction, regulating blood flow, angiogenesis, lipoprotein metabolism, and vasomotion. An important mediator in maintaining vascular homeostasis is endothelium-derived relaxing factor (EDRF) (134), which has since been found to be nitric oxide (135). The release of nitric oxide activates soluble guanylate cyclase, resulting in the formation of cyclic guanosine monophosphate (cGMP), which, in turn, activates cGMP–dependent protein kinases, resulting in relaxation of vascular smooth muscle (136,137,138,139). Alterations in the expression, release, or activity of EDRF may play an important role in the initiation and progression of both micro- and macrovascular disease. Several studies have shown that endothelial-dependent vasodilator function is impaired in patients with type 1 diabetes without hypertension and dyslipidemia (140). This impairment is in contrast to that in patients with type 2 diabetes, who have an impairment of both endothelial-dependent and endothelial-independent (smooth muscle) vasodilator function (141,142).
Although the mechanism is unknown, several possibilities are present. Acute hyperglycemia impairs endothelial-dependent vasodilation in both macro- and microvessels (143). Insulin also may play a role. Insulin results in vasodilation due in part to nitric oxide production. Glucose-clamp experiments with insulin infusion have shown that subjects with type 2 diabetes have little improvement in endothelial-dependent vasodilation relative to that in subjects without diabetes (143). As stated previously, there appears to be an increase in oxygen-derived free radicals in the diabetic state. Several studies have shown that high doses of vitamin C can improve endothelial-dependent vasodilation in patients with both type 1 and type 2 diabetes (144,145). Intensive lipid lowering by statin therapy does not improve vasoreactivity in patients with type 2 diabetes, suggesting that mechanisms other than dyslipidemia are responsible for endothelial dysfunction (146).
CLINICAL FEATURES OF CARDIOVASCULAR DISEASE IN DIABETES
Angiographic Features of Coronary Artery Disease in Patients with Diabetes
Autopsy, angiographic, and angioscopic studies have documented the severe and diffuse nature of the atherosclerotic coronary involvement in patients with diabetes. Early autopsy data have shown that patients with diabetes have a greater number of coronary vessels involved, with more diffuse distribution of atherosclerotic lesions (150,151). Large angiographic studies comparing patients with diabetes to matched controls in the setting of acute MI (152) or elective angioplasty (153) or prior to coronary bypass surgery (154) have all shown that diabetes is associated with significantly more severe proximal and distal CAD (Table 58.2). An important finding with regard to the pathogenesis of acute coronary syndromes is the autopsy (155) and angioscopic (156) evidence suggesting a significant increase in plaque ulceration and thrombosis in diabetic compared with nondiabetic patients.
TABLE 58.2. Angiographic Studies in Patients with Diabetesa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Silent Ischemia
The propensity of patients with diabetes to present with either silent or unrecognized MI is well established (157,158). Atypical symptoms such as confusion, dyspnea, fatigue, or nausea and vomiting were the presenting complaint in 32% to 42% of patients with diabetes with MI compared with 6% to 15% of patients without diabetes (157,159). Several groups have reported that the detection of silent ischemia by various noninvasive techniques, including treadmill exercise testing (160,161), ambulatory Holter monitoring (162), and exercise thallium scintigraphy (163,164,165,166), is more common in patients with diabetes than in those without diabetes. This finding, however, is not supported by all studies (167,168).
A plausible explanation for painless infarction and ischemic episodes in patients with diabetes is autonomic neuropathy with involvement of the sensory supply to the heart. In autopsies of patients with diabetes who died of silent MIs, typical diabetic neuropathic changes were found in the intracardiac sympathetic and parasympathetic fibers (169), and several studies correlated abnormalities in autonomic function in patients with silent ischemia (160,162,164,170). The anginal perceptual threshold—the time from the onset of myocardial ischemia (assessed by ST segment depression) to the onset of chest pain during exercise testing—is prolonged in patients with diabetes compared with those without diabetes. This delay in the perception of pain may be related to the impairment of autonomic nervous function (170).
Acute Coronary Syndromes in Patients with Diabetes
Acute ischemic events represent a major cause of death in the diabetic population (65). Diabetic patients who suffer an MI have a higher mortality than nondiabetic patients both in the acute phase and on long-term follow-up. Numerous studies have shown that in-hospital mortality rates from MI in patients with diabetes are 1.5- to 2-fold higher than in patients without diabetes (152,171,172,173,174). Diabetes remains an independent predictor for a poor prognosis in the thrombolytic era. In the Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) trials, the in-hospital mortality rate was nearly twice as high in patients with diabetes, with more congestive heart failure and twice the rate of clinically recognized reinfarction (152). In the
Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial, mortality at 30 days was highest among patients with diabetes treated with insulin (12.5%) compared with patients with diabetes not treated with insulin (9.7%) and nondiabetic (6.2%) patients (p <0.001) (175). Similar results have been reported from the other large studies (176,177,178). Diabetes is also a risk factor for cardiogenic shock in the setting of acute ischemic syndromes (179). Overall, despite the overall improvement in survival from an acute MI with thrombolysis, the in-hospital mortality rates in patients with diabetes remain 1.5 to 2 times higher than in patients without diabetes (175,178).
Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial, mortality at 30 days was highest among patients with diabetes treated with insulin (12.5%) compared with patients with diabetes not treated with insulin (9.7%) and nondiabetic (6.2%) patients (p <0.001) (175). Similar results have been reported from the other large studies (176,177,178). Diabetes is also a risk factor for cardiogenic shock in the setting of acute ischemic syndromes (179). Overall, despite the overall improvement in survival from an acute MI with thrombolysis, the in-hospital mortality rates in patients with diabetes remain 1.5 to 2 times higher than in patients without diabetes (175,178).
This increased in-hospital mortality among patients with diabetes with acute MI is due predominantly to an increase in the incidence of congestive heart failure (172,174,180,181), although increases in the incidence of reinfarction, infarct extension, and recurrent ischemia have also been reported (172,173,174,181,182).
Studies using serial determinations of total creatine kinase activity (180,181), radionuclide ventriculography (183), or echocardiography have found no evidence that patients with diabetes sustain more extensive infarctions than their nondiabetic counterparts (184). Thus, congestive heart failure and cardiogenic shock are more common and more severe in subjects with diabetes than would be expected from the size of the index infarction (178,180,181,183,185,186). The observation that clinical manifestations of heart failure occur in patients with diabetes despite a modest decrease in left ventricular ejection fraction (EF) led to the suggestion that preexisting diastolic dysfunction is a major culprit in the congestive symptoms (174). Indeed, subclinical diabetic cardiomyopathy, which is characterized by diastolic dysfunction (187), is likely to be an important factor in this setting.
It should be emphasized, however, that reductions in both left ventricular EF (183,188) and the regional EF of the noninfarcted myocardium (152,183,187) have been well documented in patients with diabetes following MI as compared with patients without diabetes. For example, early angiography in the TAMI trials has demonstrated worse ventricular function in the noninfarcted zone in patients with diabetes (152).
The performance of the left ventricle following MI is determined largely by the extent of coronary disease (189) and the quality of collateral circulation. Thus, the diffuse nature of coronary atherosclerosis (Table 58.2) in diabetes may contribute to systolic dysfunction of the noninfarcted myocardium. Moreover, a recent study has shown that patients with diabetes have a reduced ability to develop collateral blood vessels in the presence of CAD (190), a finding that also may explain the more frequent occurrence of postinfarction angina and infarct extension (173,174,182,184).
MEDICAL THERAPY FOR CORONARY ARTERY DISEASE IN PATIENTS WITH DIABETES
Diabetes exerts a deleterious effect on the short- and long-term course following MI through diverse mechanisms, some of which (e.g., cardiomyopathy) cannot be modified at the time of presentation. Because patients with diabetes are at greater risk, application of effective preventive and treatment measures may result in a particularly large survival benefit.
Insulin
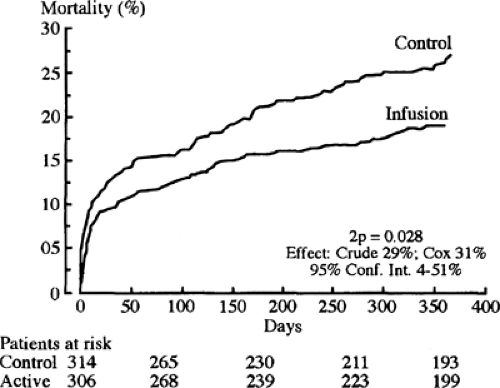
One possible mechanism for the increased mortality among diabetic patients with acute MI may be the altered metabolism of the myocardium. The diabetic state results in increased fatty acid metabolism, compromising glycolysis in both ischemic and nonischemic territories. Free fatty acids and their intermediates may potentiate ischemic injury. One way to attenuate free fatty acid oxidation is by the intravenous infusion of insulin-glucose. It was that rationale that led Malmberg and colleagues (195) to evaluate the effect of insulin-glucose infusion followed by multidose insulin treatment in patients with diabetes [Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction
(DIGAMI) Study] (Fig. 58.2). Patients with diabetes with an acute MI within the previous 24 hours were randomized to two separate arms. Insulin-glucose infusion was given for the first 24 hours and until stable normoglycemia in the experimental arm. Then subcutaneous multidose insulin was given to maintain normoglycemia for a 3-month period. Control patients received standard coronary care unit care and did not receive insulin unless clinically indicated.
(DIGAMI) Study] (Fig. 58.2). Patients with diabetes with an acute MI within the previous 24 hours were randomized to two separate arms. Insulin-glucose infusion was given for the first 24 hours and until stable normoglycemia in the experimental arm. Then subcutaneous multidose insulin was given to maintain normoglycemia for a 3-month period. Control patients received standard coronary care unit care and did not receive insulin unless clinically indicated.
The 3-month mortality was not significantly different for the control and experimental groups. However, the 1-year mortality was 18.6% in the experimental group and 26% in the control group, or a relative risk reduction of approximately 30%. This improvement in mortality continued for a total of 3.4 years, with an absolute reduction of mortality of 11% (196).
Aspirin
Studies have shown an increased platelet adhesiveness and aggregability (197), with a concomitant increased release of thromboxane A2 (115) in subjects with diabetes. On the basis of these data, several authors stated that patients with diabetes may require larger doses of aspirin to suppress the synthesis of thromboxane A2 (115,198). Furthermore, in the Second International Study of Infarct Survival (ISIS-2) study, there was no reduction in mortality in subjects with diabetes receiving 160 mg of aspirin daily (199).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree