- Diabetes affects 10–25% of elderly people (>65 years) worldwide, with particularly high rates in populations such as Pima Indians, Mexican-Americans and South Asians.
- Glucose tolerance worsens with age, the main factor being impairment of insulin-stimulated glucose uptake and glycogen synthesis in skeletal muscle.
- Factors precipitating hyperosmolar hyperglycemic syndrome include infections, myocardial infarction, stroke and drugs such as thiazides or glucocorticoids.
- Episodes of hypoglycemia resulting from insulin or sulfonylureas may be severe and prolonged, particularly because counter-regulatory responses are impaired in the elderly.
- High levels of disability are common in older people with diabetes and lead to heavy usage of health care resources and premature mortality.
- About 16% of elderly people with diabetes in the UK are registered blind or partially sighted (eight times more than the non-diabetic population) which justifies regular screening for undetected eye disease.
- Risk factors for foot ulceration affect 25% of elderly people with type 2 diabetes.
- Management strategies for many elderly people with diabetes are as in the younger population, with similar lipid-lowering and antihypertensive treatment schedules and aspirin or clopidogrel for patients with increased cardiovascular risk.
- Effective delivery of diabetes care depends on close cooperation between hospital and community, the involvement of diabetes specialists and practice nurses, and attention to all causes of disability and ill-health.
- Elderly people with diabetes in care homes are particularly vulnerable and require greater diabetes specialist input.
Introduction
Diabetes, the most common disabling metabolic disorder, imposes considerable economic, social and health burdens [1]. Many of these burdens fall heavily on older patients with diabetes, partly because type 2 diabetes mellitus (T2DM) and its co-morbidities are so much more common in that age group, and partly because of these subjects’ special needs. Older people do not accept illness without question, however, and expect equity of access to treatment and services as for younger people. As those who are above pensionable age are, in most Westernized societies, a significant proportion of the voting public, they can be very persuasive in ensuring that there are political commitments to improving the organization and delivery of health care.
Within Europe, T2DM affects 10–30% of subjects above pensionable age and in the USA about 40% of all those with diabetes fall into this category [2]. Older people with diabetes use primary care services two to three times more than their counterparts without diabetes; in one Danish study [3], insulin-treated patients accounted for over half of the services provided, mainly because of macrovascular disease. The burden of hospital care is also increased two to three times in those with diabetes compared with the general aged population [4], with more frequent clinic visits and a fivefold higher admission rate; acute hospital admissions account for 60% of total expenditure in this group [5]. In the UK, some 5–8% of general hospital beds are occupied by patients with diabetes aged 60 years or more [6,7], accounting for 60% of all inpatients with diabetes [7]. Hospital admissions last twice as long for older patients with diabetes compared with age-matched control groups without diabetes, with the totals averaging 7 and 8 days per year for men and women, respectively [4,6,8]. Introducing insulin treatment increases costs fourfold, both in the community and in hospital, where bed occupancy rises to 24 days per year [4].
The management of T2DM and its common co-morbidity of macrovascular disease is complicated in elderly subjects because of the added effects of aging on metabolism and renal function, the use of potentially diabetogenic drugs and low levels of physical activity (Figure 54.1) [9,10]. Cardiovascular risk is particularly high because many risk factors of the metabolic syndrome can be present for up to a decade before T2DM is diagnosed (Figure 54.1) [11].
Figure 54.1 Features of the metabolic syndrome. Additional considerations that apply to the elderly population are described in the text. AGE, advanced glycation end-product; HDL, high density lipoprotein; PAI-1, plasminogen activator inhibitor1.
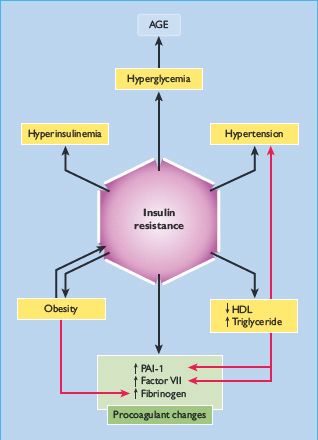
It must be remembered that older people with diabetes, particularly those who are housebound or institutionalized, have special needs (Table 54.1). Overall, the quality of diabetes care for older patients appears to be improving in various countries [12,13], although the UK still seems to be alone in developing geriatric diabetes as a subspecialty.
Table 54.1 Special characteristics of older subjects with diabetes.
| High level of associated medical co-morbidities Increased risk of cognitive dysfunction and mood disorder causing more complex decision-making Varying evidence of impaired activities of daily living and lower limb function Increased vulnerability to hypoglycemia Increased risk of inpatient mortality Unstructured specialist and primary care follow-up Increased need to involve spouses and informal carers in management |
Epidemiology of diabetes in aging populations
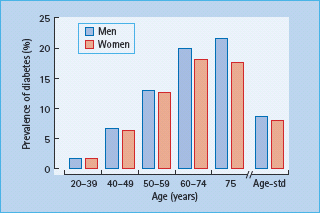
In 1997, there were approximately 124 million people with diabetes worldwide, of whom 97% had T2DM. By the time of publication of this edition, this number is projected to rise to 285 million. Aging is an important factor in the rapid worldwide rise of T2DM. In the USA, the number of new cases of diabetes in people aged 65–79 years was five times higher than in those aged less than 45 years of age [2]. The prevalence of diabetes begins to rise steadily from early adulthood, reaching a plateau in those aged 60 years or older; the data in Figure 54.2, from the Third National Health and Nutrition Examination Survey (NHANES III) in the USA [14], are representative of most developed countries. In some susceptible populations, T2DM may develop earlier; for example, among the Pima Indians prevalence peaks at 40 years of age in men and at 50 years in women, and declines after the ages of 65 and 55 years, respectively.
Figure 54.2 Prevalence of diabetes in men and women in the US population aged ≥20 years, based on the NHANES III study [14]. Subjects included those with previously diagnosed and undiagnosed diabetes (defined by fasting plasma glucose ≥7.0mmol/L). Age-std, age-standardized.
Overall prevalence rates of diabetes in the elderly are dominated by T2DM, which accounts for 95% of all cases in the UK and European countries, and for virtually all in populations such as the Pima Indians and Mexican-Americans. Some older patients who present clinically with T2DM may have slowly evolving autoimmune β-cell destruction that requires insulin treatment, so-called latent autoimmune diabetes of adults (LADA). This condition appears to be most prevalent in northern Europe and is rare in Asians and Africans.
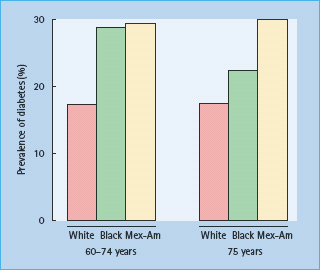
There are marked ethnic and geographic differences in the prevalence rates of diabetes amongst older people. In the UK and most developed countries, diabetes affects 9–17% of white subjects aged over 65 years and up to 25% of non-white people [15,16]; intriguingly, the prevalence of diabetes in elderly care homes in the UK is also 25% [17]. Among subjects aged over 60 years from NHANES III, Mexican-Americans showed a consistently higher prevalence of diabetes than non-Latino white and black subjects (Figure 54.3) [14].
Figure 54.3 Prevalence rates for diabetes among elderly non-Hispanic white, non-Hispanic black and Mexican-American subjects. Data from the NHANES III study [14].
Etiology of diabetes in the elderly
T2DM accounts for virtually all of the age-related increase in the prevalence of diabetes. This is attributed to various combinations of insulin resistance and impaired insulin secretion that result in a progressive age-related decline in glucose tolerance, which begins in the third decade and continues throughout adulthood [18,19]. Plasma glucose levels at 1 and 2 hours after the standard 75-g oral glucose challenge rise by 0.3–0.7 mmol/L per decade, the increase being greater in women. Consistent with this, NHANES III found the prevalence of impaired glucose tolerance (IGT) to be 12% in subjects aged 40–49 years, rising to 21% in those aged 60–74 years [14].
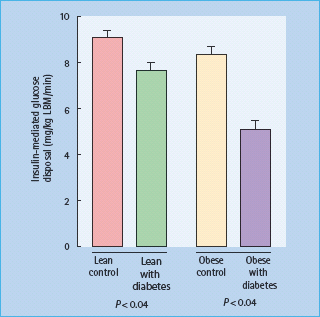
Various factors contribute to age-related glucose intolerance (Table 54.2). Perhaps the most important is impairment of insulin-mediated glucose disposal, especially in skeletal muscle [19,20], which is particularly marked in obese subjects (Figure 54.4) [21]. Insulin receptor number and binding are not consistently affected by age, and so post-receptor defects are presumably responsible. Contributory factors in some cases include increased body fat mass, physical inactivity and diabetogenic drugs such as thiazides. In contrast to younger people with T2DM, fasting hepatic glucose production does not appear to be increased in either lean or obese elderly people with T2DM [21]. The ability of insulin to enhance blood flow is also considerably reduced in obese insulin-resistant subjects with diabetes; this may be etiologically important, as insulin-mediated vasodilatation is thought to account for about 30% of normal glucose disposal.
Table 54.2 Factors contributing to glucose intolerance in old age.
Impaired glucose disposal and utilization
|
Impaired glucose-induced insulin secretion Other factors
|
NIMGU, non-insulin mediated glucose uptake.
Figure 54.4 Insulin-mediated glucose disposal is decreased in elderly patients with type 2 diabetes. The euglycemic clamp technique was used to measure the glucose disposal rate in healthy lean and obese elderly controls, and in their counterparts with diabetes. LBM, lean body mass. From Meneilly et al. [21], with permission.
As well as insulin resistance, many elderly people with glucose intolerance show impairment of glucose-induced insulin secretion, especially in response to oral rather than intravenous glucose. In addition, recent studies have shown that glucose effectiveness (i.e. the ability of glucose to stimulate its own uptake in the absence of insulin) is decreased in healthy elderly subjects [22]. This non-insulin-mediated glucose uptake (NIMGU) accounts for 70% of glucose uptake under fasting conditions (primarily into the CNS) and for 50% of post-prandial glucose uptake (especially into skeletal muscle). This is therefore a potentially important new target for therapeutic intervention, as exercise, anabolic steroids and decreased non-esterified fatty-acid (NEFA) levels can all enhance NIMGU and improve glucose tolerance, at least in younger patients [23].
Acute metabolic complications in elderly people with diabetes
Hyperglycemic states
Older subjects with diabetes can develop diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (formerly known as hyperosmolar non-ketotic coma [HONK]), which occurs predominantly in subjects aged over 50 years. In one study from Birmingham, UK, 22% of admissions with DKA were in subjects aged 60 years or more [24], while 13% of cases of hyperglycemic coma in all ages in Melbourne, Australia, were caused by hyperosmolar hyperglycemic state [25]. Many people with T2DM maintain enough residual insulin secretion to suppress lipolysis and ketogenesis, and so develop hyperosmolar hyperglycemic state instead of DKA; hyperosmolarity can worsen insulin resistance and may also inhibit lipolysis. The tendency to hyperosmolarity may be worsened in elderly people, who may not perceive thirst or drink enough to compensate for the osmotic diuresis, and are often taking diuretics [26].
Causes of hyperglycemia include infection (55% of cases in one series of hyperosmolar hyperglycemic state), myocardial infarction (MI), inadequate hypoglycemic treatment or diabetogenic drug treatment. Thiazide diuretics and glucocorticoids can increase blood glucose levels and may precipitate DKA; thiazide diuretics and furosemide (frusemide) appear particularly likely to cause hyperosmolar hyperglycemic state. A specific cause often cannot be identified – for example, in 38% of DKA cases in Birmingham [24]. Residents of care homes are at increased risk of hyperosmolar hyperglycemic state, which is associated with appreciable mortality [27].
Compared with the young, older patients have higher mortality and longer stays in hospital; they are also less likely to have had diabetes diagnosed previously, and more likely to have renal impairment and to require higher insulin regimens [28]. Death may occur from the metabolic disturbance or concomitant illnesses such as pneumonia and MI.
Investigation and treatment of hyperglycemic comas
The history and examination should pay special attention to previous diabetic symptoms, drug treatment, any precipitating infection (MI), possible MI or medication, evidence of heart failure and the degree of dehydration. Initial investigations are as for younger patients, including arterial blood gases and plasma osmolality (see Chapter 34).
Fluid, insulin and potassium replacement are discussed in general in Chapter 34. In elderly patients, intravenous saline can often be given at a rate of 500 mL/hour for 4 hours, then reducing to 250 mL/hour; faster infusion is needed if the patient is shocked, when a central line is invaluable to monitor filling pressure, particularly in the presence of cardiac failure or recent MI.
Some older subjects with hyperosmolar hyperglycemic state need very small doses of insulin to reduce plasma glucose levels, although hypercatabolic or severely insulin-resistant states will require higher dosages.
Thrombotic complications may occur, especially in subjects with hyperosmolar hyperglycemic state; prophylactic anticoagulation with low dose subcutaneous heparin is therefore recommended.
Hypoglycemia
Older patients are particularly susceptible to hypoglycemia, and this problem is often exacerbated because old people may have been given little knowledge about the symptoms and signs of hypoglycemia [29]. Even health professionals may misdiagnose hypoglycemia as a stroke, transient ischemic attack, unexplained confusion or epileptic fit, as illustrated in the case history below.
A 76-year-old man was admitted with a left-sided hemiplegia. He was unconscious and the family was told by the emergency room staff that he had had a stroke and his prognosis was very poor. At this point, no-one had thought to assess his glucose concentration. When requested by the medical registrar, it was found to be 1.4 mmol/L. Following treatment with IV glucose, the man regained consciousness and he was able to leave hospital 1 hour later. He had taken his insulin but delayed his meal.
Personal communication from Richard Holt, University of Southampton
Patients with cognitive impairment or loss of the warning symptoms of hypoglycemia are especially vulnerable, as they may not recognize impending hypoglycemia and/or fail to communicate their feelings to their carers. Multiple factors underlie the increased susceptibility to hypoglycemia in the elderly, including recent discharge from hospital with altered sulfonylurea dosages, renal and hepatic impairment, excess alcohol and insulin therapy [30]. In addition, older subjects mount a diminished counter-regulatory response to hypoglycemia [31], and this may delay recovery.
The risk of hypoglycemia is highest with insulin, but prolonged hypoglycemia is an important clinical problem for older subjects taking glibenclamide and chlorpropamide [32]. Glibenclamide-induced hypoglycemia may be more pronounced because the drug accumulates within the β-cell, and its metabolites retain some hypoglycemic activity. The long elimination half-life (approximately 35 hours) of chlorpropamide causes it to accumulate with continued dosing; steady-state is not achieved for 7–10 days. Impaired renal function further prolongs hypoglycemia secondary to sulfonylureas that are cleared through the kidneys. Short-acting sulfonylureas, gliclazide and tolbutamide, are less likely to cause hypoglycemia [33]. although glipizide is considered by some to be unsafe in the elderly [34]. Newer oral agents such as the thiazolidinediones and the meglitinides may decrease the risk of hypoglycemia in the elderly, as may both rapid- and prolonged-acting insulin analogs.
In the elderly, serious hypoglycemia appears to carry a worse prognosis and higher mortality; permanent neurologic damage may occur, presumably because of an already compromised cerebral circulation. Most sulfonylureas have caused fatal hypoglycemia, most commonly chlorpropamide or glibenclamide [35]. Other factors predisposing to fatal hypoglycemia include alcohol consumption, poor food intake, renal impairment and potentiation of hypoglycemia by other drugs.
Treatment and prevention of hypoglycemia are as described in Chapter 33. Many old people cannot treat hypoglycemia themselves [28]. The educational program should focus on detecting and treating hypoglycemia, with advice to others about how to manage cases of unresponsive hypoglycemia. In view of the additional vulnerability of older people to hypoglycemia, extra caution is required when there is a history of recurrent symptoms, drowsiness is present, the patient is on relatively large doses of insulin or when their diabetes care is delegated to an informal carer. This increased risk must be balanced by a lower threshold for admission to hospital when hypoglycemia is suspected. In this setting, a glucose level <4 mmol/L may warrant admission.
Chronic diabetic complications in the elderly
Diabetes in older subjects carries considerable morbidity, mainly through its long-term complications. For example, in a 6-year study of 188 patients aged over 60 years in Oxford [36], the reported incidence rates of ischemic heart disease, stroke and peripheral vascular disease (PVD) were 56, 22 and 146 cases per 1000 person-years, respectively. These were slightly higher than the rates in the Framingham study [37], presumably because of the older age of the Oxford patients. Retinopathy occurred at a rate of 60 cases and cataract at 29 cases per 1000 person-years, while the rate of proteinuria (albumin concentration >300mg/L) was 19 per 1000 person-years. These incidence rates appeared to be unrelated to sex or duration of diabetes, but stroke and PVD rose significantly with age.
The age-dependence of chronic diabetic complications was also investigated in a cross-sectional study of patients with T2DM aged 53–80 years [38]. Logistic regression demonstrated a significant rise in the prevalence of retinopathy with aging, independent of the effects of metabolic control, duration of disease and other risk variables. Age also increased the prevalence of peripheral neuropathy, hypertension and erectile dysfunction. An independent contribution of age per se to retinopathy, however, was not reported by Ballard et al. [39] from Minnesota, USA (who found a positive relationship with persistent proteinuria only), or by Knuiman et al. [40], who studied patients with both type 1 diabetes (TIDM) and T2DM and found independent associations of age with renal impairment, macrovascular complications and sensory neuropathy only.
Diabetic eye disease and visual loss
Cataract, age-related macular degeneration and diabetic retinopathy remain the major causes of blindness and partial-sight registration in most developed countries (see Chapter 36) [41]. All these conditions are common in elderly patients with diabetes.
Cataract, the most frequent cause of deteriorating vision in the elderly, is more common in subjects with diabetes, even at the time of diagnosis; its presence is associated with premature death [16]. Age-related macular degeneration is also frequent in older patients with diabetes and is an important cause of central visual loss [42]. Risk factors include atherosclerosis, diastolic blood pressure >95 mmHg or antihypertensive medication, and elevated serum cholesterol. Most older patients with diabetes have diabetic retinopathy of some degree, although about 5% show no evidence of retinal damage even after 15 years of the disease [42]. The main sight-threatening consequence of diabetic retinopathy in this population is maculopathy and particularly macular edema (see Chapter 36).
In Nottingham, UK, 16% of elderly people with diabetes are registered blind or partially sighted which is approximately eight times more than among their counterparts without diabetes [43]. The Welsh Community Diabetes Study [44] found that visual acuity was impaired in 40% of elderly subjects with diabetes, compared with only 31% of controls without diabetes (P <0.007). Factors significantly associated with visual loss in people with diabetes included advanced age, duration of diabetes, female sex, a history of foot ulceration and treatment with insulin. Reduced visual acuity was also significantly associated with poorer quality of life, as measured by the SF-36 questionnaire.
Screening for retinopathy
This is particularly important in the elderly, as deteriorating vision may be accepted by the patient as part of aging. Diabetic retinopathy may be the presenting feature of the disease in older people. Elderly people with diabetes need annual measurements of visual acuity and retinal photography; where the latter may not be available or feasible, patients should undergo dilated-pupil fundoscopy by experienced observers. Exudative maculopathy (hard exudates at or within one disc diameter of the macula) is easy to detect, but macular edema is practically impossible to detect by routine ophthalmoscopy; instead, slit-lamp stereoscopic fundoscopy is required to measure retinal thickness. Mydriasis is usually a short-term intervention and, in the great majority of cases, is not associated with any major problems, even in older people. It is always important to know whether patients have a history of glaucoma before mydriasis, as this requires a different approach, often under specialist supervision. This highlights the importance of measuring the corrected visual acuity, which is decreased by maculopathy (see Chapter 36). Indications for referral to an ophthalmologist are generally identical to those in younger patients (see Table 36.3).
Prevention and management of diabetic retinopathy
The benefits of tight glycemic control in slowing the progression of diabetic retinopathy have been convincingly proven in both T1DM and T2DM, by the Diabetes Control and Complications Trial (DCCT) and UK Prospective Diabetes Study (UKPDS) trials, respectively [45,46]. In the UKPDS, the risk of retinopathy progressing was reduced by 25% when HbA1c was maintained at <53 mmol/mol (7.0%) [46], while improved blood pressure control also reduced risk by 37% [47]. Although there have been no specific intervention studies in elderly patients with T2DM, it seems reasonable to extrapolate the general principles to this population. Problems with hypoglycemia and postural hypotension, however, prevent many elderly subjects from achieving tight glycemic and blood pressure control.
Laser photocoagulation should be used as indicated in Chapter 36; it halves the risk of severe visual loss with macular edema and exudative maculopathy (if visual acuity is 6/9 or better), which otherwise reaches 50–70% after 5 years [48–50]. It has been calculated that screening and treating diabetic retinopathy would prevent 56% of blind registrations resulting from this condition.
Diabetic foot disease
Amputation of a limb remains an important and expensive health problem in the diabetic population, with the elderly being particularly affected [51]. A Dutch study [52] identified increasing age and a higher level of amputation as important factors that increased both the duration and costs of hospitalization (estimated at over £10000 per hospitalization, lasting an average of 42 days). The 3-year survival following lower-extremity amputation is about 50% [53]; in about 70% of cases, amputation is precipitated by foot ulceration [54]. The principal antecedents include peripheral vascular disease, sensorimotor and autonomic neuropathy, limited joint mobility (which especially prevents older people from inspecting their feet) and high foot pressures (Table 54.3) [51]. Most elderly people with diabetes are at increased risk of developing foot ulcers. Peripheral sensorimotor neuropathy, the primary cause or contributory factor in most cases, becomes more common with increasing age and affects 25% of patients with T2DM aged 80 years or more [55]. As well as the common symptoms of numbness, neurogenic pain, “pins and needles” and hyperesthesia (ail typically worse at night), peripheral neuropathy often causes gait disturbances, falls and other foot injuries. Concomitant visual loss worsens the situation [56]. A trivial foot injury in a patient with severe neuropathy can eventually lead to Charcot arthropathy; most of these patients have had diabetes for at least 10 years, and many are elderly (see Chapter 44).
Table 54.3 Risk factors for foot ulceration in the elderly.
| Peripheral sensorimotor neuropathy Automatic neuropathy Peripheral vascular disease Limited joint mobility Foot pressure abnormalities, including deformity Previous foot problems Visual loss History of alcohol abuse |
Treatment of painful neuropathy in older patients is often difficult. Recent-onset symptoms may remit with improved metabolic control, but pain associated with loss of sensation and of greater than 6 months’ duration generally requires specific therapy. Localized pain may respond to topical application of capsaicin cream (0.075%), which depletes pain fibers of the neurotransmitter, substance P. Other drugs include tricyclic antidepressants (e.g. amitriptyline) and antiepileptics, such as carbamazepine and gabapentin (now licensed for this indication). Alternative treatments include transcutaneous nerve stimulation or acupuncture, as described in Chapter 38.
Ischemia, secondary to PVD and perhaps microcirculatory disturbances [57], is the other main contributor to diabetic foot ulceration. Macrovascular disease becomes more common with advancing age. As discussed in Chapter 39, atheromatous lesions in diabetes are more diffuse and involve vessels below the knee more often than in individuals without diabetes. Medial arterial calcification is common, especially in association with somatosensory and autonomic neuropathy; this change can affect Doppler ultrasound measurements of blood pressure in the foot and cause a misleadingly high ankle pressure index.
As in younger patients, many older people with diabetes and critical or worsening limb ischemia, or ischemic ulcers that are slow to heal, will benefit from surgical revascularization (angioplasty or arterial reconstruction). Appropriate cases should be referred early for surgery, ideally through joint protocols developed by the diabetes specialist and the vascular surgeon [58]. A reasonable life expectancy is considered important by surgeons, as concomitant cardiac and cerebrovascular disease kill 50% of patients within 5 years [59]. Following proximal arterial reconstruction, the 5-year patency averages 70% and may exceed life expectancy in patients with major co-morbidities; for distal reconstruction surgery, 5-year limb salvage rates approach 85% [59].
Strategies to prevent diabetic foot ulceration are based on a multidisciplinary approach to identifying, educating and treating high-risk patients; as discussed in Chapter 44, these measures can effectively reduce diabetes-related amputation rates. Many elderly patients have great difficulty in performing the most basic routine foot care [60], often because of poor vision and reduced mobility. In such cases, spouses and other carers must be involved to prevent and treat foot lesions. Education needs to be concise and repeated regularly; video presentations may also be helpful.
Coronary heart disease and arterial disease
Angina, MI, heart failure, stroke and intermittent claudication are substantially more common in elderly subjects with diabetes than in age-matched control groups without diabetes. Mortality is particularly high following MI, especially because of acute pump failure and later onset of left ventricular failure.
Many elderly patients with diabetes may present with atypical symptoms, including “silent ischemia” (which carries a worse prognosis than in the non-diabetic population); even MI may be painless and present non-specifically as a fall, breathlessness, malaise or hypotension.
Investigation and management of cardiovascular disease is covered in detail in Chapter 41.
Erectile dysfunction
Erectile dysfunction becomes more common in older adults and occurs earlier and more commonly in men with diabetes after 60 years of age; 55–95% of men with diabetes are affected, compared with 50% of their counterparts without diabetes [61]. It is often neglected, or attributed to a diminished quality of life or depressive illness. Vasculopathy, autonomic neuropathy, hormonal dysregulation, endothelial dysfunction and psychogenic factors have all been implicated, as have certain drugs (notably cimetidine, betablockers and spironolactone) and a high alcohol intake.
Investigation should generally proceed as for younger men, beginning with an interview with the patient and his partner, where appropriate. For many older patients, extensive testing is often avoided. Treatments include oral sildenafil (which achieved erections in 67% of elderly men in one study [61]), a vacuum tumescence device, misoprostol urethral pellets and self-administered intracorporeal injection of vasoactive drugs (e.g. prostaglandin E1). The latter is relatively successful, but up to 50% of men eventually discontinue because of pain or loss of effect or interest [62].
Mental illness in elderly people with diabetes
Cognitive impairment and dementia
Diabetes and cognitive dysfunction are related and have evoked some interest over the last decade (Table 54.4). Impaired cognitive function has been demonstrated in elderly subjects with diabetes, but these studies were mostly not population-based, excluded subjects with dementia and generally used a large battery of tests to show the deficit [63]. Community-based studies in the UK (Melton Mowbray [64]. Nottingham [43] and South Wales [65]) have shown worse cognitive function in elderly subjects with diabetes, using simple instruments such as the Folstein Mini-Mental State Examination (MMSE), Hodkinson’s Abbreviated Mental Test (AMT), and the Clock Test. These are easily learned, bedside screening tests of mental status which test several cognitive domains such as memory, orientation, calculation, language and, in the case of the MMSE and Clock Tests, also test planning skills and visuospatial function.
Table 54.4 Background to relationship between diabetes and cognitive disorders. Reproduced from European Diabetes Working Party for Older People [92], with permission.
| Professional and public concern about the impact of diabetes on cognition Long-term influence of hyperglycemia and hypoglycemia on cerebral function unknown Pathophysiologic mechanisms involved uncertain, but may involve both vascular, inflammatory and neuronal mechanisms No current agreement on the most optimum method to detect or assess cognitive deficits in diabetes Clinical relevance of the changes observed uncertain |
Impaired glucose tolerance has also been shown to be associated with cognitive dysfunction [66]. It has been suggested that certain components of the metabolic syndrome (hyperglycemia, dyslipidemia, hypertension) may each contribute to memory disturbance in T2DM [67], and that hyperinsulinemia is associated with decreased cognitive function and dementia in women [68]. The Rochester study [69] has demonstrated that the overall risk of dementia is significantly increased for both men and women with T2DM; excess risk for Alzheimer disease achieved significance in men only. Poor glucose control may be associated with cognitive impairment, which recovers following improvement in glycemic control [70]. In other cases, vascular or “mixed” dementias are probably responsible.
Cognitive dysfunction in older subjects with diabetes has wide implications including increased hospitalization, less ability for self-care, reduced likelihood of specialist follow-up and increased risk of institutionalization [71]. Impaired cognitive function should be borne in mind when treating elderly subjects with diabetes, as it has implications for their safe treatment; it may cause difficulty with glycemic control because of erratic taking of diet and medication, including hypoglycemia when the patient forgets earlier administration of hypoglycemic medication and takes more.
Depression
Depression in diabetes is a serious co-morbidity associated with poor outcome and high health care expenditure (see Chapter 55). The presence of a major depressive disorder significantly increases the risk of diabetes [72], this association being apparently independent of age, gender or coexistent chronic disease [73]. Moreover, depression was the single most important indicator of subsequent death in a group of people with diabetes admitted into hospital [74]. Failure to recognize depression can be serious, as this is a long-term life-threatening disabling illness that can significantly damage quality of life. It is also associated with worsening diabetic control [75] and decreased treatment compliance (see Chapter 55) [76].
The relationship between diabetes and depression is complex and may result from the presence of a chronic medical condition in a susceptible individual. There are also complex neuroendocrine and cytokine changes in both conditions that may provide an explanation to link these two conditions (see Chapter 55).
Diabetes and depression share similar symptomatology (e.g. fatigue, irritability and sexual dysfunction). This may delay or confuse the diagnosis, although the commonly used diagnostic assessment scales are unlikely to be invalidated. Enquiries about well-being, sleep, appetite and weight loss should be part of the routine history, with a more comprehensive psychiatric evaluation if appropriate. A Geriatric Depression Scale (GDS) score of >5 can be regarded as indicative of probable depression. 77]. While the GDS-15 may be the scale of choice for older people without cognitive impairment, it does not perform as well in those with dementia. Depression in diabetes can be treated successfully with pharmacotherapy, and/or psychologic therapy, but blood glucose levels should be monitored closely especially with pharmacotherapy. Goals for treating patients with depression and diabetes are twofold:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







