- An estimated 700 000 children have diabetes worldwide; 100 000 new cases are diagnosed annually; the incidence has been increasing by up to 5% annually, over decades.
- Type 1a (autoimmune) diabetes mellitus (T1aDM) accounts for almost all cases in children younger than 10 years, for >90% among older children of European ancestry and for 20–70% of diabetes in children older than 10 years from other ethnic groups.
- The presence of two or more of the islet autoantibodies predicts the development of diabetes in those less than 10 years, in most cases; despite enormous research effort, prevention of T1aDM is still elusive.
- Diabetes in children is diagnosed based on polyuria, polydipsia, weight loss, fatigue and random blood glucose >200mg/dL (11.1 mmol/L). Oral glucose tolerance testing is rarely needed.
- Diabetic ketoacidosis (DKA) in a thin dehydrated child with Kussmaul breathing, abdominal pain, vomiting and impaired neurologic status is present at diagnosis in fewer than 30% of cases in developed countries today.
- There are significant differences in management of DKA in children, compared with adults, with the primary focus on prevention of cerebral edema.
- After diagnosis, childhood diabetes is managed in the outpatient setting by a team including a pediatrician specializing in diabetes, a diabetes nurse educator, a dietitian, a pediatric social worker and/or a pediatric psychologist trained in childhood diabetes.
- In-depth initial and continuing education of parents and patients in the self-management of diabetes is the cornerstone of lowering the risk of acute and long-term complications.
- Insulin pump or basal bolus multiple daily injections are the standard of insulin therapy in children.
- Nutrition planning should be based on carbohydrate counting or exchange system; the macronutrient and micronutrient composition of the diabetic diet is similar to that for healthy children without diabetes.
- While regular exercise is recommended for all children with diabetes, it requires careful planning of insulin and nutritional management to prevent severe hypoglycemia.
- Severe hypoglycemia is largely preventable, but is still the most common serious complication of childhood diabetes.
- Health care providers should equip families with the tools necessary to avoid dehydration, uncontrolled hyperglycemia or DKA, and hypoglycemia during routine infections.
- HbA1c levels below 58 mmol/mol (<7.5%) are the target currently recommended and achievable by most children and adolescents; the gap between recommended and actual HbA1c levels is the widest among adolescents.
- Age-specific psychologic care should include screening for and treatment of family dysfunction, developmental maladjustments, communication problems, disordered eating and sleep patterns, as well as psychiatric disorders both in the patients and their care providers.
- All children with diabetes should be screened at an appropriate age and duration of diabetes for dyslipidemia, microalbuminuria, elevated blood pressure, retinopathy, celiac, thyroid and Addison disease.
Spectrum of diabetes in children
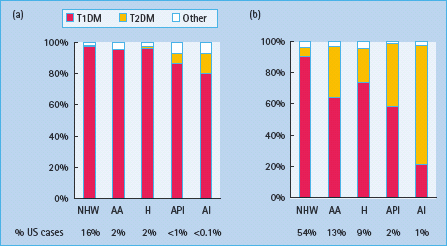
In Europe and North America, 1 in 300 children develops diabetes by the age of 20 years. While the rates are lower elsewhere, there are an estimated 700 000 children with diabetes worldwide and 100 000 new cases are diagnosed annually. Diabetes is a heterogeneous disease at any age. Newborn babies and infants rarely develop the disease (1 in 250 000 in those younger than 6 months) and the etiology is not autoimmune, but usually monogenic (see Chapter 15). From the age of 9 months to 10 years, almost all diabetes is caused by islet autoimmunity (see Chapter 9). Type 1a (autoimmune) diabetes mellitus (T1aDM) accounts also for more than 90% of the cases among older children of European ancestry, but in other ethnic groups 20–70% of older children may have type 2 diabetes mellitus (T2DM; Figure 51.1). With the increasing prevalence of obesity in the general population, a significant proportion of children with T1aDM present with a phenotype that masquerades for T2DM. Measurement of autoantibodies to insulin, GAD65, IA-2 and ZnT8 at diagnosis, C peptide after the initial metabolic stabilization and HLA-DR,DQ typing may be necessary to assess appropriate long-term treatment.
Figure 51.1 Type-specific proportions of prevalent cases of diabetes in the US population, according to age group (a, 0–9 years; b, 10–19 years) and race/ ethnicity. AA, African American; AI, American Indian; API, Asian/Pacific Islander; H, Hispanic; NHW, Non-Hispanic White. Adapted from SEARCH for Diabetes in Youth Study, 2001 [30] .
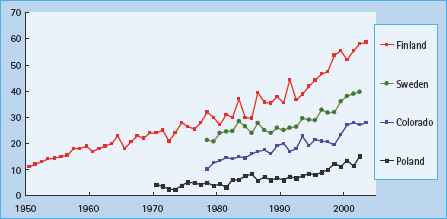
This chapter focuses on the practical aspects of the management of T1aDM in children, while Chapter 52 addresses management of diabetes in adolescents and transition to adult care. For type 1b diabetes (clinical presentation as in T1aDM, but absent islet autoantibodies) and monogenic diabetes see Chapters 9b and 15, respectively. The epidemiology and etiology of diabetes are addressed in Chapters 3 and 9, respectively. Briefly, T1aDM is caused by the interplay of genetic and environmental factors. The initial step – development of islet autoimmunity marked by the presence of islet autoantibodies – is believed to be driven by one or more environmental triggers [1], Over the past 40 years, the incidence of childhood T1DM – worldwide has increased by 3–5% annually (Figure 51.2). Elimination of the environmental trigger(s) responsible for this epidemic would be the most efficient approach to primary prevention; however, there is lack of consensus about which environmental factors initiate and promote islet autoimmunity. Efforts to prevent T1DM have been recently reviewed else where [2]. After the initiation of islet autoimmunity, most patients have a long preclinical period that offers an opportunity for secondary prevention of the progression to clinical diabetes. The presence of more than one of the autoantibodies combined with susceptibility HLA-DR,DQ genotypes identifies those at high-risk of developing diabetes. There may be a “point of no return” in the autoimmune destruction of the islets, rendering some interventions effective only at the earlier stages of the process. Once tolerance is broken to more than one of islet autoantigens, most individuals progress to diabetes within 10 years. A period of mild asymptomatic hyperglycemia, detectable by oral glucose tolerance testing (OGTT) [3] or HbA1c [4], may precede by months or years overt insulin dependence among persons with islet autoantibodies. Intervention at this “dysglycemic” stage may also theoretically preserve endogenous insulin secretion and prevent the acute and long-term complications of T1DM. Preservation or regaining of residual insulin secretion after diagnosis of diabetes might also help, but the immunomodulatory agents used so far in tertiary prevention may carry unacceptable long-term risks.
Manifestation, diagnosis and initial treatment
Clinical presentation and diagnosis
The cardinal symptoms at the diagnosis of diabetes include polyuria (96% of children, often with nocturia or bed-wetting), polydipsia, weight loss (61%) and fatigue [5]. The classic presentation of diabetic ketoacidosis (DKA) in a thin dehydrated child with Kussmaul breathing, abdominal pain, vomiting and impaired neurologic status affects fewer than 30% of cases presenting in developed countries today [5,6]. with the increasing community recognition of diabetes, most children present with milder hyperglycemia of shorter duration; however, 75% of the children (63% below age 5) have the symptoms for more than 2 weeks, suggesting that the diagnosis could be made earlier in many cases. A young child may have a less specific presentation, for example with vomiting or rapid breathing during the course of an infection. Diabetes should always be considered in ill children; urine or blood testing for glucose and ketones leads to an early diagnosis and may prevent DKA and hospitalization. Nearly all patients admitted with severe DKA have been seen hours or days earlier by health care providers who missed the diagnosis.
While most children do not require intravenous fluids or insulin infusion at the diagnosis of diabetes, many are hospitalized for a few days. These hospitalizations could be avoided if safe outpatient alternatives and adequate reimbursement existed for this initial care. For instance, the availability of an outpatient care center has helped to decrease hospitalization at diagnosis from 88% in 1978–1982 to 46% in 1998–2001, with the proportion of hospitalizations secondary to DKA increasing from 44% to 63% [6].
The diagnostic criteria are the same in children and adults (see Chapter 2); however, most children are quite symptomatic and do not require an extensive work-up. In a symptomatic child, plasma glucose ≥11.1mmol/L (200mg/dL) at any time of day, without regard to time since the last meal, or fasting plasma glucose ≥7.0 mmol/L (126 mg/dL) is diagnostic. Blood glucose results obtained using a glucose meter should be immediately confirmed in a laboratory, before the initiation of insulin treatment. By contrast, if marked hyperglycemia and blood or urinary ketones are present, treatment is urgent; waiting another day to confirm the diagnosis may be dangerous if DKA is allowed to develop. In a well child, the diagnosis must not be based on a single plasma glucose test or borderline results obtained using a glucose meter. In such cases, the authors check the HbA1c level; if this is normal, further monitoring of the fasting and/or 2-hour post-prandial blood glucose is recommended for several days. In children progressing to overt diabetes, hyperglycemia after dinner is usually the initial abnormality detectable by self-monitoring of blood glucose at home. OGTT should not be performed if fasting, random or post-prandial criteria are met as it is unnecessary and excessive hyperglycemia can result.
Hyperglycemia detected incidentally or during acute infection, trauma or other illness may be transient, especially if typical symptoms of diabetes are absent or equivocal. Children with transient hyperglycemia may be more likely to develop diabetes, but the reported progression rates vary from 0 to 32%. Testing for islet autoantibodies helps to rule in diabetes in cases with mild presentation; however, it is important to consider that the quality of commercial assays for islet autoantibodies varies widely and testing for at least three autoantibodies (to insulin, GAD65 and IA-2) provides 80% predictive value.
Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) may also be detected in a child with islet autoimmunity progressing to overt diabetes [3]. OGTT is rarely needed in prepubertal children, however, except to reassure the family that a metabolic decompensation is not imminent. In older children, especially obese teenagers with equivocal symptoms, the OGTT may have a role in the earlier diagnosis of T2DM, IGT and IFG.
Diabetic ketoacidosis
The clinical presentation of DKA includes abdominal pain, nausea and vomiting which can mimic an acute abdomen. The patients are mildly to moderately dehydrated (5–10%), may have Kussmaul respiration and become progressively somnolent and obtunded.
DKA results from absolute or relative deficiency of circulating insulin and a corresponding increase in the levels of counter-regulatory hormones, such as catecholamines, cortisol, glucagon and growth hormone (see Chapter 34). This combination leads to a catabolic state with increased glucose production by the liver and kidneys, increased lipolysis, ketogenesis with ketonemia and metabolic acidosis. Absolute insulin deficiency occurs in patients with previously undiagnosed T1DM or in established patients with omission of or inadequate insulin regimens. Relative insulin deficiency occurs during acute illness and stress if the increase in counter-regulatory hormones is not balanced by an appropriate increase in insulin dosage. The severity of DKA is categorized by the degree of acidosis:
- Mild: venous pH 7.2–7.3 or bicarbonate <15mmol/L
- Moderate: venous pH 7.1–7.2 or bicarbonate <10mmol/L
- Severe: venous pH <7.1 or bicarbonate <5mmol/L
Diabetic ketoacidosis at diagnosis of diabetes
In the USA, patients younger than 20 years with a clinical diagnosis of T1DM and T2DM presented in DKA in 29% and 10% of the cases, respectively [6]. In Europe, the rates for T1DM cases ranged 15–67% correlating inversely with the local incidence of T1DM [7]. DKA is more often found among younger children and in children with lower socioeconomic status who encounter barriers in accessing medical care [6]. Intensive community intervention to raise awareness of the signs and symptoms of childhood diabetes among school teachers and primary care providers may help to reduce the prevalence of DKA at diagnosis [8].
Diabetic ketoacidosis in established patients
In a large cohort of children with established T1DM, on average, eight patients per 100 developed DKA every year [9]; however, nearly 60% of the DKA episodes occurred in 5% of children with recurrent events. Recurrent DKA was predicted by poor metabolic control, previous episodes of DKA, psychiatric and eating disorders, difficult family or social circumstances or limited access to medical care.
Treatment of ketoacidosis
Hydration status should be assessed and fluid deficit and osmolality calculated to help guide fluid and electrolyte replacement. Serum electrolytes, glucose, blood urea nitrogen (BUN), creatinine, calcium, magnesium, phosphorous and blood gas testing should be repeated every 2–4 hours or more frequently in severe cases. The calculations are shown below.
Patients with DKA have a 5–10% deficit in extracellular fluid volume which has developed slowly. Avoid rapid or overzealous fluid replacement. Initial volume expansion should occur over the first 1–2 hours with IV infusion of 10–20 mL/kg normal saline (0.9%) or Ringer solution. The bolus rarely needs to be repeated and should not exceed a total of 40 mL/kg over the first 4 hours of treatment. Subsequent fluid deficit replacement should occur over the next 48 hours using 0.5–0.75 normal saline 0.9%. Once blood glucose concentrations reach 250 mg/dL (14 mmol/L), 5–10% dextrose should be added to the IV solution to maintain the blood glucose concentration between 150 and 250 mg/dL (8–14 mmol/L) and avoid saline overload and hyperchloremic acidosis.
Continuous IV infusion of soluble human insulin at a dose of 0.1 units/kg/hour should commence after the patient has received initial volume expansion. An IV bolus of insulin is unnecessary and may increase the risk of cerebral edema [10]. The insulin infusion should allow for a gradual decrease in blood glucose concentration by 50–100 mg/dL/hour. If the blood glucose levels decrease too quickly or become too low before acidosis has resolved, IV dextrose concentration may be increased to 12.5% to prevent hypoglycemia while continuing to correct the metabolic acidosis with insulin. Unless the patient is truly hypoglycemic, the insulin infusion rate should not be decreased to less than 0.05 U/kg/hour as this is likely to prolong the time needed to suppress ketogenesis. Bedside monitoring of blood ketones (β-hydroxybutyrate) is more helpful than blood gases in adjusting insulin and glucose infusion rates.
Total body potassium is usually depleted, but serum levels at presentation may be normal or high secondary to efflux of intracellular potassium into the extracellular space in the presence of acidosis. Once the serum potassium is found to be normal or low, and urine output is confirmed, IV fluids should include 20–40 mEq/L potassium in the form of K acetate, K2HPO4 or a combination of these. No more than half of the potassium replacement should be given as K2HPO4 because excessive phosphorous administration may result in hypocalcemia following the suppression of parathyroid hormone.
Similar to serum potassium levels, serum phosphorus levels may be initially elevated only to fall rapidly during treatment of DKA. Clinical problems resulting from low phosphorus levels have not been substantiated; however, severe hypophosphatemia (<1 mg/dL) should be treated. If hypocalcemia develops, administration of phosphate should be decreased or stopped.
Severe acidosis is reversible with fluid and insulin replacement. Bicarbonate therapy may paradoxically cause CNS acidosis and hypokalemia from rapid correction of acidosis and is a risk factor for cerebral edema. Bicarbonate therapy is not recommended unless the acidosis is profound (pH <6.9) and likely to interfere adversely with the action of epinephrine during resuscitation. If bicarbonate is considered necessary, 1–2 mmol/kg can be cautiously administered over 60 minutes.
Cerebral edema
Neurologic status must be monitored at frequent and regular intervals. Subclinical cerebral edema occurs in most children with DKA. Severe clinical edema affects 0.5–1% of the children and is fatal in over 20% [10]. Typically, cerebral edema occurs at 4–12 hours, but has been reported as late as 24–28 hours after the initiation of IV fluid treatment. Potential risk factors for symptomatic cerebral edema in children include:
- More profound dehydration, hyperventilation and acidosis at presentation;
- Bicarbonate therapy;
- Excessive and rapid fluid administration, especially if initial serum osmolality is >320mOsm/L;
- Failure of serum sodium to rise as hyperglycemia resolves; and
- Initial IV insulin bolus or too early initiation of insulin infusion.
Signs and symptoms of cerebral edema include headache, change in mental status or behavior, incontinence, focal neurologic findings, sudden normalization of heart rate in a previously tachycardic dehydrated patient or a worsening clinical course in a patient with improved laboratory values. Bradycardia, hypertension and irregular respiration (Cushing triad) are signs of greatly increased intracranial pressure. Early intervention is essential. Treatment includes administration of mannitol (1 g/kg over 30 minutes), decreasing fluid rate to 75% or less of maintenance rate, and elevation of head of bed. Mannitol therapy may need to be repeated. Intravenous hypertonic saline has also been used as an alternative to mannitol. Radiographic studies (such as head CT) should be obtained after rather than during treatment of cerebral edema.
Frequent monitoring of blood glucose levels should prevent hypoglycemia. For acute hypoglycemic episodes while on continuous insulin infusion, the insulin infusion may be temporarily discontinued for up to 15 minutes if necessary.
Transition to subcutaneous insulin regimen
Patients may be transitioned to an appropriate subcutaneous insulin regimen once DKA has resolved and they are able to eat. To prevent rebound hyperglycemia, the insulin infusion should not be discontinued until 15–30 minutes after the first subcutaneous injection of rapid-acting insulin has been administered. Long-acting insulin analogs achieve therapeutic levels able to replace insulin infusion 4–6 hours after the subcutaneous injection. Bedside monitoring of blood ketones helps to titrate insulin dose and prevent a relapse.
Pediatric ambulatory diabetes care
Diabetes is primarily managed in the outpatient setting by a team including a pediatrician specializing in diabetes, a diabetes nurse educator, a dietitian, a pediatric social worker trained in childhood diabetes and/or a pediatric psychologist with knowledge of childhood diabetes and chronic illness. In communities with low population density and low prevalence of childhood diabetes, such a care team may not be available and care will primarily be provided by the child’s primary care physician. In these instances, these physicians should work closely with and have access to a regional diabetes care team.
Health care providers and the diabetes care team must always be cognizant of and sensitive to the cultural needs and barriers to care that may arise with minority children of recent immigrants. Interpreters should be utilized when needed.
Initial education
Initial education should provide a basic understanding of the pathophysiology of diabetes and its treatment to ensure that families feel confident in providing diabetes care at home (Table 51.1) [11].
Table 51.1 Initial education curriculum.
| An explanation of how the diagnosis was made and reason for symptoms Discussion regarding normal blood glucose levels and targets, the need for immediate insulin treatment and its mechanism of action Practical skills including how to draw up and administer insulin, blood glucose testing, blood and urine ketone testing Basic dietary guidelines Simple explanation of symptoms and management of hypoglycemia Diabetes at school Importance of medical alert identification Psychologic adjustment to the diagnosis Emergency telephone contacts |
At many institutions, initial education at time of diagnosis occurs in an inpatient setting regardless of whether or not the child presents acutely ill in DKA. In some centers with appropriate outpatient resources, initial diabetes education and initiation of insulin therapy can occur in the ambulatory setting which has been shown to be cost effective.
Continuing education
In the first 6 months following diagnosis, close contact in the form of frequent outpatient visits, home visits, telephone communication and other methods of communication is essential for addressing the frequently changing requirements during this time (Table 51.2) [11].
Table 51.2 Continuing education curriculum.
| Insulin types, actions and adjustments based on self-monitoring of blood glucose Monitoring and treatment goals Mechanisms for coping with and adjusting to the diagnosis of diabetes Nutrition, including carbohydrate counting Management of diabetes during illness Management of hyperglycemia and ketosis and prevention of DKA Prevention, recognition and management of hypoglycemia Exercise, travel and holiday planning Microvascular and macrovascular complications of diabetes and their prevention Up-to-date information on research in diabetes and new therapies and technologies in diabetes care when age-appropriate, discuss diabetes and driving, smoking, alcohol, drugs, college and employment |
DKA, diabetic ketoacidosis
Diabetes education is a continuous process and must be repeated to be effective. It must be adapted and appropriate to the age of the child. Infants and toddlers often have unpredictable eating and activity patterns. There is often more difficulty in distinguishing normal behavior from mood swings related to hypoglycemia or hyperglycemia. Needle phobia can present a significant issue with the perception of pain inflicted by the care-giver. Hypoglycemia is more common in this age group and the prevention, recognition and management of hypoglycemia is a priority. School-aged children will have increased understanding and involvement with their diabetes management. Providers should address school-aged children directly in addition to speaking with their parents or care providers. Education includes monitoring of blood glucose levels and injections at school, particularly during meal times, exercise and extracurricular activities. There should be increased recognition and awareness of hypoglycemic symptoms. Education should also focus on age-appropriate step-wise handover of diabetes responsibilities. This becomes particularly important in adolescence during which there is a critical balance between promoting independent responsible management of diabetes while maintaining parental involvement.
Once established, it is common practice for children to be seen in the ambulatory setting at least every 3 months; visits should be more often if the patient does not meet the treatment goals or intensifies treatment, for example if insulin pump treatment is initiated. During these visits, overall health and well-being is assessed, growth and vital signs are monitored, and a physical examination is performed. There should be routine screening for diabetes-associated complications and co-morbidities. Blood glucose records, including a check of HbA1c, medications and school plans are reviewed. This will allow for the insulin doses to be adjusted and provide a template for continued diabetes education.
The dietitian may review dietary habits and provide ongoing nutrition education as needed. The social worker or psychologist assesses and monitors psychosocial problems and family dynamics and the impact of diabetes care. At the conclusion of these visits, an individualized plan should be developed for each child and their family and a written copy of this plan should be provided.
The advent of new technology including downloadable glucometers, insulin pumps and continuous glucose sensors has made it increasingly possible for the diabetes care team to gain insight into home management of diabetes; however, this should not replace self-monitoring and regular review of blood glucose data at home by the patient and their family.
Diabetes management in school
Children with diabetes spend a significant portion of their day in school; therefore, diabetes management in school is a critical portion of their diabetes management plan [12]. The child has the right to receive adult support for diabetes care from school personnel during school hours, outdoor school activities and school-sponsored events away from school. School personnel must be trained to provide or supervise all diabetes care prescribed by the diabetes team and be supportive of providing diabetes care and encourage diabetes management during school hours, including:
- Insulin administration by injection or with an insulin pump;
- Testing blood glucose in young children and older, newly diagnosed children and adolescents until they are capable of performing the task independently; and
- Identification and treatment for hypoglycemia, both mild–moderate and severe.
Children with diabetes should have a school health plan in place. The health plan should include contact information for the child’s family as well as their diabetes care providers. It should also contain information regarding routine management of diabetes (blood sugar monitoring, insulin administration and dosing, snack times) and an emergency plan for management of hypoglycemia and hyperglycemia. Issues specific to insulin pumps include remembering to activate insulin bolus with food, disconnecting the pump during vigorous exercise or in the event of severe hypoglycemia, pump failures and pump alarms.
Extracurricular activities are an important component of a child’s school experience and children with diabetes should be allowed to participate and their needs accommodated accordingly. Field trips, field days and overnight trips often require advanced planning, but a child’s diabetes should never be a cause for exclusion from any school-sanctioned activity.
Insulin treatment
The overarching goal of insulin replacement is to provide just enough insulin at an appropriate time to provide sufficient basal insulin levels as well as higher insulin levels after meals [13]. The choice of insulin regimen depends on the individual’s age, duration of diabetes, dietary and activity patterns, ability to cope and metabolic targets. Patient and family preferences should also be respected.
Insulin pump therapy
Insulin pump therapy is the best way to restore the body’s physiologic insulin profile. The pump delivers a variable programmed basal rate that corresponds to the diurnal variation in insulin needs. Prepubertal children require a higher basal rate in the early part of night, while post-pubertal patients who experience the “dawn phenomenon” require higher rates in the morning.
The user initiates bolus doses before meals and to correct hyperglycemia. Most pumps can receive wireless transmission of test results from glucose meters, but the patient or caregiver must still manually enter the amount of carbohydrate being consumed. The pump calculates the amount of insulin needed for a meal or correction based on previously entered variables which include: insulin: carbohydrate ratios, insulin sensitivity factor, glycemic target and duration of insulin action (set at 2–8 hours to protect from accumulating too much insulin). The user may override the suggestion or press a button to initiate the bolus.
Rapid-acting insulin analogs perform better in pumps than regular insulin, both in terms of mimicking the first-phase insulin release after meal and avoidance of post-prandial hypoglycemia. Even with the analogs, however, insulin has to be administered at least 10–15 minutes before meal to reach effective levels in time. A longer lead time may be needed if preprandial blood glucose is higher than 150mg/dL. Young children, picky eaters and disorganized patients may struggle with these requirements. In addition, they are often unable to predict the size of the meal. In these cases, one may administer half of the usual meal bolus in advance, with the other half, if needed, after meal. Compliance problems include infrequent blood glucose testing, not reacting to elevated blood glucose, incorrect carbohydrate counting or missing the boluses altogether.
Patients and their families must be instructed on troubleshooting and treatment of hyperglycemia, particularly if ketones are present, as this may be an indication of a pump malfunction. If the flow of insulin becomes interrupted, ketonemia will develop within 4 hours; this is particularly dangerous at night as there is no long-acting insulin on board. Syringes should always be available so that insulin may be administered via injection in the event of a pump failure.
Most clinical trials have demonstrated better HbA1c and less severe hypoglycemia with pump therapy, compared with multiple daily injections (MDI). Pump therapy can improve the quality of life in children who have trouble with or fear of injections or who desire greater flexibility in their lifestyle (e.g. with sleeping in, sports or irregular eating). Insulin pumps can be particularly helpful in young children or infants who have multiple meals and snacks and require multiple small doses of rapid-acting insulin. The newer generation of insulin pumps can deliver as little as 0.025 U/hour, but higher rates using diluted insulin may be needed for uninterrupted flow.
Disposable pumps are already available and much smaller “patch” pumps are in development. In the near future, one may expect a “closed-loop” system allowing the insulin pump to be directed automatically by a continuous glucose sensor with minimal human input; however, a number of issues remain to be solved, including the biocompatibility of the sensors and infusion sets, limitations of the systemic versus intraportal administration of insulin, lack of counterbalancing delivery of glucagon and optimal delivery algorithms for various meals and activities.
Currently, the most frequent complications of insulin treatment include failures of insulin delivery because of a displaced or obstructed infusion set, local skin infections and DKA. Insulin pump treatment is significantly more expensive than regimens based on injections. For some patients, pumps may be too difficult to operate or comply with the multiple testing and carbohydrate counting requirements, or may be unacceptable because of body image issues or extreme physical activity (e.g. swimming, contact sports).
Subcutaneous insulin injection regimens
Injections regimens, in order of worsening HbA1c outcomes, include:
- Basal bolus regimen: 40–60% of the total daily dose as basal insulin analog (glargine, detemir) in 1–2 doses a day with rapid-acting insulin analog 10–15 minutes before each meal; soluble human insulin is less preferable and requires administration at least 20–30 minutes prior to each meal.
- Intermediate-acting human insulin twice daily and soluble human insulin 20–30 minutes prior to each meal.
- Two injections daily of a mixture of short or rapid and intermediate-acting insulin before breakfast and before the evening meal.
- Three injections using some variation of the following: a mixture of short or rapid and intermediate-acting insulin before breakfast, rapid or soluble human insulin before the afternoon snack or evening meal, and intermediate or basal/long-acting insulin before bed.
Intermediate-acting insulins such as NPH are often mixed with soluble human (regular) or rapid-acting analogs (aspart, lispro or glulisine). Patients and families should be taught how to mix the insulin properly to avoid contamination. It is generally taught to draw up clear (regular or short-acting) insulin prior before drawing up cloudy insulin (NPH). Per the manufacturer’s instructions, glargine or detemir insulins should not be mixed with any other insulin.
Premixed insulins contain a mixture of regular (or rapid-acting) insulin and NPH insulin in various fixed ratios. These preparations may be useful for children who do not want to draw insulin from separate vials prior to injecting. They may also be useful in reducing the number of injections when compliance is an issue, especially among teenagers. Premixed insulins are also available for use in pen injector devices. The main disadvantage to using premixed insulin preparations is the lack of flexibility in adjusting the separate insulin doses, which is often necessary with varied food intake or during illness or exercise.
Nutrition
Nutritional management in children with diabetes remains a key component of diabetes care and education; if available, a pediatric dietitian should be a part of the diabetes care team. The management does not require a restrictive diet, just a healthy dietary regimen that the children and their families can benefit from. Current guidelines target optimal glycemic control, reduction of cardiovascular risk, psychosocial well-being and family dynamics [14,15]. A thorough dietary history should be obtained including the family’s dietary habits and traditions, the child’s typical meal times and patterns of food intake.
Insulin pump and MDI therapy utilize carbohydrate counting in which the grams of carbohydrate to be eaten are counted and a matching dose of insulin is administered. This plan allows for the most freedom and flexibility in food choices, but it requires expert education and commitment and may not be suitable for many families or situations (e.g. school lunches, teenagers). Exchange planning teaches that it is not necessary to count precise grams. Exchanges are taught as either 10 or 15-g servings of carbohydrate. The exchange plan can enable a more consistent daily intake of carbohydrate. The constant carbohydrate meal plan was used often in the past with insulin regimens based on NPH and regular insulin, where carbohydrate intake and the amount of insulin were kept relatively constant from day to day. It has been perceived as too restrictive and a potential source of conflict.
The use of the glycemic index has been shown to provide additional benefit to glycemic control. Low glycemic index carbohydrate foods, such as wholegrain breads, pasta, temperate fruits and dairy products may lower post-prandial hyperglycemia. A glycemic load approach to predicting the post-prandial blood glucose response, based on the glycemic index of the food and the portion size, has not been fully explored in children. Regardless of which meal plan is chosen, helpful principles are shown in Table 51.3.
Table 51.3 Principles of dietary planning in children with diabetes.
1 Eat a well-balanced diet, with daily energy intake distributed as follows:
|
| 2 Eat meals and snacks at the same time each day, if possible |
3 Use snacks to prevent and treat hypoglycemia, but avoid overtreatment:
|
4 Gauge energy intake to maintain appropriate weight and body mass index:
|
| 5 Recommended fiber intake for children older than 1 year: 2.8–3.4g/MJ; children older than 2 years should eat = (age in years + 5)g/day fiber |
| 6 Avoid foods high in sodium that may increase the risk of hypertension; target salt intake – to less than 6 g/day (sodium chloride) |
| 7 Avoid excessive protein intake (athletes should not require protein supplements) |
| 8 Children with diabetes have the same vitamin and mineral requirements as other healthy children; however, hypovitaminosis D is common and screening and supplementation are recommended |
| 9 There is no evidence of harm from an intake of artificial sweeteners in doses not exceeding acceptable daily intakes |
| 10 Specially labeled diabetic foods are not recommended because they are not necessary, are expensive, are often high in fat and may contain sweeteners with laxative effects. These include the sugar alcohols such as sorbitol |
| 11 while alcohol intake is generally prohibited in youth, teenagers continue to experiment with and sometimes abuse alcohol. Alcohol may induce prolonged hypoglycemia in young people with diabetes (up to 16 hours after drinking). Carbohydrate should be eaten before, during and/or after alcohol intake. It may be also necessary to lower the insulin dose, particularly if exercise is performed during or after drinking (e.g. dancing) |
| 12 Approximately 10% of patients with type 1 diabetes have serologic evidence of celiac disease. Those with positive intestinal biopsy or symptomatic have to be treated with GFD. Products derived from wheat, rye, barley and triticale are eliminated and replaced with potato, rice, soy, tapioca, buckwheat and perhaps oats. while most of the children are asymptomatic, the long-term consequences of untreated celiac autoimmunity may warrant GFD |
GFD, gluten-free diet.
Age-specific advice
Breastfeeding in infants should be encouraged. Insulin pump therapy should be considered, particularly in patients who require very small doses of insulin. Toddlers are often picky eaters and are more likely to eat frequent smaller meals throughout the day; their insulin regimen should match this eating pattern. Food refusal can be a significant source of distress, particularly if an insulin dose has already been administered. In school-aged children, meal plans may need to be adjusted depending on the school schedule. Food intake among teenagers is often chaotic. Breakfasts are skipped and binges may happen at any time of day or night. weight loss or failure to gain weight may be associated with insulin omission for weight control and may be indicative of a disordered eating behavior. while insulin pump or MDI treatment may help some, simplification of the management plan to avoid extreme mismatches between food intake and insulin are sometimes the only viable option.
Exercise
Children with diabetes derive the same health and leisure benefits from exercise as children without diabetes and should be allowed to participate with equal opportunities and equal safety [16]. Physiologically, during exercise in children without diabetes, there is a decrease in pancreatic insulin secretion and an increase in counter-regulatory hormones resulting in an increase in liver glucose production (see Chapter 23). This matches skeletal muscle uptake of glucose during exercise, maintaining stable blood glucose concentrations under most conditions. In children with T1DM, there is no pancreatic regulation of insulin in response to exercise and there may be impaired counter-regulation. These factors combine to increase the risk of hypoglycemia and hyperglycemia during exercise. It is helpful to keep an exercise record noting the most recent insulin dose, timing and type of exercise, blood glucose levels before and after exercise, snacks eaten and the time of any episode of hypoglycemia.
Factors affecting a child’s response to exercise include:
- Duration, type, timing and intensity of activity;
- Overall metabolic control and ambient blood glucose level;
- Type and timing of insulin injections and its absorption;
- Type and timing of food;
- Muscle mass and conditioning; and
- Degree of stress.
Preventing hypoglycemia
Blood glucose levels should be checked before, during and after the exercise. Children should consume carbohydrates prior to exercise, with the amount depending on the blood sugar level prior to exercise and the duration and intensity of exercise. For short duration activity, sports drinks with simple sugars provide optimal absorption and usually prevent hypoglycemia for the next 30–60 minutes. For activity of longer duration, solid foods containing carbohydrates are digested more slowly and should be consumed in addition to a liquid with simple sugars. Extra snacks should always be available to the child during exercise. The child’s coaches and teammates or other responsible adult and peers should be aware of the signs and symptoms of hypoglycemia. Often, children will require adjustments to their insulin dosing when exercise is anticipated. The site of insulin injection should also be taken into account. Exercise increases blood flow in the part of the body being used, increasing insulin absorption if that area is where the insulin injection was administered. For example, prior to running the insulin dose should not be administered in the legs.
Insulin adjustments
For exercise anticipated within the first hour after eating, the dose of rapid-acting insulin before the meal may need to be decreased by 25–75% (depending on the intensity of the exercise) in addition to consuming 10–15 g of fast-acting carbohydrate prior to exercise. For daywl ong activities (such as camps, hiking or skiing), consider a 30–50% reduction in the long-acting insulin dose (or in the basal rate if using an insulin pump) the night before and on the day of the activity. For children using insulin pumps, there are numerous options for insulin adjustments with exercise. If the pump will be worn during exercise, the basal rate can be reduced by 30–50% beginning 30–90 minutes prior to exercise and continuing for up to 30 minutes or longer after the exercise. For some types of activities (e.g. contact sports), children may need to disconnect from the pump and do one of the following:
- Bolus part of the basal insulin to be missed prior to exercise (particularly if the pre-exercise blood sugar level is elevated) and the remainder after exercise;
- Bolus half of the insulin missed while disconnected after exercise;
- Bolus all of the insulin missed while disconnected after exercise.
In general, the pump should not be disconnected for longer than 2 hours. If necessary, the pump should be reconnected briefly and a bolus administered prior to disconnecting again.
Delayed hypoglycemia
Hypoglycemia can occur several hours after exercise secondary to increased glucose transport into the skeletal muscle, the late effect of increased insulin sensitivity, and the delay in replenishing liver and muscle glycogen stores. Blood glucose levels must be monitored for several hours following exercise, at bedtime and sometimes during the night on days with strenuous exercise. Consider a longer lasting snack (containing a solid carbohydrate, protein and fat) at bedtime and reducing the insulin dose as discussed above.
Ketones and exercise
In situations of underinsulinization, whether it be from poor glycemic control or from illness, exercise may be dangerous because of the effect of uninhibited action of the counterregulatory hormones. Children with diabetes should not participate in strenuous exercise if the pre-exercise blood sugar level is high and urine ketones (small or more) or blood ketones (0.5 mmol/L or more) are present.
Hypoglycemia
Hypoglycemia is the most common acute complication in the treatment of T1DM and responsible for a significant proportion of deaths in people with diabetes aged under 40 years of age (see Chapter 33) [17]. while hypoglycemia in persons with diabetes is defined as plasma glucose below 70 mg/dL (4 mmol/L), severe hypoglycemia (seizure or loss of consciousness) usually does not occur until prolonged exposure to levels of 40–50 mg/dL (2.2–2.7 mmol/L) or lower. Severe hypoglycemia happens to one in five children every year on average, but 80% of the events occur among the 20% of children who have recurrent events [6]. Younger age, longer diabetes duration, barriers in access to care and presence of psychiatric disorders or chaotic family environment increase the risk. while lower HbA1c is generally a risk factor for hypoglycemia, appropriate intensive insulin treatment can lower the risk by improving timing of insulin in relationship to food intake and exercise [18].
Signs and symptoms
- Autonomic signs and symptoms: trembling, pounding heart, cold sweatiness, pallor;
- Neuroglycopenic signs and symptoms: difficulty concentrating, blurred or double vision, disturbed color vision, difficulty hearing, slurred speech, poor judgment and confusion, problems with short-term memory, dizziness and unsteady gait, loss of consciousness, seizure, death;
- Behavioral signs and symptoms: irritability, erratic behavior, nightmares;
- Non-specific symptoms: hunger, headache, nausea, tiredness; Early warning signs and symptoms of hypoglycemia are much more difficult to identify in young children.
Treatment
In mild or moderate symptomatic hypoglycemia, after documenting a blood glucose of ≤70 mg/dL (3.9mmol/L):
- Provide immediate oral, rapidly absorbed 5–15 g glucose or sucrose: glucose tablets, “Smarties” or 4oz (100 mL) of sweet drink (juice, soda).
- 1 g glucose should raise the blood glucose by 3 mg/dL (0.17 mmol/L) for the average adult and proportionally more in a child; target the rise of blood glucose level to 100 mg/dL (5.6 mmol/L).
- Retest blood glucose in 10–15 minutes, if no response or inadequate response – repeat as above.
- For initially lower glucose values, as symptoms improve and euglycemia is restored, ingest solid snack or meal (e.g. fruit, bread, cereal) to prevent recurrence.
- Retest blood glucose in 20–30 minutes to confirm that target glucose has been maintained.
In severe hypoglycemia, where the child has an altered mental status and is unable to assist in their care, may be unconscious and/or seizing, urgent treatment with parenteral glucagon or dextrose is required.
Glucagon
Glucagon is given intramuscularly or subcutaneously (10–30μg/kg body weight):
- 0.3 mg for children younger than 6 years
- 0.5 mg for those 6–12 years
- 1 mg for those older than 12 years or heavier than 100lb (45 kg)
Glucagon injection may be repeated in 5–10 minutes, if response was inadequate; however, it is likely to be ineffective after prolonged fasting. Side effects include vomiting and tachycardia.
Dextrose
Dextrose can be given intravenously by trained medical staff if glucagon is unavailable or recovery is inadequate in a hospital setting or by paramedics:
- Intravenous dextrose should be administered slowly over several minutes (e.g. dextrose 10–30% at a dose of 200–500 mg/ kg; dextrose 10% is 100 mg/mL).
- Rapid administration or higher concentration may result in an excessive rate of osmotic change, phlebitis and extensive tissue damage, if extravasated.
Close observation and monitoring of blood glucose is essential because vomiting is common and hypoglycemia may recur. If recurrent hypoglycemia occurs, the child may ultimately require an IV infusion of dextrose 10% at 2–5 mg/kg/minute until stable.
Severe headache and transient paresis lasting up to 24 hours are not uncommon and generally do not require radiologic work-up.
Hypoglycemia unawareness
Hypoglycemia unawareness occurs when there is reduced awareness of the onset of hypoglycemia. A single hypoglycemic episode can lead to hypoglycemia unawareness secondary to a decrease in counter-regulatory responses, but it is usually seen in patients who have multiple periods of blood glucose <70 mg/dL. Avoiding subsequent hypoglycemia for 2–3 weeks may reverse this loss of awareness.
Prevention
Hypoglycemia occurs more frequently:
- When the treatment regimen or lifestyle is altered (increased insulin, less food, more exercise);
- In younger children;
- With lower HbA1c levels;
- When there are frequent low blood glucose levels;
- When there is hypoglycemia unawareness;
- During sleep; or
- After alcohol ingestion.
Patients and families should be aware of the above risk factors so that glucose monitoring and insulin regimens can be changed accordingly. There is an increased risk for hypoglycemia during, immediately after and up to 2–12 hours after exercise. Untreated celiac disease and Addison disease may also increase the risk of hypoglycemia.
Nocturnal hypoglycemia is often asymptomatic and should be suspected if the morning blood glucose is low and/or there are episodes of confusion, nightmares or seizures during the night, or if there is impaired thinking, altered mood or headaches upon awakening. Nocturnal hypoglycemia can be confirmed with blood glucose monitoring during the night and may be prevented by including more protein and fat in the bedtime snack. Care should be taken that this does not occur at the expense of high overnight blood glucose levels.
Studies have shown an association between hypoglycemia and decrease in cognitive functioning in children with T1DM, particularly in children diagnosed before the age of 5–6 years. More recently, there has been increased interest in the potential role of chronic hyperglycemia on cognitive functioning in young children. Even mild–moderate hypoglycemia may impair school functioning and overall well-being. A reduced awareness of hypoglycemia and the risk of injury or accident often lead to a significant fear of hypoglycemia and decreases in insulin dosing, which in turn results in increased HbA1c. Severe hypoglycemia can increase parental and patient’s worry, poor sleep, emergency room visits, hospitalizations, excessive lowering of insulin doses and subsequent worsening of glycemic control. Long-term follow-up of the Diabetes Control and Complications Trial (DCCT) participants has been reassuring that there was no evidence for permanent neurocognitive changes related to hypoglycemia in adolescent and young adult individuals, suggesting that the effect of severe hypoglycemia on long-term neuropsychologic functioning may be age-dependent [19].
Ultimately, hypoglycemia is frequently predictable and should be prevented. Children and their caregivers must be taught to recognize the symptoms of hypoglycemia and treat this immediately and appropriately. Children with diabetes should always carry around a source of rapid-acting glucose and should wear identification noting that they have diabetes. The diabetes care provider should be notified if a child is having recurrent episodes of symptomatic hypoglycemia or if there is hypoglycemia unawareness. This will facilitate discussions to adjust insulin regimens, food intake patterns, blood glucose goals and monitoring. Continuous glucose monitoring helps to detect and to avoid hypoglycemia.
Sick day management
Children with diabetes in good metabolic control should not experience more illness or infections than children without diabetes; however, they will go though their share of routine infections which can be challenging for their caregivers. The influenza vaccine and other routine childhood immunizations are recommended for all children with diabetes.
Health care providers should equip families with the tools necessary to avoid dehydration, uncontrolled hyperglycemia or ketoacidosis, and hypoglycemia. Face to face education and written instructions are important, but most parents require telephone advice when first facing sickness in their child and some may need repeated support. Over time, most parents should be able to manage sick days independently as well as identify appropriate times when to seek help from their diabetes provider or emergency services. Patients should immediately seek medical attention if:
- Blood glucose concentrations continues to rise despite extra insulin;
- Blood glucose concentrations remains persistently below 3.5 mmol/L (70 mg/dL);
- Blood ketones are higher than 1.5 mmol/L or ketonuria is severe and persistent; or
- The child becomes exhausted, confused, dehydrated or develops difficulty in breathing, severe abdominal pain or a severe hypoglycemic reaction.
Missed insulin injection, inactivated insulin or interruption of insulin delivery from pump may lead to “sick days” as well, especially in older children. while treatment is essentially the same as for hyperglycemia in the course of an infection, the differential diagnosis is important for prevention of recurrent events.
Hyperglycemia is seen in many illnesses, particularly those associated with fever, as a result of elevated levels of stress hormones, which promote gluconeogenesis and insulin resistance. Severe illness increases ketone body production secondary to inadequate insulin action or insufficient oral intake of carbohydrates. By contrast, illnesses associated with vomiting and diarrhea can lead to hypoglycemia secondary to decreased food intake, poor absorption and slower gastric emptying.
In general, during illness, blood glucose concentrations must be monitored more frequently – at least every 3–4 hours and more often when blood glucose concentrations are outside the target range (e.g. 80–200 mg/dL). Urinary or blood ketones must be checked at least twice daily and always when blood glucose concentration exceeds 300 mg/dL (17.6 mmol/L). If available, the authors recommend testing blood ketones (β-hydroxybutyrate, using Precision Xtra/Exceed meter) over urine ketone testing as a more specific and timely marker of ketosis:
- The presence of ketones when blood glucose concentrations are persistently elevated above 200 mg/dL (11.1 mmol/L) indicates the need for supplemental insulin and fluids.
- The presence of ketones when blood glucose concentrations are low or normal, especially during gastrointestinal illness, indicate insufficient oral intake of carbohydrates (starvation ketones). In this case, ketones do not reflect insulin deficiency, but rather a physiologic response and may protect the patient from severe hypoglycemia as β-hydroxybutyrate is the only alternative fuel to glucose for the brain. Supplemental insulin is contraindicated as it will likely cause hypoglycemia; the correct treatment includes fluids with glucose.
Insulin therapy must never be stopped during a sick day, although the dose may need to be decreased if the child is vomiting or eating less than usual. A fasting patient still requires approximately 40% of the usual daily insulin dose, as long-acting basal insulin, to cover basic metabolic needs and prevent ketoacidosis; however, infections associated with normal food intake often require an increase of basal insulin by 10–15%. In addition, extra doses of rapid-acting insulin are usually needed to correct hyperglycemia, prevent ketoacidosis and avoid hospital admission. These doses may be repeated every 2–4 hours as needed based on the results of blood glucose and ketone monitoring.
With blood glucose concentrations greater than 200 mg/dL (11.1 mmol/L), the authors recommend:
- Usual high blood glucose correction (e.g. 1 unit of rapid-acting insulin for each 50 mg/dL above 100 mg/dL, if blood ketones <0.6 mmol/L or urine ketones negative/small;
- Injection of rapid-acting insulin in the amount of 10% of the total daily dose, if blood ketones 0.6–1.5 mmol/L or urine ketones are moderate or large;
- Injection of rapid-acting insulin in the amount of 10–20% of the total daily dose if blood ketones >1.5 mmol/L or urine ketones are moderate or large;
- As acidosis is present in most patients with hyperglycemia and blood ketones >3.0mmol/L, this warrants referral to an emergency department.
Patients using insulin pumps who develop hyperglycemia and moderate or large urine ketones (or greater than 1.0 mmol/L blood ketones) must always take into consideration the possibility of an interruption in insulin delivery. If blood glucose levels do not decrease appropriately after an insulin bolus from the pump, the correction bolus of short-acting insulin should be given as injection by pen or syringe and the pump infusion set should be changed. A temporary increase in the basal rate by 20% or more may be required until blood glucose concentrations begin to normalize and ketones clear.
If hypoglycemia <70 mg/dL (<3.9mmol/L) persists and the patient is unable to tolerate any oral intake, an injection of low dose glucagon may reverse the hypoglycemia and enable oral fluid intake to resume. Glucagon is mixed as usual but given using an insulin syringe with the dose being one unit per year of age up to 15 years [20]. Upon mixing with water, glucagon remains stable for at least 48 hours at 4°C. The small dose of glucagon can be repeated every 2–4 hours; however, it is likely to be less effective with prolonged fasting. This dose of glucagon is not to be used for the emergency treatment of severe hypoglycemia.
Hydration status must be followed closely. Fever, hyperglycemia with osmotic diuresis, and ketonuria all increase fluid losses. Households should maintain a supply of sugar and electrolyte containing fluids:
- Oral water is sufficient to prevent dehydration in uncomplicated cases of hyperglycemia.
- If there is an ongoing fluid loss from diarrhea or vomiting, hydration liquids should contain salt in addition to water (e.g. Pedialyte, Rehydralate). These preparations contain 25–30 g/L glucose, 45–90 mEq/L sodium, 30mEq/L bicarbonate and 20–25 mEq/L potassium. Oral rehydration fluid can be made at home by mixing half of a flat teaspoon of salt (∼3g of NaCl = ∼50 mEq sodium), 7 teaspoons of sugar (28g) and (optionally) 100 mL (4 oz) of orange juice into 1 L water.
- If there is difficulty eating or keeping food down and the blood glucose is falling below 200mg/dL (11.1mmol/L), sports drinks should be administered. They contain less electrolytes but higher amounts of glucose (e.g. Gatorade contains 255 g/L glucose, 20 mEq/L sodium, 3 mEq/L bicarbonate and 3 mEq/L potassium).
- If the blood glucose is falling below 100mg/dL (5.6mmol/L), fluids with higher concentration of sugar are recommended (e.g. juice or non-carbonated regular soda containing approximately 70 g glucose per 100 mL. These fluids contain almost no sodium and are not appropriate in large amounts for children with diarrhea.
The required volume of oral fluid replacement is the sum of maintenance volume, deficit and ongoing losses. In practical terms, infants and toddlers with diabetes who vomited more than twice or have multiple loose stools should be referred to an emergency department for evaluation and intravenous fluids. Those with milder symptoms can be given oral fluid therapy at home; small amounts (5 mL) of cold fluids every 5 minutes. Most children with vomiting can by successfully orally rehydrated with persistent gentle encouragement of parents.
Antiemetic medication at home is generally contraindicated, especially in young children, as it may mask acute abdominal processes such as appendicitis, volvulus or intussusception and may have significant adverse effects. Ondansetron (Zofran) can be used to contain vomiting in patients properly evaluated by a physician and selected older children presenting without abdominal pain.
Estimate of 24-hour maintenance fluid volume
Estimation based on age:
|
Estimation based on body weight:
|
| For example, a child weighting 30 kg needs 1000 + 500 + 200 = 1700 mL maintenance water for 24 hours or 70 mL/hour, not counting past or ongoing losses |
Monitoring and goals of diabetes management
Hemoglobin A1c (HbA1c) is the only measure of mid to long-term glycemic control for which robust outcome data are available (see Chapter 25). Elevated HbA1c predicts long-term microvascular and macrovascular complications but has its limitations. In the DCCT, an HbA1c of 53 mmol/mol (7.0%) corresponded to a higher average blood glucose concentration (measured seven times a day) of 192 mg/dL in the conventionally treated patients compared with 163 mg/dL in the intensively treated patients. Consequently, the same HbA1c level conferred significantly higher risk of microvascular complications and hypoglycemia in the conventionally treated patients compared with intensively treated patients [21]. HbA1c can only be one of the measures of optimal glycemic control, along with documented hypoglycemia and quality of life. Ideally, there should be four to six measurements per year in younger children and three to four measurements per year in older children.
Self-monitoring of blood glucose (SMBG) provides immediate and daily documentation of hyperglycemia and hypoglycemia, helps to determine immediate and daily insulin requirements and detects hypoglycemia and assists in its management. Blood glucose is best measured during the night, after the overnight fast, at anticipated peaks and troughs of insulin action, 2 hours after a meal and in association with vigorous sport or exercise – typically 4–6 times a day. The frequency of SMBG is associated with improved HbA1c in patients with T1DM [21]. Patient acceptance of SMBG may be enhanced by including the opportunity for testing alternative sites in addition to the fingertips (e.g. the palm of the hand or the forearm); however, alternative sites may be slower to reflect falling blood glucose.
A logbook or some type of electronic memory device has to be used to record patterns of glycemic control and adjustments to treatment. The record book is useful at the time of consultation and should contain time and date of blood glucose reading, insulin dosage, together with a note of special events (e.g. illness, parties, exercise, menses, hypoglycemic episodes and episodes of elevated blood or urinary ketones).
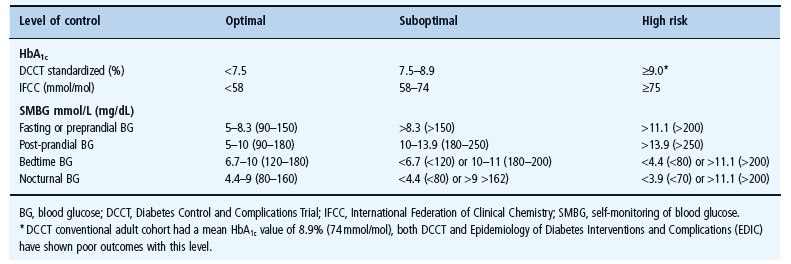
At present, the safest recommendation for glycemic control in children is to achieve the lowest HbA1c that can be sustained without severe hypoglycemia as well as frequent moderate hypoglycemia or prolonged periods of significant hyperglycemia where blood glucose levels exceed 250 mg/dL (14 mmol/L). Each child should have targets individually determined. Targets for HbA1c and SMBG recently proposed by the International Society for Pediatric and Adolescent Diabetes (ISPAD) [21] are summarized in Table 51.4.
Table 51.4 Biochemical targets of glycemic control. These targets are intended as guidelines, each child should have their targets individually determined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree