- Diabetes is associated with an increased overall risk of infections.
- The presence of diabetes also modifies the course of many infections and increases morbidity and mortality.
- Multiple disturbances in innate immunity have a role in the pathogenesis of the increased prevalence of infections in people with diabetes.
- Impaired phagocytosis by neutrophils, macrophages and monocytes, impaired neutrophil chemotaxis and bactericidal activity, and impaired innate cell-mediated immunity appear to be the most important disturbances of the immune system.
- Humoral immunity appears relatively unaffected, hence plasma levels of antibodies and responsiveness to vaccination are relatively unaffected.
- In general, better regulation of the diabetes leads to an improvement in cellular immunity and function.
- Increased skin and mucosal carriage of Staphylcoccus aureus and Candida species may increase risk of infection with these organisms.
- Some microorganisms become more virulent in a high glucose environment; examples include certain Klebsiella serotypes and Burkholderia pseudomallei.
- Viral infections such as hepatitis C are associated with a higher prevalence of diabetes.
- Highly active antiretroviral therapy therapy for HIV/AIDS may also precipitate diabetes.
- Vascular disease such as microangiopathy can further impair the expression of the immune response as well as affecting the overall function of the microcirculation. It is commonly a factor in severe infections such as malignant otitis externa, emphysematous pyelonephritis and necrotizing fasciitis.
- Urinary tract infections and asymptomatic bacteriuria are more common in people with diabetes. Autonomic neuropathy is a common and important underlying factor.
- Skin and soft tissue infections are more common, with the infected diabetic foot as a prime example. Vascular disease and diabetic neuropathy are important underlying factors in the vulnerability of the foot to infection. Skin infection or infections of the external genitalia are common presenting features of diabetes. Necrotizing fasciitis is also associated with diabetes.
- Some uncommon but life-threatening infections occur almost exclusively in people with diabetes. Examples include the rhinocerebral form of mucormycosis, malignant otitis externa, Fournier gangrene and emphysematous forms of cystitis, pyelonephritis and cholecystitis.
- Diabetes increases the risk of tuberculosis and also increases the risk of treatment failure. Unusual or extrapulmonary sites of infection may be important and cavitatory disease more common.
- Other underlying factors that can predispose to infection include renal failure, obesity, need for hospitalization, indwelling catheters and delayed wound healing.
Introduction
People with diabetes develop infections more often than those without diabetes and the course of the infections is also more complicated. Historically, infections have been well recognized as an important cause of death in diabetes and remain a very important cause of morbidity and mortality in people with diabetes. This is particularly true in less well-developed countries and areas, where infections are commonly the first presenting feature of previously unknown diabetes. The infected diabetic foot remains a prime example of this phenomenon despite its potential preventability.
While the association between diabetes and infections is well recognized, the relationships are complex, not always clear-cut and often controversial. Data on the true incidence of certain infections are lacking and a number of factors complicate efforts to assess risk of infections and outcomes. Studies are often retrospective and uncontrolled in nature.
Some infections, which occur predominantly in people with diabetes, are uncommon and inevitably have limited data. Examples include malignant otitis externa, mucormycosis, emphysematous forms of cholecystitis, cystitis and pyelonephritis, and Fournier gangrene.
In the case of more common infections that, while not limited to people with diabetes, have diabetes as a complicating factor, many potential variables make for considerable heterogeneity in the clinical course. Examples of such factors include duration of disease, presence of diabetic complications, glycemic control (both recent and longer term), access to and provision of medical services, and presence or absence of other concurrent illnesses.
A recent carefully controlled study from Utrecht, the Netherlands, has confirmed that, in general terms, patients with type 1 (T1DM) and type 2 diabetes mellitus (T2DM) are at increased risk of lower (but not upper) respiratory tract infection, urinary tract infection and skin and mucous membrane infections [1]. In this study, the well-documented increased risk of urinary infection was extended to include both risk of recurrence in both sexes and risks in males (perhaps explained by prostatitis).
Another recent study, conducted in Ontario, Canada, compared people with diabetes with matched control subjects without diabetes [2]. The investigators calculated the risk ratios, both for contracting an infection and for death from infection. Forty-six percent of all people with diabetes had at least one hospitalization or outpatient visit for infections compared with 38% of those without diabetes, the relative risk ratio being 1.21. The risk ratios for infectious disease-related hospitalization or death were noticeably higher at 2.17 and 1.92, respectively. This may be attributable to increased severity and presence of complications. In the case of hospitalization, it could also reflect a lower threshold on the part of physicians to admit people with diabetes to hospital when they have intercurrent illnesses. A separate study also from Canada, in this case from the Calgary Health Region, has conducted a population-based assessment of severe bloodstream infections requiring intensive care admission. Demographic and chronic conditions that were significant risk factors for acquiring severe bloodstream infection included diabetes, with a relative risk ratio of 5.9. The most common organisms were Staphylococcus aureus, Escherichia coli and Streptococcus pneumoniae [3].
Data from a study by Bertoni et al. [4] suggest that adults with diabetes are at greater risk for infection-related mortality, and that the excess mortality risk may be mediated by cardiovascular disease (CVD). When diabetes was combined with CVD, the relative mortality risk was 3.0 (1.8–5.0), whereas individuals with diabetes without CVD had a risk of 1.0 (0.5–2.2).
Evidence that the presence of diabetes can worsen the outcome or prognosis of infections comes from a number of sources. There is evidence that the presence of T2DM is associated with an increased mortality from community-acquired pneumonia. While much of this may be explained by factors such as age and coexisting comorbid illnesses, admission hyperglycemia has been shown to be a particularly important predictor of death. Also, even in patients without previously diagnosed diabetes, glucose levels in general assume importance [5]. During the outbreaks of severe acute respiratory syndrome (SARS) in 2003 in Toronto, the presence of diabetes was an independent risk factor for poor outcomes (intensive care unit admission, mechanical ventilation and death) with a threefold increase in relative risk [6].
Both host- and organism-specific factors appear to be implicated in the increased susceptibility to particular infections. From the host perspective, defects in innate immunity are important, notably decreased functions (chemotaxis, phagocytosis and killing) of neutrophils, monocytes and macrophages. Other factors include effects of diabetic complications, poor wound healing and the presence of chronic renal failure. Frequent hospitalizations, with the attendant risk of nosocomial infection, can also be contributory.
Infections, as well as leading to considerable morbidity and mortality in people with diabetes, may also precipitate metabolic derangements, producing a bidirectional relationship between hyperglycemic states and infection. Some infections may also be implicated more directly in the etiology of diabetes.
Physicians working in primary care need to have high levels of awareness of the relationships between diabetes and infection, and of the important infections that may be involved. Infections involving the foot, soft tissues, skin and nails, as well as the urinary tract, are of particular importance in the setting of primary care. These infections are commonly encountered in people with diabetes, may be present at diagnosis and may be the presenting feature that leads to the diagnosis of diabetes being suspected. Infections of the foot and skin will receive additional attention elsewhere in this textbook so, in order to avoid duplication, coverage in this chapter is curtailed. This should not be taken as an indication of lack of relative importance, the opposite is the case. The other chapters concerned should be taken as forming part of the overall coverage of the topic of diabetes and infections (see Chapters 44 and 47).
Diabetes, the immune system and host factors
Host immune response
Although the increased susceptibility of people with diabetes to bacterial (and other) infections is well established, the mechanisms remain incompletely understood. Deficiencies in the host innate immune response are apparent and appear more important than changes in adaptive immunity.
The presence of diabetes has multiple effects upon innate immune responses, including effects upon neutrophils, monocytes and other components of innate immunity. These disturbances have important roles in the increased prevalence and enhanced severity of infections in people with diabetes. The effects include reduced chemotaxis, phagocytosis and impaired bactericidal activity.
Some disturbances in the complement system and in cytokine responses have been described in people with diabetes (e.g. low complement factor 4 and decreased cytokine responses after stimulation), but their role in the increased susceptibility to infection is less clear [7]. Consistent defects have not been demonstrated. No clear disturbances in adaptive immunity have been described. Humeral adaptive immunity, in particular, appears relatively unaffected as exemplified by the relatively normal antibody responses to most vaccinations and the fact that serum antibody concentrations and responses in patients with diabetes are generally normal. For example, people with diabetes respond to pneumococcal vaccine equally as well as controls without diabetes [8,9].
The complexity of the component systems involved in the immune system makes comparison between studies difficult and it is obviously simplistic to study individual components of the immune system in isolation given the interdependency of these components. Many studies have relied upon in vitro or animal model methodology.
Investigations to identify the mechanisms of immune impairment in animal models of diabetes and in vitro experiments have been numerous. A full review of these animal and in vitro studies is beyond the scope of this chapter; however, the observations that follow may serve as examples from within the range of abnormalities that have been found, although many questions remain as to the nature of the defects produced by diabetes and their effects upon infection risk.
Neutrophil chemotaxis, neutrophil adherence to vascular endothelium, phagocytosis, intracellular bactericidal activity, opsonization and other aspects of innate immunity are all depressed in hyperglycemic patients with diabetes, although adaptive immunity appears relatively unaffected [ 10–12]. These changes lead to reduced host defense in response to infection with extracellular bacteria. Both impaired chemotaxis and phagocytosis have been described in monocytes of people with diabetes [13]. Defects in innate immunity have also been shown to predispose db/db mice to S. aureus infections. Interestingly, these mice show a heightened inflammatory response which occurs in association with an impaired neutrophil respiratory burst, and the recruited neutrophils fail to resolve the infection [14]. A possible role for advanced glycosylation end-products (AGEs) is postulated as a component in the pathogenesis of the impaired neutrophil function.
While release of tumor necrosis factor α and interleukin 1β (IL-1β) from lipopolysaccharide-stimulated macrophages has been shown to be reduced in diabetic mice compared with control mice [15], study of monocytes from patients with diabetes indicates upregulation of the secretion of the same inflammatory mediators [16]. This further illustrates the difficulty in comparing studies conducted in different settings and species. Some innate (e.g. cytokines, complement) immune functions are decreased while others remain the same in patients with diabetes as those without diabetes. For example, while unstimulated cytokine concentrations may be higher, cytokine responses to stimuli are often reduced [17]. Interpretation of the complexity of the underlying mechanisms may also need to take into account potential underlying proinflammatory effects associated with diabetes itself.
The level of macrophage inflammatory protein 2, a mediator of lung neutrophil recruitment, is significantly decreased in diabetic mice compared to control mice [18]. The deficiency causes a delay in neutrophil recruitment in the lungs. This may be an important factor influencing the susceptibility of people with diabetes to infections of the lower respiratory tract.
Hyperglycemia impairs opsonophagocytosis by diverting nicotinic acid adenine dinucleotide phosphate (NADPH) from superoxide production into the aldose reductase-dependent polyol pathway [19], providing one further example of a mechanism by which hyperglycemia directly impairs phagocytosis.
Diabetic mice show greater than twofold induction of genes that directly or indirectly induce apoptosis [20]. By contrast, it is known that blocking of apoptosis allows for a significant improvement in wound healing and bone growth. This may influence many aspects of responses to infection, including impaired wound healing, which are important in the setting of diabetes.
These examples, while somewhat piecemeal, serve to demonstrate the range of abnormalities in the innate immune system that result from hyperglycemia. Of particular importance are those spanning macrophage, monocyte and neutrophil function, and which impair adherence to endothelium, chemotaxis, phagocytosis and bacteriocidal activity. They also point to other abnormalities (e.g. involving apoptosis), wound healing and cytokine responses to infection. The antioxidant systems involved in bacteriocidal activity may be compromised. These impairments, which are exacerbated by hyperglycemia and acidemia, may be reversed substantially, if not entirely, by normalization of pH and blood glucose levels. It needs to be emphasized, however, that the severity of effects correlates somewhat unpredictably with direct contemporaneous measures of glycemic control such as HbAlc, perhaps reflecting longer term or persistent changes such as accumulation of AGEs [21]. In general, a better regulation of the diabetes leads to an improvement of the cellular aspects of immune function.
Other host-related factors
Other host-specific factors, over and above direct diabetes-related impairment of immune defenses, can further the predisposition to infection. These include vascular insufficiency (microangiopathies and macroangiopathies), sensory peripheral neuropathy, autonomic neuropathy, and skin and mucosal colonization with pathogens such as S. aureus and Candida species. Abnormalities of the structure and function of the microcirculation can also have additional indirect adverse effects on the immune responses themselves. Thus, immunologic responses may be further compromised by microangiopathy, and additional factors related to diabetes complications specifically increase the risks of certain infections, especially those involving the foot and the urinary tract.
While beyond the direct scope of this chapter, obesity, which is commonly associated with diabetes, also increases the risk of certain infections. These include nosocomial infections, wound and surgical site infections, respiratory infections and infections involving the gastrointestinal tract. The presence of obesity also correlates with infected diabetic foot ulcers in people with diabetes [22].
Diabetic complications
Vascular disease is an important component in the etiology of the diabetic foot and the attendant complications of infection, ulceration and gangrene. Vascular disease is very common in diabetes and macrovascular disease may be premature, extensive, severe and present in unusual sites. Vascular insufficiency results in local tissue ischemia that can, in turn, enhance the growth of microaerophilic and anaerobic organisms, while simultaneously depressing the oxygen-dependent bactericidal functions of leukocytes. The antioxidant systems involved in bacteriocidal activity may be compromised by the combination of microvascular disease and the diabetic metabolic derangement itself. Hyperglycemia and acidemia are important predisposing factors to this latter effect and are reversed substantially by normalization of pH and blood glucose levels. Vascular disease related to diabetes may also further impair the local inflammatory response and may also impair the tissue penetration of antibiotics.
Neuropathy, both peripheral and autonomic, also contributes to the risk of foot infections and ulceration, as well as to certain other infections. Sensory peripheral neuropathy masks the recognition of trauma. Minor local trauma in patients with diabetes-associated peripheral neuropathy may result in skin ulcers, which, in turn, lead to diabetic foot infections. Skin lesions are often either unnoticed or ignored until infection occurs. Autonomic neuropathy contributes to the etiology of diabetic foot infections by mechanisms such as decreased sweating, which predisposes to drying and fissuring of the skin, and by further exacerbating abnormalities in the control of the microcirculation. Both sensory and motor neuropathy can lead to deformity and alter the dynamics of the function of the foot. Fuller discussion of the etiologic factors related to sepsis and the diabetic foot is provided in Chapter 44.
Patients with diabetes-associated autonomic neuropathy may develop urinary retention and stasis in association with loss of innervation to the bladder. This predisposes them to urinary tract infections. This risk is particularly high in females. Autonomic neuropathy can also impair the function of the gastrointestinal tract, predispose to certain gastrointestinal tract infections and contribute to risk of aspiration pneumonia in the context of gastroparesis. Renal papillary necrosis can also contribute to the risk of renal failure as well as to infection within the urinary tract.
Thus, a number of factors contribute to the vulnerability of people with diabetes to infections. In addition to the abnormalities of the immune response, vascular disease and neuropathy can greatly enhance susceptibility (e.g. in the lower limb and urinary tract). Hyperglycemia per se may specifically heighten susceptibility to certain fungal and bacterial infections.
Organism-specific factors
Certain organisms may show increased adherence to diabetic cells [12] and others may demonstrate increased virulence in hyperglycemic environments. Specific factors that predispose people with diabetes to infection with specific organisms include the following examples.
Candida albicans and fungi
Glucose-inducible proteins produced by Candida albicans are homologous to a complement receptor on phagocytes. These proteins may promote adhesion of C. albicans to buccal or vaginal epithelium. This adhesion, in turn, impairs phagocytosis, giving the organism an advantage over the host [12].
Ketone reductases produced by Rhizopus species allow these species to thrive in high glucose, acidic conditions typically present in patients with diabetic ketoacidosis [23].
Klebsiella spp.
A bacterial genus of note in the context of diabetes is Klebsiella. Klebsiella infections are the second most common causes of Gram-negative sepsis (after E. coli). In a report from Taiwan, diabetes was the most commonly associated underlying condition in patients presenting with community-acquired K. pneumoniae bacteremia (whereas neoplastic diseases were more commonly associated with nosocomial infections) [24]. The percentage of patients with underlying diabetes was 49%, which is even higher than in earlier reports [25,26]. Apart from the high proportion with diabetes, associations were also observed with serotype K1 (associated with impaired phagocytosis), liver abscess and other metastatic complications (endophthalmitis, meningitis, brain abscess) [24]. Primary liver abscess in other parts of Asia is also increasing in incidence, with 40% reportedly associated with diabetes [27]. Bacteremia is present in 50%, and 8–10% have meta-static complications (endophthalmitis, meningitis, brain abscess, pneumonia, skin and soft tissue infections, lung abscess, septic arthritis, renal abscess and prostatic abscess).
Melioidosis
A combination of organism-specific factors together with the changes in innate immunity may explain the increased susceptibility of people with diabetes to melioidosis. About 50% of cases of melioidosis occur in people with diabetes. The responsible organism, Burkholderia pseudomallei, has been shown to be selectively resistant to phagocytosis compared to Salmonella typhimurium and E. coli in subjects with diabetes.
In a study from Thailand, a country where melioidosis is relatively common, neutrophil responses to B. pseudomallei, in both healthy subjects and people with diabetes showed that B. pseudomallei displayed reduced phagocytosis by neutrophils compared to S. enterica typhimurium and E. coli. In addition, intracellular survival of B. pseudomallei was detected throughout a 24-hour period, indicating intrinsic resistance of B. pseudomallei to killing by neutrophils. Furthermore, neutrophils from subjects with diabetes displayed reduced migration in response to IL-8 and an inability to delay apoptosis. Thus, B. pseudomallei appears to be intrinsically resistant to phagocytosis and killing by neutrophils. When added to the impaired migration and apoptosis seen in diabetes, the combination seems sufficient to explain the increased susceptibility to melioidosis in people with diabetes [28].
Bidirectionality: the effect of infections on diabetes
Bidirectionality exists in the relationship between diabetes and infections. The effect of infections upon diabetes includes the effects of certain infections on the etiology and pathogenesis of diabetes itself, adverse effects upon hyperglycemia in established diabetes and exacerbation of diabetes complications. The importance of the potential adverse effects upon hyperglycemia in established diabetes needs to be stressed. Infections remain an important predisposing cause of both diabetic ketoacidosis and hyperosmolar hyperglycemia syndrome.
The importance of certain viral infections in the possible etiology of diabetes has received increasing attention in recent years with respect to both T1DM and T2DM.
Viral infections have been implicated in the etiology of T1DM for many years. Although this complicated topic is beyond the general scope of this chapter and is considered in detail in Chapters 3 and 9. it is noteworthy that type 1a (autoimmune) diabetes is increasing in prevalence globally, providing strong evidence that environmental factors are involved in the clinical expression of the disease. Viruses have long been included in the list of putative environmental triggers. Enteroviruses (especially coxsackie B viruses, B4 in particular), rubella, mumps, rotavirus, parvovirus and cytomegalovirus have all been implicated and continue to be reported [29,30]. Although correlations between the presentation of diabetes and the occurrence of a preceding viral infection have been recognized, a direct causal relationship, with fulfillment of Koch postulates, between viral infection and the diabetogenic process, remains difficult to prove, possibly because other inflammatory factors are also required. In this context, it is interesting to note that the process may be associated with a dominant CD4 T-helper type 1 immune response, whereas the dominance of a T-helper type 2 response, as seen in the face of certain infectious and parasitic agents, may protect against T1DM and other autoimmune diseases. Thus, the increasing freedom from such infections, especially in developed areas of the world, may allow the increased expression of an underlying genetic predisposition, and infection by certain viruses, such as the coxsackie B viruses, may then be associated with the appearance of (and persistence of) β-cell antigens, mediated by mechanisms such as molecular mimicry and activation of Toll-like receptors. Lack of exposure to infection and infestation in early childhood appears to dilute the ability of the innate immune system to withstand autoimmune responses and challenges. T1DM is not alone in this respect and the general concept has become known as the “hygiene hypothesis” [31].
The high prevalence of T2DM in association with hepatitis C infection and the progression of certain diabetes complications (e.g. diabetic nephropathy) in association with hepatitis B are other noteworthy examples. The treatment of HIV/AIDS with protease inhibitors predisposes to diabetes, metabolic syndrome and increased cardiovascular risk. The public health implications of these issues are considerable given the concordance of the diabetes epidemic with these other highly prevalent diseases.
All infections, especially if severe, have the potential to exacerbate hyperglycemia by a number of mechanisms (e.g. worsening of insulin resistance by production of “stress” or counter-regulatory hormones and production of cytokines such as IL-1 and tumor necrosis factor) [32]. Infection remains a major factor in the pathogenesis of diabetic ketoacidosis or hyperosmolar hyperglycemia. Infections can also precipitate hypoglycemia if symptoms, such as anorexia, nausea and vomiting, lead to reduced food intake. Malaria and its treatment with quinine can also produce hypoglycemia.
Hepatitis C
A number of reports from North America, Europe and the Middle East consistently demonstrate an increased prevalence of diabetes (ranging from 24% to 62%) among patients with chronic hepatitis C virus (HCV) infection compared both with persons with other forms of liver disease and with other control groups. Among HCV-infected individuals reported prevalence of diabetes ( 21–50%) is much higher when compared with other forms of chronic liver disease ( 2–12%) and with control subjects (26%) [ 33–38].
In the USA, T2DM occurs more often in persons with HCV infection who are older than 40 years of age, particularly in the range of 40–49 years where the relative risk ratio is 3.77. Apart from age, the prevalence of diabetes is greatest in subjects who are non-white, have a high body mass index, are below the poverty level and have a family history of diabetes. The prevalence of T1DM appears to be unaffected [39].
The suggestion that HCV infection predisposes to T2DM as a result of the progressive liver damage is supported by the observations that the association is most marked in the older age groups (>40 years), and that there is higher risk among patients with advanced HCV cirrhosis. The presence of diabetes is also associated with worse hepatic fibrosis; however, the higher prevalence of diabetes compared to other forms of liver disease suggests an additional mechanism specific to hepatitis C. Tumor necrosis factor α has been suggested as one possible candidate for this role [40].
HCV infection is also strongly associated with diabetes among intravenous drug users and this is independent of HIV infection or use of highly active antiretroviral therapy (HAART) [41]. Thus, it is important to monitor patients with chronic HCV infection for development of diabetes. Bidirectionality again applies with weight loss and good glycemic control improving hepatitis outcomes.
HIV/AIDS
Although HIV/AIDS has not in itself been reported to increase the risk of diabetes, the treatment of HIV/AIDS with HAART predisposes to T2DM, other metabolic risks and premature cardiovascular disease. This has become a major problem in the management of this already very complicated disease. The effects occur via disturbances in lipid homeostasis and fat partitioning (lipodystrophy), insulin resistance, insulin secretion and mitochondrial function. The underlying cellular mechanisms are complex and incompletely understood. Combination HAART for HIV-1 infection is frequently complicated by lipodystrophy (peripheral fat loss and relative visceral obesity), dyslipidemia and insulin resistance. HIV-infected adults receiving HAART have an increased incidence of elevated blood pressure and cardiovascular morbidity.
Whether antiretroviral therapy-naive patients have altered risk of subsequent CVD or T2DM is unclear, particularly in light of emerging data indicating that rates of CVD may also be higher in people with HIV who are not treated with antiretriviral therapy.
Exposure to antiretroviral therapy for more than 1 year is associated with increasing risk of diabetes. Although the risk is greatest among individuals treated with a protease inhibitors (PI) (attributed to a direct inhibitory effect on cellular glucose transport by PI medications) [41], an increased prevalence of diabetes among those receiving a PI-sparing regimen has also been found and insulin resistance has been reported among PI-naive persons with HIV infection, in association with fat redistribution. Nucleoside analog-induced mitochondrial toxicity is probably of importance. Cessation of PI appears to have little beneficial effect in reversing lipodystrophy, although it may improve the metabolic control in diabetes. Alteration of thymidine analog nucleosidase reverse transcriptase inhibitors may, however, confer benefit on lipodystrophy [42,43].
In a study of almost 900 HIV-infected patients, initiation of antiretroviral therapy was evaluated for prevalence and incidence of metabolic syndrome (using the National Cholesterol Education Program, Adult Treatment Panel III [NCEP ATP-III] and the International Diabetes Federation [IDF] criteria) and subsequent diagnosis of CVD and T2DM over a 3-year period. The prevalence of baseline metabolic syndrome was 8.5% and 7.8% (ATP-III and IDF criteria, respectively). Substantial progression to metabolic syndrome occurred within 3 years following initiation of antiretroviral therapy. The presence of metabolic syndrome at baseline was significantly associated with an increased risk of T2DM while metabolic syndrome occurring during the 3-year period was associated with an increased risk of both CVD and diabetes [44,45].
In another study [46], 123 of 6513 HIV-infected persons developed diabetes during 27 798 person-years of follow-up, resulting in an incidence of 4.4 cases per 1000 person-years of follow-up. An increased incidence rate ratio was found for male subjects, older age, obesity, Afro-American or Asian ethnicity. A weaker, although still significant, association of the incidence rate was also found with Centers for Disease Control and Prevention (CDC) disease stage C. Strong associations were observed with treatment using nucleoside reverse-transcriptase inhibitors (NRTI), NRTI plus PI and NRTI + PI + non-nucleoside reverse-transcriptase inhibitors (NNRTI), but not with an NRTI + NNRTI regimen.
Lipodystrophy is a crucial aspect of the association of HAART with insulin resistance, leading to relative preponderance of visceral fat, hepatic steatosis and fat deposition at other “ectopic” sites. HIV-infected persons with lipodystrophy, compared with those without lipodystrophy, have a reduction in plasma adiponectin and adipose tissue adiponectin mRNA levels of approximately 50%, correlating with insulin resistance and increased cytokine levels [45].
Because baseline and incident metabolic syndrome identifies individuals at risk for both CVD and T2DM, evaluation in all patients commencing HAART is warranted. A fasting plasma glucose concentration should be checked before initiation of therapy and monitored every 3–6 months, especially in patients receiving changes in treatment or who have significant risk factors for insulin resistance. An oral glucose tolerance test may be required, particularly in the presence of risk factors or equivocal glucose concentrations. Dietary guidelines established for the general population remain relevant for the management of glucose disorders in the context of HIV infection. Weight loss through increased activity and caloric restriction should be recommended for overweight HIV-infected patients.
Metformin improves insulin sensitivity in patients with HIV lipodystrophy and is an effective antidiabetic medication; however, it should be used with caution in patients receiving an NRTI, and in persons with impaired renal function because of the possibility of lactic acidosis. Thiazolidinediones also improve insulin sensitivity in patients with HIV lipoatrophy, although rosiglitazone treatment may worsen hyperlipidemia. Insulin therapy should be used according to standard recommendations. Substitution of an NNRTI for a PI has been observed to improve insulin resistance but this needs to be balanced against any risk to virus control. Careful discussion with the HIV physician is therefore essential and it may be deemed safer to increase the antihypoglycemic treatment rather than changing the components of the HAART regime [ 47–49].
Hepatitis B
Although, in contrast to hepatitis C, hepatitis B virus (HBV) has been less consistently associated with an increased prevalence of diabetes, the presence of hepatitis B markers may nevertheless influence the natural history of diabetes and its complications and the relationship may be bidirectional as the presence of diabetes is associated with more severe fibrosis and cirrhosis. However, there is uncertainty as to cause and effect given the general association of diabetes with liver cirrhosis [50].
Chinese HBV-infected patients with T2DM have been shown to be more likely to develop end-stage renal disease than non-HBV infected patients with T2DM (8.7 vs 6.4%) with a hazard ratio of 4.5. The association of chronic HBV infection with increased risk of end-stage renal disease was independent of other potential confounding factors. HBV-infected patients also reported earlier onset of diabetes and had a higher frequency of diabetic retinopathy than non-HBV-infected patients (28% compared to 22%). Cardiovascular complications appeared unaffected [51].
Specific infections either strongly associated with diabetes or in which the presence of diabetes is important
Infections involving the head and neck
Two head and neck infections that are associated with high rates of morbidity and mortality, malignant otitis externa and rhinocerebral mucormycosis, are particularly noteworthy in people with diabetes.
Malignant otitis externa
Malignant otitis externa is an invasive infection of the external auditory canal and skull base that typically arises in elderly people with diabetes. An early series of patients was described in 1968 [52]. Most cases ( 86–90%) have been reported in patients with diabetes. Pseudomonas aeruginosa is nearly always the causal organism (>98% of cases) although Aspergillus species are occasionally responsible. Microangiopathy in the ear canal has been suggested as a predisposing factor.
Presenting features include severe intractable headache and otalgia, otorrhea and deafness. Patients often report a duration of weeks to months of these symptoms. Intense cellulitis is combined with edema of the ear canal. Focal neurologic signs and cranial nerve palsies may occur. The pain may involve the temporomandibular joint and be aggravated by chewing. Osteomyelitis of the skull base and temporomandibular joint is a potentially life-threatening complication and the mortality in the pre-antibiotic era exceeded 50%. On otoscopy, granulation tissue may be seen in the floor of the ear canal, often in association with edema and intense cellulitis. The tympanic membrane is usually intact. Computed tomography (CT) and magnetic resonance imaging (MRI) studies are essential for defining the extent of bone and soft tissue involvement, together with the bony destruction of the skull base that may be seen in advanced cases.
Systemic antipseudomonal antibiotics are the primary therapy. Early referral to an otorhinolaryngologist is essential and allows diagnostic confirmation by surgical biopsy. Débridement of necrotic tissue can also be carried out if necessary, although the introduction of effective antibiotic therapy has reduced the requirement for surgery. With the introduction of quinolones, the cure rate has increased to 90%, with few adverse effects reported and oral therapy rendered possible. Prolonged treatment for 6–8 weeks is recommended, as for osteomyelitis [53]. Thus, treatment consists of prolonged administration ( 6–8 weeks) of an antipseudomonal agent (typically, an orally administered quinolone). The emergence of ciprofloxacin resistance is a potential problem. It is recommended that systemic quiniolone use be reserved for treatment of invasive ear infections. An example of invasive aspergillosis involving the skull base is shown in Figure 50.1.
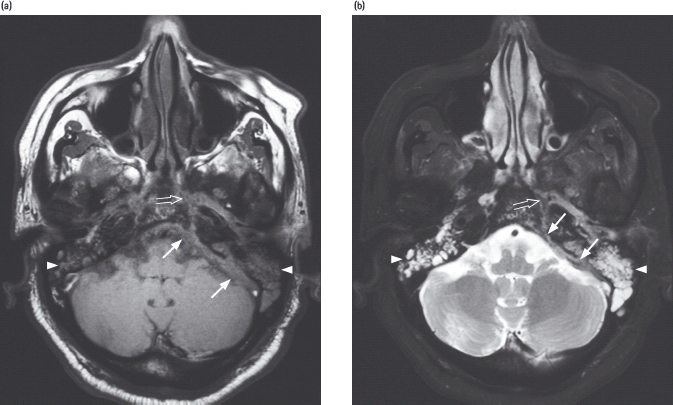
Figure 50.1 Magnetic resonance imaging (MRI) scan of skull base in a 59-year-old male with a 20-year history of diabetes (with nephropathy), treated with insulin who developed severe extensive invasive aspergillosis. He presented with headache and vertigo followed by left sixth and seventh nerve palsies. He subsequently developed bilateral sensorineural hearing impairment and blindness secondary to extensive skull base infiltration by the invasive aspergillosis. MRI demonstrated enhancing soft tissue closely related to the left posterolateral wall of the nasopharynx with parapharyngeal, skull base, perineural and dural infi ltration. Biopsy showed inflamed fibrous tissue with degenerated fungal filaments. Culture confirmed Aspergillus flavus . He is receiving lifelong therapy with voriconazole. He remains blind. MRI of skull base in the axial plane with: (a) T1-weighted; (b) fat-saturated T2-weighted; and (c) post-gadolinium T1-weighted sequences. These show marked dural thickening (arrows) with enhancement in the left posterior cranial fossa. An abnormal signal with enhancement is also noted in the adjacent left petrous apex (open arrow). Note also the presence of infl ammatory fl uid within both mastoid air cells (arrowheads). Acknowledgements to Dr. K.T. Wong, Consultant Radiologist, for preparation and reporting of the fi gures, and to Professor A. Ahuja for permission to use the fi gures. Both are placed at the Department of Diagnostic Radiology and Organ Imaging, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







