Development of the Hypothalamic–Pituitary–Thyroid Axis
Pilar Santisteban
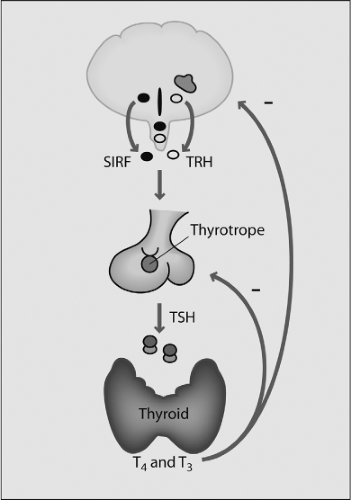
The thyroid gland is the most anterior organ that buds from the gut tube; it is mainly composed of endoderm-derived follicular cells that are responsible for thyroid hormone production in all vertebrates. The main regulator of thyroid function is thyroid-stimulating hormone (TSH), a glycoprotein synthesized and secreted from the thyrotrope cells of the anterior pituitary gland, which in turn is under the control of thyrotropin-releasing hormone (TRH) secreted by the hypothalamus. Thyroid hormone secretion and serum concentrations of thyroxine (T4) and 3,5,3′-triiodothyronine (T3) are maintained by a negative feedback loop involving inhibition of TSH and TRH secretion by T4 and T3. Thus, the regulation of thyroid function depends on the normal development of the hypothalamic–pituitary–thyroid axis, which occurs independently but coordinately during embryonic and neonatal life.
This chapter will focus mainly on the formation of the thyroid gland with some comments and references on the development of the hypothalamic–pituitary axis. The role of several transcription factors expressed specifically in the hypothalamus–pituitary–thyroid has helped to define the molecular events responsible for the development of this axis. Most of the recent advances in the understanding of the formation of this axis come from studies in animal models, but wherever possible this information will be integrated with what is known in humans. Human transcription factors and proteins will be depicted in all capitals, and a capital followed by lowercase will be used for mice; italics will be used when referring to genes.
The Hypothalamic–Pituitary Axis
The vertebrate embryo relies on complex inductive interactions to orchestrate pattern formation, organogenesis, and ultimately, the development of integrated organ systems. The hypothalamic–pituitary axis is a prime example of such a system and is considered a unit that acts as a neuroendocrine transducer that mediates trafficking between the brain and the peripheral hormone-secreting target glands. The concept that the pituitary is centrally regulated by the hypothalamus required contributions from several disciplines, including
neuroanatomy, biochemistry, and physiology (1). Summary information about the most important events in the development of the hypothalamus–pituitary axis will be given in this chapter (1,2,3,4).
neuroanatomy, biochemistry, and physiology (1). Summary information about the most important events in the development of the hypothalamus–pituitary axis will be given in this chapter (1,2,3,4).
The regulated functions of the hypothalamus and pituitary are intrinsically linked throughout life, and they result from the coordinated development of these two organs. Experimental embryology and molecular genetic studies have yielded evidence that the determination, development, and differentiation of the pituitary gland and hypothalamus are intimately coupled. How both glands, with different embryonic origins, codevelop introduces a level of complexity that is still not well understood.
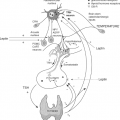
The hypothalamus constitutes less than 1% of brain volume and emerges from the ventral-caudal prosencephalon (diencephalon) during the sixth week of gestation in humans, and between embryonic days (E) E11 and E18 in rats. The hypothalamic sulcus defines the upper border and extends from the interventricular foramen to the cerebral aqueduct, above which lies the thalamus. The primitive hypothalamic region is initially localized at the most rostral region of the neural tube, and later has a more caudal and ventral position. Hypothalamic neuronal bodies that produce factors controlling the pituitary are clustered in different nuclei, including the paraventricular, supraoptic, and arcuate nuclei. The hypothalamic hormone is often produced in more than one nucleus and even a single nucleus may express several hormones. The nuclei involved in pituitary regulation are generally located in the medial hypothalamus, and the median eminence is the major functional link between the hypothalamus and the pituitary. Two different neurosecretory systems are organized in the hypothalamus, one consisting of magnocellular neurons whose axons migrate directly into the posterior lobe of the pituitary gland, and the other consisting of parvocellular neurons that synthesize, among other neuropeptides, TRH for regulating the TSH-thyroid axis (1) (Fig. 2.1).
In the ventral hypothalamus, the infundibulum evaginates to give rise to multiple structures, including the posterior pituitary gland, the median eminence, and the pituitary stalk that connects the hypothalamus and the pituitary gland. The endocrine part of the pituitary gland derives from the most anterior segment of surface ectoderm. The most anterior of the middle surface ectoderm, the anterior neural ridge, is the presumptive pituitary. Fate-mapping studies show that the adjoining neural territory will form the ventral diencephalon and the hypothalamus. When head development begins and the neuroepithelium expands to form the brain, the anterior neural ridge is displaced ventrally and eventually occupies the lower facial and oral area. The midline portion of the oral ectoderm invaginates to give rise to the pituitary anlage, the so-called Rathke’s pouch, which in mice appears at around day E8.5 and detaches from the ectoderm by E12.5 (1,2,3,4). Even before the pouch is visible, certain cells are committed to developing into the anterior pituitary. Thus, the pituitary gland originates from two embryonic tissues: The anterior and intermediate lobes (adenohypophysis) are derived from the oral ectoderm, and the posterior lobe (neurohypophysis) from the neural ectoderm (5). During this process, there is a direct association between the neuroectoderm of the diencephalon and Rathke’s pouch. The close apposition of these tissues suggests that cell–cell contact and inductive tissue interactions may be important for gland specification or determination (6). These terms, in developmental biology, underscore the fact that some cells are “specified or determined” to undergo a final cellular fate (7).
In fact, a number of transcription factors expressed in the developing diencephalon and infundibulum at E10.5, but not in the pituitary itself, such as Titf1/Nkx2.1, Sox3, and Lhx2 are required for proper diencephalon development and secondarily affect pituitary formation. These data support the importance of signal exchange between diencephalon and the forming pituitary for the development of both tissues.
It is important to note that the homeodomain transcription factor Titf1/Nkx2.1 (see later in this chapter) is expressed in the embryonic ventral forebrain and certain hypothalamic areas, such as the median ganglionic eminence that gives rise to the pallidal component of the basal ganglia at E10.5. Consistent with this expression, Titf1 null mice do not form the pallidal structures due to a ventral to dorsal transformation of the pallidal primordium into a striatum-like structure (8,9). Interestingly, it has been shown that Titf1 plays key roles in the developing telencephalon where it regulates the identity of progenitor
cells in the medial ganglionic eminence. In addition, this factor controls cell-fate determination regulating neuronal migration by direct transcriptional regulation of guidance receptors in postmitotic cells (10). This is important, as it has been demonstrated that in humans Titf1 mutations cause, besides hypothyroidism, benign chorea due to problems with the migration of the basal ganglia (11,12).
cells in the medial ganglionic eminence. In addition, this factor controls cell-fate determination regulating neuronal migration by direct transcriptional regulation of guidance receptors in postmitotic cells (10). This is important, as it has been demonstrated that in humans Titf1 mutations cause, besides hypothyroidism, benign chorea due to problems with the migration of the basal ganglia (11,12).
The role of the pituitary primordium in the development of the hypothalamus has been less extensively studied, but contact between the two structures is important for proper formation of the ventral hypothalamus and median eminence, and for differentiation and proliferation of the different types of pituitary cells.
Signaling and Transcriptional Mechanisms Involved in Pituitary Organogenesis
The development of the pituitary provides an instructive model system for elucidating the molecular mechanisms by which different cell types arise from a common progenitor lineage in response to multiple extrinsic and intrinsic signals.
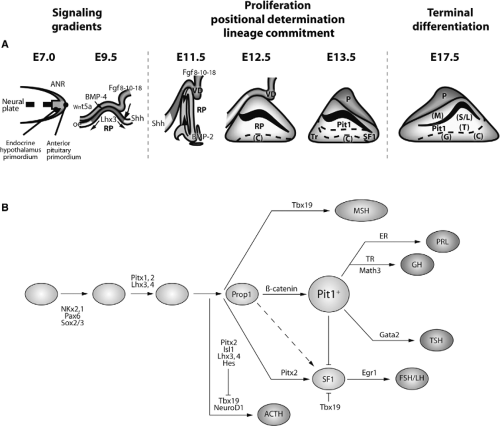
The functions of this gland are carried out by six cell types located in the anterior and intermediate lobes of the pituitary gland. These cell types are defined by the hormone they produce and secrete. Thus, corticotropes secrete adrenocorticotropic hormone (ACTH), thyrotropes secrete TSH, somatotrophs secrete growth hormone (GH), lactotrophs secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and melanotropes secrete melanocyte-stimulating hormone (MSH) (3,4,13). These cells arise from progenitors in Rathke’s pouch, in a precisely orchestrated spatial and temporal fashion, during pituitary development; there are multiple signals that regulate progenitor cell proliferation, positional determination, lineage commitment, cell-fate specification, and terminal differentiation (Fig. 2.2). The somatotrophs, lactotrophs, and thyrotropes derive from precursor cells expressing the transcription factor Pit1, a pituitary-specific POU-class homeodomain factor (14). The induction of Pit1 expression is dependent on a paired-like transcription factor named Prop1 (from Prophet of Pit1) (15). Terminal differentiation of corticotropes and melanotropes is dependent of the T-box transcription factor Tbx19 (16).
Morphogenic Signals Involved in Early Steps of Pituitary Development
The initial step of Rathke’s pouch formation as an invagination of the oral ectoderm and ventral neuroectoderm, respectively, is dependent on the bone morphogenetic protein BMP4. This protein is expressed in the overlying ventral diencephalon at E8.5–9, subsequently in the infundibulum, and then its levels decrease (Fig. 2.2). BMP4 is a member of the transforming-growth factor-β (TGF-β) superfamily that plays critical roles in patterning and cell-type specification in several species. BMP4 is one of the early signals required for the initial commitment of some cells of the oral ectoderm to form the pituitary gland. Blockade of the BMP4 signal results in arrest of pituitary gland development and absence of all pituitary endocrine cell types (17,18,19). Nevertheless, it has also been suggested that the initiation of Rathke’s pouch and infundibulum formation precedes their commitment to the pituitary fate, and that this may be induced by morphogenetic signals from the prechordal plate/notochord. The process of induction is contact-dependent and requires the presence of mesenchyme. The nature of the inducing signals is unknown and it is unlikely that the same growth factors and morphogens responsible for the stratification of the anterior pituitary primordium such as Shh, Fgf8 and -10, Bmp2/4, and Wnt factors are involved, as these factors either alone or in combination fail to induce any signs of pituitary differentiation (4).
Signals Involved in Pituitary Cell-type Specification
The signals that control pattern formation in the anterior pituitary primordium are composed of a complex of morphogenetic factors originating in the ventral diencephalon/infundibulum and in Rathke’s pouch itself. These signals are essential for the stratification of Rathke’s pouch and for the spatial and temporal organization of endocrine cell types. The best characterized are Sonic hedgehog (Shh) and members of the fibroblast growth factor (FGF), bone morphogenetic protein (BMP), Notch, and Wnt families.
Sonic Hedgehog
The secreted factor Shh plays an important role in organogenesis as a whole. Its ablation results in changes in embryo axis patterning and among other defects, Rathke’s pouch is absent. However, this effect seems to be a consequence of a general defect of midline structures including diencephalon/infundibulum rather than a defect in the anterior pituitary primordium itself. In pituitary development in mice, Shh is expressed in the ventral diencephalon and oral ectoderm, although not in the region that forms Rathke’s pouch (20). It may act in a signaling cascade with BMP2 in the determination of ventral pituitary cell lineages (Fig. 2.2). The zinc finger transcription factors Gli1, Gli2, and Gli3 that are downstream of Shh are expressed in the ventral diencephalon and developing Rathke’s pouch. Gli1 and Gli2 inactivation results in pituitary absence reviewed in (3).
Fibroblast Growth Factors
Several members of the FGF family play different roles in cell-type differentiation during organogenesis. Thus, Fgf8, -10, and -18 are expressed in the ventral diencephalon and in the posterior lobe of the pituitary during early development. Fgf8 and Fgf10 control pituitary proliferation and positional-restricted determination of pituitary cell lineage (17,21). In mice null for Fgf10 for its receptor Fgfr2IIb, Rathke’s pouch initially forms but immediately undergoes apoptosis and in consequence there is agenesis of the anterior pituitary at E14.5, suggesting that Fgf10 signaling is essential for cell survival. Besides, inactivation of Fgf8 causes early lethality at E8.5 before pituitary development. The infundibulum can stimulate proliferation and induce Lhx3 expression. The patterning activity of the infundibulum can be mimicked at least in part by Fgf8. Thus, overexpression of Fgf8 leads to increased Lhx3 expression and pituitary hyperplasia. The function of Fgf8 is to maintain proliferation and to oppose the ventral BMP2 differentiation signal. The requirement for Fgf8 has been further suggested by studies in mice lacking Titf1/Nkx2.1, in which the pituitary is completely absent at birth (8). Titf1 is expressed in the ventral diencephalon and in Titf1 null mice the region that expresses Fgf8 and Fgf10 is deleted, leading to an impairment of Lhx3 and Lh4 induction. The absence of Fgf8 is linked to the absence of the Rathke’s pouch–specific transcription factor Rpx (22).
Bone Morphogenetic Proteins
BMP signaling is essential for anterior pituitary development. BMP4 and BMP2 participate in the development of the anterior pituitary and, as explained above, BMP4 is critical for the invagination of Rathke’s pouch (22). The oral ectoderm invagination occurs in Titf1 null mice, in which the expression domain of BMP4 is maintained at E9.5 despite the absence of Fgf8 and Fgf10. Studies in different mouse models have demonstrated that BMP4 signaling is critical for the continued progression of pituitary development. It remains to be determined whether BMP4, like Fgf8, also regulates the expression of Lhx3.
BMP2 controls the expression of different, ventrally expressed, pituitary transcription factors, and also the expression of the gene for the α subunit of the glycoprotein hormones, which is an early marker of cells fated to become thyrotropes and gonadotropes. Together, these data suggest that BMP2 signaling specifies the progenitors that will later give rise to the ventral pituitary cell types providing a ventralizing positional cue for pituitary development (17,21).
Notch Pathway
The signal that is known to regulate the initial process of specification of the pituitary anlage is the Notch
pathway, an evolutionary conserved mechanism in development. Ligand and receptor of Notch signaling are cell surface proteins mediating cell–cell communication (23). The best characterized Notch signaling targets include Hes1 and Hes5, members of the Hairy enhancer of split (Hes) family. During pituitary development, Notch signaling and Hes1/5 are required for maintaining pituitary progenitors. Notch signaling directly regulates Prop1 expression and is essential for the emergence of Pit1 precursor cells. This signal also plays a role in specifying the intermediate and posterior lobes of the pituitary gland. The attenuation of Notch signaling in Pit1 precursor cells is necessary for the emergence of distinct Pit1 lineages and for ensuring proper terminal differentiation (24,25)
pathway, an evolutionary conserved mechanism in development. Ligand and receptor of Notch signaling are cell surface proteins mediating cell–cell communication (23). The best characterized Notch signaling targets include Hes1 and Hes5, members of the Hairy enhancer of split (Hes) family. During pituitary development, Notch signaling and Hes1/5 are required for maintaining pituitary progenitors. Notch signaling directly regulates Prop1 expression and is essential for the emergence of Pit1 precursor cells. This signal also plays a role in specifying the intermediate and posterior lobes of the pituitary gland. The attenuation of Notch signaling in Pit1 precursor cells is necessary for the emergence of distinct Pit1 lineages and for ensuring proper terminal differentiation (24,25)
Overexpression of Notch in thyrotropes and gonadotropes leads to defects in their differentiation processes (26). Decreased expression of the bHLH transcription factors Mash1 and Mash3 accounts for some of the defects induced by ectopic Notch activation (24). Mash1 executes roles in the terminal differentiation of thyrotropes, gonadotropes, corticotropes, and melanotropes. The expression of Mash3 in the pituitary is regulated by Pit1, starts at E13.5, is maintained in adulthood, and regulates the response to Ghrh.
Wnt/β-catenin Pathway
Other morphogenic factors have also been implicated in the formation of different cell types within the pituitary. The Wnt proteins are a family of secreted proteins that play an important role in the control of embryonic patterning, cell-fate determination, and homeostasis. Activation of the canonical Wnt/β-catenin pathway stabilizes the β-catenin protein, which subsequently translocates to the nucleus and functions as a co-activator of DNA-binding transcription factors, Lef/Tcf in most cases, by displacing HDAC and TLE/groucho corepressor complexes and recruiting p300/CBP and Brg1, to stimulate the expression of target genes (27,28). The Wnt/β-catenin pathway is required for Pit1 lineage determination and pituitary gland growth; thus, the Wnt/β-catenin pathway is active between E11.5 and E15.5 in the pituitary as indicated by the expression Axin2, a direct downstream target gene. Temporal control by Wnt/β-catenin signaling is essential for correct pituitary development, as premature activation of β-catenin leads to Hesx1 repression and in consequence, pituitary gland agenesis at E13.5 (29).
Other components of the Wnt/β-catenin signaling pathway, including frizzled-2 (Fzd2), Lef1, Tcf3, and Tcf4, are expressed during pituitary development. Fzd2 is detectable at E12.5 in Rathke’s pouch and the infundibulum. Tcf3 is detectable between E9.0 to E14.5 and is restricted to the Pit1-expressing caudomedial region of the gland. Tcf4 is detectable in the early pituitary as well as in surrounding tissues and is markedly decreased at E13.5. Lef1 has a biphasic expression, transiently at E9.0 in Rathke’s pouch and later reappearing in the anterior and intermediate lobes of the gland at E13.5.
Inactivation of Tcf4 results in hyperplasia of the anterior lobe and inactivation of Lef1 leads to elevated expression of Pit1 as well as GH and TSH-β, consistent with a role of Lef1 in inhibiting Prop1/β-catenin-mediated Pit1 activation (29,30). Other Wnt genes are detected in the developing pituitary on E12.5. Two of them, Wnt4 and Wnt5a, are specifically associated with the early development of the anterior pituitary. At E9.5, Wnt5a is expressed in the ventral diencephalon and infundibulum, and Wnt4 is detected in Rathke’s pouch where it is sustained until E14.5. Mice lacking Wnt4 have a hypocellular pituitary gland that is differentiated but incompletely expanded ventrally (29). In cultured Rathke’s pouch cells, Wnt5 and BMP4 induce expression of the α-subunit gene, suggesting that both signals may act in synergy to expand pituitary cell lineages and induce cell determination (31,32)
All morphogens and growth factors that contribute to pituitary development are important for thyroid development and function, because pituitary TSH is the main regulator of thyroid gland function.
Transcription Factors Controlling the Early Phases of Pituitary Development
Many transcription factors responsible for the differentiation of pituitary cells have been identified (2,3,4), establishing a hierarchy of expression during pituitary development (Fig. 2.2).
1. Three related Pitx transcription factors have been identified. These factors have a homeodomain similar to that of bicoid (formerly referred to as P-Otx and Pitx-1). Pitx3 is critical for the proper development and survival of mesodiencephalic dopaminergic neurons in mammals.
Pitx1 and Pitx2 display overlapping but different expression patterns during pituitary development reviewed in (2,3,4,33). These two factors are required for cell proliferation, survival, and differentiation in a dosage-sensitive manner. Pitx2 plays a more prominent role than Pitx1, and both act redundantly controlling Lhx3 expression. Pitx-1 interacts with the amino-terminal domain of the pituitary-specific POU-domain protein Pit-1 (see later), and is expressed in the earliest stages of pituitary organogenesis in the anterior region of the neural plate, the oral ectoderm. Targeted disruption of Pitx-1 leads to decreased expression of terminal differentiation markers for gonadotropes and thyrotropes (34). The PITX-2 gene was identified through positional cloning of the gene responsible for the Rieger syndrome in humans (35). Both Pitx genes, Pitx-1 and -2, are expressed in most pituitary cell types from early to late pituitary ontogeny, and also in other developing organs. Pitx-2 null mice have many severe developmental defects, including arrest of the early stage of pituitary development after establishment of the contact between the infundibulum and Rathke’s pouch, and arrest of the subsequent signaling gradients; the pituitary gland fails to develop after E10.5, which results in marked hypoplasia of the gland.
These transcription factors bind within the promoters of the α-subunit of glycoproteins, TSHβ, LHβ, FSHβ, GnRHR, PRL, and GH.
2. The LIM homeodomain proteins (LIM-HD) have two tandemly repeated LIM domains located between the N-terminus and the DNA binding homeodomain. The LIM domain provides a protein–protein interaction interface that recruits cofactors mediating LIM-HD functions (36). Multiple members of the LIM homeodomain family of transcription factors are expressed in Rathke’s pouch, including Lhx-3, Lhx-4, and Isl-1. Lhx-3 is specifically expressed in Rathke’s pouch on E9.5 in mice, and it plays a decisive role, together with Lhx-4, in the earliest phases of pituitary organogenesis. Lhx-3 knockout mice have a rudimentary Rathke’s pouch, but the ectoderm fails to continue to proliferate and all pituitary cell types are absent with the exception of a few corticotrophs (36). Isl-1 is initially
expressed in the oral ectoderm at E8.5, then in Rathke’s pouch at E9.5, and at E10.5 it has become restricted to the most ventral region of the pouch where it co-localizes with the α-subunit of glycoproteins in the rostral thyrotrope cells. The expression of this gene is activated by BMP4 and BMP2 and is repressed by Fgf8/Fgf2, two opposing ventral/dorsal signals that are critical for pituitary development. Isl1 null mice die at E10 with multiple defects in the heart, pancreas, and motor neurons. At E9.5, the primitive pouch forms in these mice but with an aberrant epithelium, suggesting that Isl1 is necessary for proliferation and differentiation of pituitary progenitors.
expressed in the oral ectoderm at E8.5, then in Rathke’s pouch at E9.5, and at E10.5 it has become restricted to the most ventral region of the pouch where it co-localizes with the α-subunit of glycoproteins in the rostral thyrotrope cells. The expression of this gene is activated by BMP4 and BMP2 and is repressed by Fgf8/Fgf2, two opposing ventral/dorsal signals that are critical for pituitary development. Isl1 null mice die at E10 with multiple defects in the heart, pancreas, and motor neurons. At E9.5, the primitive pouch forms in these mice but with an aberrant epithelium, suggesting that Isl1 is necessary for proliferation and differentiation of pituitary progenitors.
The similarity of the phenotypes of Pitx-2 null and Lhx-3 null mice suggests that these two classes of homeodomain factors collaborate to regulate the same pituitary-specific genes. Indeed, both factors act synergistically to activate the expression of the α-subunit gene (37). In summary, the induction of Lhx-3 expression in response to infundibular Fgf signals is the critical step in the selection of oral ectoderm for development into the pituitary gland, and it acts synergistically with Pitx-2 to direct pituitary-specific gene expression.
3. The transcription factor Hesx1 is a member of the paired-like class of homeodomain genes. It is first expressed in the prechordal plate precursor and later becomes restricted to the ventral diencephalon and Rathke’s pouch at E9.5 reviewed in (2,3,4). Expression in the developing pituitary persists until E12, when Hesx1 is downregulated in a spatial and temporal manner that coincides with the rise of Prop1 transcripts and progressive differentiation of pituitary-specific cell types. Hesx1 expression is regulated by Lhx3 and Prop1. Hesx1 null mice have different pituitary phenotypes with pituitary agenesis in about 5% of the embryos.
Hesx1 is a transcriptional repressor with a repressor domain in the N-terminal region and another one in the homeodomain region. The homeodomain recruits the NcoR/Sin3/HDAC corepressor complex and the N-terminal eh1 motif interacts with the groucho-related Tle corepressor. This association is critical for Hesx1 function as temporal regulation of the Hesx1/groucho (Tle) complex is fundamental for pituitary organogenesis (38).
The Rathke’s pouch factor Rpx is a homologue of Hesx-1. Its expression is restricted to the oral ectoderm and to the pouch. The attenuation of Rpx/Hesx-1 expression by different corepressors coincides with the appearance of terminal differentiation markers for anterior pituitary cell types, suggesting that downregulation of this gene is required for progression of pituitary development. Rpx can dimerize with Prop-1 and inhibit Prop-1 activity, suggesting that Rpx/Hesx-1 acts to antagonize Prop-1 function (39).
4. The paired homeodomain factor Prop1 is essential for pituitary development. It was identified by positional cloning in Ames dwarf mice and is called Prophet of Pit-1 (Prop-1). This factor has a C-terminal transactivator domain and an N-terminal repressor domain (reviewed in 2–4,33), suggesting that Prop1 function is both activating and repressive. In agreement with this function is the fact that the Prop1/β-catenin complex acts as a transcriptional activator of Pit1 and as a transcriptional repressor of Hesx1, depending on the associated cofactors.
The onset of Prop1 expression coincides with the closure of Rathke’s pouch at E-10.5; expression peaks at E12.5 followed by downregulation on day E15.5 to E16.5, at the time of terminal differentiation of pituitary-specific cells. Notch signaling is required for maintaining high levels of Prop1 expression at E12.5. Prop1-deficient mice do not express Pit-1, and consequently lack somatotrophs, lactotrophs, and thyrotropes. Prop-1 is also required for the generation of gonadotropes and corticotrophs, indicating that it is important for the expansion of all anterior pituitary cell lineages. Mutations in the PROP-1 gene are the cause of combined pituitary hormone deficiency in humans (reviewed in 40) (see Chapter 38).
5. The Six genes are homeodomain transcription factors homologous to the sine oculis gene in Drosophila. The genes Six1, Six3, Six4, and Six6 are expressed in the developing pituitary. Six1 and Six4 are co-expressed in many embryonic primordium structures including Rathke’s pouch. Pituitary development is not impaired in Six1 or Six4 null embryos. Six1 regulates transcription of its target genes by recruiting cofactors, repressors, or activators via the Six domain (41) and reviewed in (4).
Six3 promotes cell proliferation counteracting Wnt and BMP signaling and specifying anterior identity. Six6 is expressed in a dorsoventral gradient in the early stages of pituitary development, but at E13.5 it decreases. Six6 null mice have pituitary and retina hypoplasia with a reduced number of terminally differentiated cells due to defects in precursor cell proliferation.
6. The paired-domain factor Pax-6 is important in the early development of Rathke’s pouch. This gene is transiently expressed in the dorsal part of the pouch, and is downregulated when cell-type differentiation starts. Thus, Pax6 is expressed early in the anterior neural plate that will become telencephalon, diencephalon, eyes, and pituitary. During pituitary development, Pax6 is expressed in the oral ectoderm at E9.0, but is excluded from the Shh expressing region. At E10-E12, it is detected in Rathke’s pouch with an apparent dorsal/ventral gradient; it becomes downregulated at E13.5 and disappears when cells reach terminal differentiation at E17.5. In the pituitary glands of Pax6 null mice, there is an expansion of the ventral cell types that express α-glycoprotein subunits, predominantly thyrotropes, with a corresponding loss of the more dorsal somatotrophs and lactotrophs. Thus, Pax6 is required for delineating the dorsal/ventral boundaries between the thyrotrope/gonadotrope and the somatotroph/lactotroph progenitor regions of the pituitary gland (42,43) and reviewed in (3).
Transcription Factors Controlling Specification of Different Pituitary Cell Types
As explained at the beginning of this chapter, Pit1 is a member of the family of POU domain-containing transcription factors that consists of an N-terminal POU-specific domain and a C-terminal POU-homeodomain separated by a short linker. Both subdomains contain helix-turn-helix motifs with a high DNA binding affinity. Also referred to as GHF1, Pit-1 was originally identified through analysis of the nuclear proteins regulating the transcription of GH and PRL. Later, Pit1 was found to be required for the generation and cell-type specification of three pituitary cell lineages: Somatotrophs, lactotrophs, and thyrotropes, as well as for repression of gonadotropes. Pit-1 binds to the promoter region of the genes encoding GH, PRL,
the β-subunit of TSH, GHRHR, the type 1 somatostatin receptor, and the TRH receptor, where it interacts with other transcription factors to form functionally active heterodimers. Pit1 also interacts with various members of the nuclear-receptor family including thyroid hormone receptors (TR) and retinoic acid receptors (RAR). The early activation of the Pit1 gene at E13.5 requires the actions of Prop1 and Wnt/β-catenin signaling. Pit1 function is conserved in evolution. In humans, mutations in the PIT gene are associated with congenital defects (40).
the β-subunit of TSH, GHRHR, the type 1 somatostatin receptor, and the TRH receptor, where it interacts with other transcription factors to form functionally active heterodimers. Pit1 also interacts with various members of the nuclear-receptor family including thyroid hormone receptors (TR) and retinoic acid receptors (RAR). The early activation of the Pit1 gene at E13.5 requires the actions of Prop1 and Wnt/β-catenin signaling. Pit1 function is conserved in evolution. In humans, mutations in the PIT gene are associated with congenital defects (40).
The Pit1-dependent cell lineage, the thyrotropes, shares some common features with the non-Pit1-dependent gona-dotropes. Both arise from the ventral portion of the gland and secrete heterodimeric hormones containing the common α-glycoprotein subunit and specific β-subunits. These two cell lineages diverge at an early stage of development. Their plasticity is dependent on Pit1 as has been demonstrated in several genetic models where gonadotropes can be converted into thyrotropes and vice versa. This stresses the critical function of Pit1 in lineage choice.
The zinc finger transcription factor GATA2, whose expression is under the control of ventral to dorsal BMP2 gradient, interacts with Pit1. This interaction is critical for the development of thyrotropes and for the activation of thyrotrope-specific genes such as the gene encoding the β-subunit of TSH which ultimately regulates thyroid function (see Chapter 10A).
In gonadotropes, Pit-1 inhibits GATA-2 binding to promoters not containing an adjacent Pit-1 site. In Snell dwarf mice, in which the Pit1 gene is mutated, Pit1 and GATA2 do not interact, and the thyrotropes assume the fate of gonadotropes (reviewed in 3,4).
Taken together, these studies indicate that there are extrinsic and intrinsic signaling mechanisms that govern the early and late aspects of the development of the hypothalamus and the pituitary gland (Fig. 2.2). Coordination between signaling molecules and between transcription factors is necessary for the early patterning, proliferation, and positional determination of pituitary cell types, including the thyrotropes.
The Thyroid Gland
The thyroid in mammals emerges from a diverticulum of the primitive pharynx. It consists of two lobes connected by an isthmus and is located in the neck region. The gland synthesizes thyroid hormone and calcitonin through two distinct cell types, the epithelial or follicular cells, and the parafollicular or C cells, respectively. The epithelial cells, the most numerous cell population, derive from the endoderm (44), while the C cell precursors derive from the neural crest bilaterally to the fourth pharyngeal pouches and become localized in the ultimobranchial bodies (45). The gland’s name derives from its topographic relationship to the laryngeal thyroid cartilage, whose shape resembles a Greek shield or “thureos.”
We will focus mainly on the epithelial cells that form the thyroid follicles, spherical structures serving as storage for thyroid hormones that enable a controlled release of the hormones. Thyroid epithelial cells phylogenetically derive from the primitive iodide-concentrating gastroenteric cells in an area named endostyle, which during evolution migrated and specialized in the uptake and storage of iodine in follicular cellular structures. This enabled organisms from an iodine-rich sea environment to adapt to an iodine-deficient land environment.
Thyroid Bud Formation
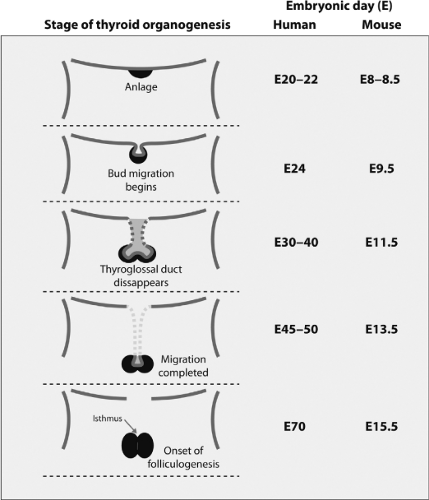
The thyroid gland of vertebrates has a dual embryonic origin, which is reflected in the two endocrine functions of the gland. The most abundant cells, the thyroid follicular cells, arise from the embryonic endoderm as a thickening in the ventral wall on the primitive pharynx floor (Fig. 2.3). It is the first endocrine structure that becomes recognizable in humans. This thickening primordium, called anlage in embryology, is located on the midline of the embryonic mouth cavity in its posterior part, between the first and second branchial arches (Fig. 2.4A). The anlage emerges as a visible bud on E20–22 in humans and on E8–8.5 in mice, and this phenomenon, as explained above in the pituitary development section, is called specification or determination in developmental biology. Thyroid cell specification occurs in parallel to morphological and biochemical changes that make these cells clearly different from their neighboring cells. Thickening in a restricted region of the embryonic cell layer is a common event in the initiation of organogenesis and is essential for the generation of signals required for this process to continue. The initial thickening expands and gives rise to a small pit, the thyroid bud, on E8.5–9 in mice; this is followed by outpouching from the endoderm adjacent to the newly differentiating myocardium (Fig. 2.4A).
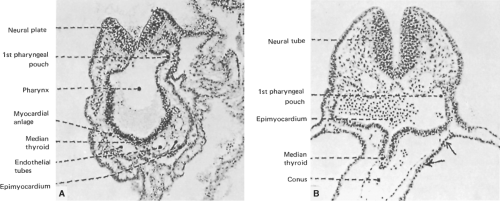 Figure 2.4 Sections of human embryos showing thyroid development. A: Section through a 2-somite embryo (x150). B: Section through the thyroid anlage of a 10-somite embryo (x150). |
The signals that induce the origin of the thyroid follicular cells specification are still unknown. As the ventral pharyngeal endoderm lies in close apposition to the cardiogenic mesoderm, it has been proposed that signals originating in the heart primordium may be responsible for thyroid cell specification. The existence of cardiogenic signals has been shown for other endoderm-derived organs such as liver and pancreas (46). In addition, defects in the foregut secondary to defects in heart organogenesis have been described (47), and furthermore cardiac malformations represent the most frequent birth defects associated with congenital hypothyroidism (48). Altogether, these observations suggest a role for neighboring cells in thyroid specification, with the primitive cardiac cells being the most firm candidates. Future experiments should resolve this question.
Thyroid Migration
The thyroid follicular progenitor cells proliferate and start to migrate downward on E24 in humans and on E9.5 in mice. Subsequently, the thyroid bud expands ventrally as a diverticulum, with a rapid proliferation of the cells at its distal end (Fig. 2.3), but it remains attached to the pharyngeal floor by a tubular stalk called the thyroglossal duct (Fig. 2.4B). This structure fragments and degenerates between E30–40 in humans and at E11.5 in mice, and the primitive thyroid thus loses its connection with the floor of the pharynx. The foramen cecum at the base of the tongue is a small hole located at the site of the thyroid’s origin in the pharyngeal floor and is the remnant of the anlage in the floor of the primitive pharynx (Fig. 2.5). During migration, the thyroid cells proliferate laterally, which leads to the formation of the characteristic bilobed structure connected by an isthmus. The thyroid reaches its definitive position in the
trachea and migration is completed at E45–50 in humans and at E13.5 in mice (Fig. 2.3).
trachea and migration is completed at E45–50 in humans and at E13.5 in mice (Fig. 2.3).
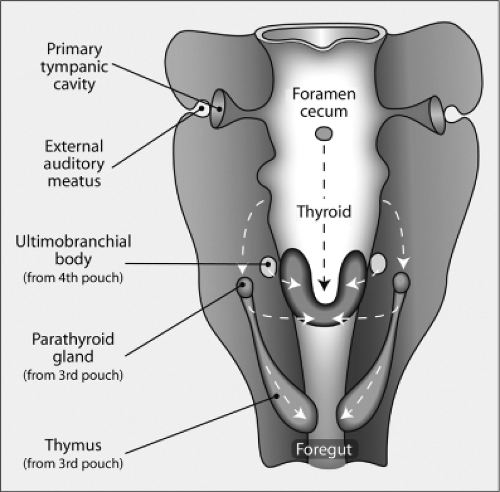 Figure 2.5 Frontal view of the structures derived from the pharynx organ. The thyroid gland forms from the posterior migration of the thyroid anlage, which as shown in Figure 2.3, is derived from the ventral floor of the pharynx. As it migrates downward, it is joined by the ultimobranchial bodies (derived from the fourth branchial pouch), which contain C cells, to form the mature thyroid gland. The foramen cecum is an opening formed by the evagination of the thyroid anlage that closes later in development. The parathyroid glands and thymus are derived from the third branchial pouch. The tympanic cavity and external auditory meatus are formed from the first pouch. (Modified from Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development 1995;121:1989.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|