Iodine Deficiency and Endemic Cretinism
Michael B. Zimmermann
Introduction
Bernard Courtois, a French chemist, accidentally discovered iodine (atomic weight 126.9) in 1811 while producing gunpowder for Napoleon’s army. He was extracting sodium carbonate from seaweed ash to use for manufacture of saltpeter (nitrate). Applying sulfuric acid to clean out the metal vats used to burn the seaweed, he produced an intense violet-colored vapor that cooled into dark violet crystals (Fig. 11D.1) based on the following reaction:
He gave the crystalline material to Gay-Lussac who identified it as a new element and named it ‘iode’, from the Greek ιωδης (i.e., violet) (1). In 1819, Jean-François Coindet, in Geneva, successfully treated goiter with tincture of iodine. In 1851, Gaspard Chatin, the director of the School of Pharmacy in Paris, published his hypothesis that iodine deficiency was the cause of goiter. In 1883, Semon suggested that myxedema was due to thyroid insufficiency and the link between goiter, myxedema, and iodine was established in 1896, when Baumann and Roos discovered iodine in the thyroid.
In the first two decades of the 20th century, pioneering studies by Swiss and American physicians demonstrated the efficacy of iodine prophylaxis in the prevention of goiter and cretinism in populations. The first use of salt iodization as a general public health measure was by the surgeon H. Eggenberger in northern Switzerland in the early 1920s (1) (Fig. 11D.2); the Swiss iodized salt program, running continuously since then, has eliminated endemic goiter and cretinism from the country and become the model for salt iodization efforts worldwide (2).
 Figure 11D.1 Iodine sublimating. (http://images-of-elements.com/iodine.php) See color plate. |
The ecology of iodine
Iodine is a halogen and its high electronegativity and reactivity allow it to form iodides with most elements, in which iodine possesses the formal oxidation state –I. Iodine (as iodide) is widely but unevenly distributed in the earth’s environment (3). In many regions, leaching from glaciations, flooding, and erosion have depleted surface soils of iodide, and most iodide is found in the oceans; the concentration of iodide in seawater is
≈50 μg/L. Its concentration in brown seaweeds is particularly high; these have been used as raw material for iodine production since the early 1800s. Today, iodine for medical and industrial uses is produced from brines in natural gas fields (mainly in Japan) and from Chilean caliche deposits (3).
≈50 μg/L. Its concentration in brown seaweeds is particularly high; these have been used as raw material for iodine production since the early 1800s. Today, iodine for medical and industrial uses is produced from brines in natural gas fields (mainly in Japan) and from Chilean caliche deposits (3).
Iodide ions in seawater and coastal seaweed beds are oxidized to elemental iodine, which volatilizes into the atmosphere and is returned to the soil by rain. However, this iodine cycle is slow and incomplete in many regions, leaving soils and drinking water depleted of iodine. Crops grown in these soils will be low in iodine, and humans and animals consuming food grown in these soils become iodine deficient. In plant foods grown in deficient soils, iodine concentration may be as low as 10 μg/kg dry weight, compared to ≈1 mg/kg in plants from iodine-sufficient soils. Iodine-deficient soils are common in mountainous areas (e.g., the Alps, Apennines, Andes, Atlas, and Himalaya ranges) and areas of frequent flooding (e.g., the Ganges River plain of Northeastern India). Many inland areas are iodine deficient, including Central Asia and Africa, Central and Eastern Europe, and the Great Lakes region of the United States and Canada. Iodine deficiency in humans and animals in these areas will persist until iodine enters the food chain through addition of iodine to foods (e.g., iodization of salt) or dietary diversification using foods produced outside the iodine-deficient area (4).
Dietary sources, absorption, and metabolism of iodine
The native iodine content of most foods and beverages is low; commonly consumed foods provide 3 to 80 μg per serving (5,6). Foods of marine origin have higher iodine content because marine plants and animals concentrate iodine from seawater. However, the so-called ‘sea salt’, harvested during evaporation of salt water in coastal areas, typically contains negligible iodine; nearly all the native iodine is lost by sublimation due to prolonged sunlight exposure during production. Major dietary sources of iodine in industrialized countries like the United States and Switzerland are bread (containing iodized salt) and milk (5,7). Most of the iodine in dairy products is adventitious; milk has low native iodine content but this is often increased by iodine supplements given to livestock as well as residues of iodine disinfectants used in dairying. In the United States, the median intake of iodine from food in the mid-1990s was estimated to be 240 to 300 μg/day for men and 190 to 210 μg/day for women (8). In Switzerland, based on direct food analysis, mean dietary intake of iodine is ≈140 μg/day (5). In many countries, iodized salt used for cooking and seasoning of foods is the main dietary source. Boiling, baking, and canning of foods containing iodized salt cause only small losses (≤10%) of iodine content (9). Dietary supplements often contain iodine. In the U.S. Third National Health and Nutrition Examination Survey (NHANES III), 12% of men and 15% of nonpregnant women reported regular consumption of an iodine-containing supplement, and the median adult intake of iodine from supplements was ≈140 μg/day (8). Other sources of iodine include some water purification tablets, erythrosine (a red coloring agent high in iodine that is used in foods, cosmetics, and pharmaceuticals but is poorly absorbed), radiographic contrast media, and medicines (e.g., a 200 mg tablet of amiodarone, an antiarrhythmic drug, contains 75 mg). Exposure can also occur from iodine and iodophors used as disinfectants; these have a wide range of antimicrobial action against gram-positive and -negative bacteria, fungi, and viruses. For example, povidone-iodine, the common surgical scrub and skin disinfectant, contains ≈10 mg iodine/mL.
Iodine is ingested in several chemical forms. In healthy adults, iodide is rapidly and nearly completely absorbed (>90%) in the stomach and duodenum (8). Iodide absorption in the gut is thought to be mainly passive, but active absorption may also occur through the sodium/iodide symporter (NIS)
(10) and the sodium/multivitamin transporter (SMVT) (11); both are present in intestinal mucosa. Iodate, widely used in salt iodization because of its high stability during storage in humid conditions, is rapidly reduced in the stomach and absorbed as iodide (12). Organically bound iodine is typically digested and the released iodide absorbed, but some forms may be absorbed intact; for example, ≈70% of an oral dose of thyroxine (T4), is absorbed intact (13). Iodine is cleared from the circulation mainly by the thyroid and kidney, and while renal iodine clearance (RIC) is fairly constant, thyroid clearance varies markedly depending on iodine intake. In conditions of adequate dietary iodine supply, the healthy thyroid usually takes up <20% of absorbed iodine. In chronic iodine deficiency, this fraction can exceed 80% (14,15,16). During lactation, the mammary gland concentrates iodine and secretes it into breast milk to provide for the newborn. The salivary glands, gastric mucosa, and choroid plexus also take up small amounts of iodine.
(10) and the sodium/multivitamin transporter (SMVT) (11); both are present in intestinal mucosa. Iodate, widely used in salt iodization because of its high stability during storage in humid conditions, is rapidly reduced in the stomach and absorbed as iodide (12). Organically bound iodine is typically digested and the released iodide absorbed, but some forms may be absorbed intact; for example, ≈70% of an oral dose of thyroxine (T4), is absorbed intact (13). Iodine is cleared from the circulation mainly by the thyroid and kidney, and while renal iodine clearance (RIC) is fairly constant, thyroid clearance varies markedly depending on iodine intake. In conditions of adequate dietary iodine supply, the healthy thyroid usually takes up <20% of absorbed iodine. In chronic iodine deficiency, this fraction can exceed 80% (14,15,16). During lactation, the mammary gland concentrates iodine and secretes it into breast milk to provide for the newborn. The salivary glands, gastric mucosa, and choroid plexus also take up small amounts of iodine.
Iodine in the blood is turned over rapidly; under normal circumstances, plasma iodide has a half-life of ≈10 hours, but this is shortened in iodine deficiency or hyperthyroidism (14,15,16). The body of a healthy adult contains up to 20 mg of iodine, of which 70% to 80% is in the thyroid (17). In chronic iodine deficiency, the iodine content of the thyroid may fall to <20 μg. In iodine-sufficient areas, the adult thyroid traps ≈60 μg of iodine/day to balance losses and maintain thyroid hormone synthesis. The NIS, a transmembrane protein in the basolateral membrane of the thyrocyte, transfers iodide into the thyroid at a concentration gradient 20 to 50 times that of plasma (18). The human NIS gene is located on chromosome 19 and codes for a protein of 643 amino acids (19). The NIS concentrates iodine by an active transport process that couples the energy released by the inward translocation of sodium down its electrochemical gradient to the simultaneous inward translocation of iodine against its electrochemical gradient (18). Metabolism of circulating thyroid hormone in peripheral tissues releases iodine that enters the plasma iodine pool and can be taken up by the thyroid or excreted by the kidney. More than 90% of ingested iodine is ultimately excreted in the urine, with only a small amount appearing in the feces.
Goitrogens
Although iodine deficiency is usually the cause of endemic goiter, a variety of naturally occurring compounds and environmental pollutants may be goitrogenic (20). Most goitrogens are likely to be clinically relevant only if dietary iodine supply is limited and/or goitrogen intake is high and prolonged. These compounds belong to the following chemical groups (see Chapter 57):
Sulfurated organics (like thiocyanate, isothiocyanate, goitrin, and disulfides)
Flavonoids (polyphenols)
Polyhydroxyphenols and phenol derivatives
Pyridines, phthalate esters, and metabolites
Polychlorinated (PCB) and polybrominated (PBB) biphenyls
Organochlorines (e.g., DDT)
Polycyclic aromatic hydrocarbons (PAH)
Inorganic iodine (in excess)
Lithium
Goitrogens can be broadly divided into agents acting directly on the thyroid gland and those causing goiter by indirect action (20). The former group is subdivided into those inhibiting NIS and the transport of iodide into the thyroid (e.g., thiocyanate and isothiocyanate), those acting on the intrathyroidal oxidation, binding and/or coupling of iodide (e.g., phenolics, disulfides, and goitrin) and those interfering with proteolysis, dehalogenation, and hormone release (e.g., lithium). Cigarette smoking is associated with higher serum levels of thiocyanate that may compete with iodine for uptake via the NIS into both the thyroid and the secretory epithelium of the lactating breast; smoking during the period of breastfeeding is associated with reduced iodine levels in breast milk (21). Perchlorate is a competitive inhibitor of NIS and iodine uptake (22), but 6-month exposure to perchlorate at doses up to 3 mg/day has no effect on thyroid iodide uptake or serum levels of thyroid hormones (23).
Various dietary components have been implicated as goitrogens (20). Cruciferous vegetables, including cabbage, kale, cauliflower, broccoli, turnips, and rapeseed, contain glucosinolates; their metabolites compete with iodine for thyroidal uptake. Similarly, cassava, lima beans, linseed, sorghum, and sweet potato contain cyanogenic glucosides; these may be metabolized to thiocyanates that compete with iodine for thyroidal uptake. For example, linamarin is a thioglycoside found in cassava, a staple food in many developing counties. If cassava is not adequately soaked or cooked to remove the linamarin, it is hydrolyzed in the gut to release cyanide, which is metabolized to thiocyanate. Soy and millet contain flavonoids that may impair thyroid peroxidase activity. Use of soy-based formula without added iodine may produce goiter and hypothyroidism in infants, but in healthy adults, soy-based products appear to have negligible effects on thyroid function (24).
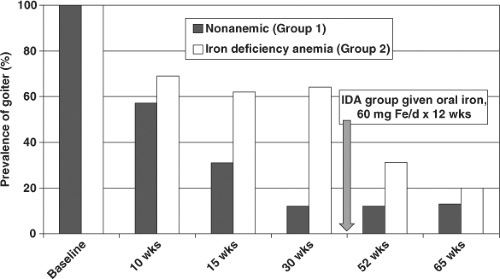
Deficiencies of selenium, iron, and vitamin A exacerbate the effects of iodine deficiency. Glutathione peroxidase and the deiodinases are selenium-dependent enzymes. In selenium deficiency, accumulated peroxides may damage the thyroid and deiodinase deficiency impairs thyroid hormone synthesis; these effects have been implicated in the etiology of myxedematous cretinism (25). Iron deficiency reduces heme-dependent thyroperoxidase activity in the thyroid and impairs production of thyroid hormone. In areas of endemic goiter, iron deficiency anemia blunts the efficacy of iodine prophylaxis while iron supplementation improves the efficacy of iodized oil and iodized salt (Fig. 11D.3) (26). Pregnant women are highly vulnerable to iron deficiency anemia, and poor maternal iron status predicts both higher thyroid stimulating hormone (TSH) and lower T4 concentrations during pregnancy in areas of borderline iodine deficiency (27). Vitamin A deficiency in iodine-deficient children increases TSH stimulation and risk for goiter through decreased vitamin A–mediated suppression of the pituitary TSHβ gene (28,29).
Table 11D.1 Recommendations for Iodine Intake (μg/day) by age or Population Group | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
Iodine requirements
Several methods have been used to estimate the requirement for iodine. Daily uptake and turnover of radioactive iodine can be used to estimate the requirement, provided that the subjects tested have adequate iodine status and are euthyroid (17,30,31). Several studies have estimated requirements from balance studies (32,33,34,35,36), but these have limitations: Many ingested
substances contain unrecognized iodine and strict control of iodine intake is difficult. Moreover, because of the need to consider the iodine in the thyroidal compartment in addition to iodine intake and excretion, even in prolonged balance studies equilibrium may not be clearly established (32). There are several methods for describing the iodine requirement (Table 11D.1). The U.S. Institute of Medicine (IOM) (8) uses the Estimated Average Requirement (EAR), the Recommended Dietary Allowance (RDA), and the Adequate Intake (AI). The EAR is the daily iodine intake that meets the requirement of half of the healthy individuals in a particular life stage. The EAR is not meant to be used in the assessment of intake in individuals, but can be used for groups. The RDA is the average daily intake sufficient to meet the iodine requirement of 97% to 98% of healthy individuals in a life stage. Individuals can use it as a goal for daily iodine intake. The RDA is derived from the EAR considering the estimated variability in individual requirements; for iodine, the corresponding RDAs are defined as the EAR plus twice the coefficient of variation (≈20%) in the population. The AI is used if there is insufficient scientific evidence to calculate an EAR. The AI is expected to meet or exceed the amount of iodine needed in “essentially all” individuals in the specified population group, and can be used as a goal for individual intake. The World Health Organization (WHO) (37) uses the Recommended Nutrient Intake (RNI) to define iodine requirements; the RNI is the intake estimated to cover the needs of “nearly all” healthy individuals in the specified life stage.
substances contain unrecognized iodine and strict control of iodine intake is difficult. Moreover, because of the need to consider the iodine in the thyroidal compartment in addition to iodine intake and excretion, even in prolonged balance studies equilibrium may not be clearly established (32). There are several methods for describing the iodine requirement (Table 11D.1). The U.S. Institute of Medicine (IOM) (8) uses the Estimated Average Requirement (EAR), the Recommended Dietary Allowance (RDA), and the Adequate Intake (AI). The EAR is the daily iodine intake that meets the requirement of half of the healthy individuals in a particular life stage. The EAR is not meant to be used in the assessment of intake in individuals, but can be used for groups. The RDA is the average daily intake sufficient to meet the iodine requirement of 97% to 98% of healthy individuals in a life stage. Individuals can use it as a goal for daily iodine intake. The RDA is derived from the EAR considering the estimated variability in individual requirements; for iodine, the corresponding RDAs are defined as the EAR plus twice the coefficient of variation (≈20%) in the population. The AI is used if there is insufficient scientific evidence to calculate an EAR. The AI is expected to meet or exceed the amount of iodine needed in “essentially all” individuals in the specified population group, and can be used as a goal for individual intake. The World Health Organization (WHO) (37) uses the Recommended Nutrient Intake (RNI) to define iodine requirements; the RNI is the intake estimated to cover the needs of “nearly all” healthy individuals in the specified life stage.
Infancy and childhood
Because no functional criteria are available that reflect iodine intake in infants, the AI from the IOM is based on mean iodine intake of healthy full-term infants fed human milk. The current AI is based on a median breast milk iodine content (BMIC) of women in the United States in the early 1980s of 146 μg/L, providing ≈115 μg/day to the fully breastfeeding infant (8). Thus, the AI for infants aged 0 to 6 months is set at 110 μg/day (8). But because iodine intakes in the United States population were excessive in the early 1980s (38), the BMIC used was at the upper end of the range of 78 to 167 μg/L reported for iodine-sufficient countries (39). Although high maternal iodine intakes can increase BMIC, iodine intakes by the infant greater than his or her requirements will simply be excreted in the urine. Thus, iodine requirements during lactation should be based on infant balance studies rather than the measured but variable amount excreted in breast milk from women in iodine-sufficient countries. Balance studies in full-term infants fed 20 μg/kg/day of iodine found that iodine retention was 7.3 μg/kg/day (40). If the reference body weight at 6 months of age is 7 kg (8), daily
retention of iodine in a 6-month old infant in positive balance is ≈50 μg. Thus, it is likely that the current AI of 110 to 130 μg/day and the WHO RNI of 90 μg/day exceed actual infant requirements.
retention of iodine in a 6-month old infant in positive balance is ≈50 μg. Thus, it is likely that the current AI of 110 to 130 μg/day and the WHO RNI of 90 μg/day exceed actual infant requirements.
In a balance study in children aged 1.5 to 2.5 years (41), the median iodine intake was 63.5 μg/day, and the average iodine balance was +19 μg/day. Children 8 years of age who consumed ≈40 μg/day of iodine were in negative iodine balance (-23 to -26 μg/day), suggesting that the average minimum requirement is approximately 65 μg/day (42). Therefore, an EAR of 65 μg/day was set for ages 1 to 8 years (8). For the remainder of childhood and adolescence, there are few data available for estimating requirements so the EAR was set by extrapolating down from adult data (8). WHO recommends a daily intake of iodine of 90 μg for preschool children (0 to 59 months) and 120 μg for schoolchildren (6 to 12 years) (37).
Adulthood
Iodine turnover, thyroidal radioiodine uptake, and balance studies in euthyroid adults have suggested the average daily requirement for iodine is 91 to 96 μg/day (17,30,32). There is no evidence to suggest that the average iodine requirement in adults varies with age. Thus, the EAR for iodine for men and nonpregnant, non-lactating women ≥14 years from the IOM has been set at 95 μg/day (8). The corresponding RDA is 150 μg/day (8). This agrees with the WHO recommendation for adequate daily iodine intake of 150 μg/day for men and nonpregnant, non-lactating women (37).
Pregnancy and Lactation
The iodine requirement during pregnancy is increased due to: (1) an increase in maternal T4 production to maintain maternal euthyroidism and transfer thyroid hormone to the fetus early in the first trimester, before the fetal thyroid is functioning, (2) iodine transfer to the fetus, particularly in later gestation, and (3) an increase in RIC (43). Balance studies have found that the average iodine retention of full-term infants is 7.3 μg/kg/day (40); the mean retention of a healthy fetus with a weight of 3 kg would be ≈22 μg/day. Estimated daily fetal iodine retention added to the EAR of 95 μg/day for nonpregnant women would yield an EAR of 117 μg/day, but this would not take into account the iodine needed to increase maternal T4 production and balance additional urinary losses. Dworkin et al. (32) found that five pregnant women were at balance when consuming ≈160 μg/day, with no significant differences pre- and postpartum. Several authors have roughly estimated iodine requirements during pregnancy by correlating the effects of iodine supplementation with changes in thyroid volume during pregnancy: In Italian (44) and Danish (45) pregnant women, total daily iodine intakes of 200 to 280 μg/day prevented an increase in thyroid volume, while in Belgian pregnant women (46), total daily iodine intake of ≈150 μg/day was insufficient to prevent an increase in thyroid size. The IOM set the EAR at 160 μg/day for pregnancy in women ≥14 years, and the RDA at 220 μg/day (8). WHO recommends a daily iodine intake of 250 μg/day for pregnant women, a value ≈10% higher than the RDA (37). On the basis of mean breast milk excretion of 0.78 and 0.6 L/day in the first and second 6 months of infancy, respectively (8), and a mean BMIC of 146 μg/L in iodine-sufficient women from the United States, the average daily loss of iodine in breast milk has been estimated to be ≈115 μg/day (8). Added to the EAR for nonpregnant women of 95 μg/day, the EAR for lactating women ≥14 years is set at 209 μg/day by the IOM (8), and the RDA is 290 μg/day. WHO recommends a daily iodine intake of 250 μg/day for lactating women (37).
Assessment of iodine deficiency
Four methods are generally recommended for assessment of iodine nutrition in populations: Urinary iodine concentration (UIC), the goiter rate, TSH, and thyroglobulin (Tg). These indicators are complementary, in that UIC is a sensitive indicator of recent iodine intake (days) and Tg shows an intermediate response (weeks to months), whereas changes in the goiter rate reflect long-term iodine nutrition (months to years).
Table 11D.2 Epidemiologic Criteria from the world Health Organization for Assessment of Iodine Nutrition in A Population Based on Median or Range of Urinary Iodine Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Urinary Iodine Concentration
Because >90% of dietary iodine eventually appears in the urine (36,47), UIC is an excellent indicator of recent iodine intake. UIC can be expressed as a concentration (μg/L), in relation to creatinine excretion (μg iodine/g creatinine), or as 24-hour excretion (μg/day). For population surveys, UIC can be measured in spot urine specimens from a representative sample of the target group, and expressed as the median, in μg/L (37). Variations in hydration among individuals generally even out in a large number of samples, so that the median UIC in spot samples correlates well with that from 24-hour collections. For national, school-based surveys of iodine nutrition, the median UIC from a representative sample of spot urine collections from ≈1,200 children (30 sampling clusters × 40 children per cluster) can be used to classify a population’s iodine status (37) (Table 11D.2). Although the median UIC does not provide direct information on thyroid function, a low value suggests that a population is at higher risk of developing thyroid disorders. Daily iodine intake for population estimates can be extrapolated from UIC, using estimates of mean 24-hour urine volume and assuming an average iodine bioavailability of 92% using the formula (8):
Using this formula, a median UIC of 100 μg/L in a group of adults corresponds roughly to an average intake of 150 μg/day.
Although the median UIC is a good indicator of iodine status in populations, its value for assessing individual status is limited by high day-to-day variability in iodine intakes. In European adults, intra-individual variation in UIC is ca. 35% for both 24-hour collections and spot samples, so that urine samples from ≈10 different days are needed to assess individual iodine status with 20% precision (48). In population studies, the median UIC is often misinterpreted: A common mistake is to assume that all subjects with a spot UIC <100 μg/L are iodine deficient. But because individual iodine intakes are highly variable from day-to-day, on any given day, it is inevitable that some individuals will have a low UIC, despite average daily intakes that are adequate to maintain thyroidal iodine stores (48).
Thyroid Size
Two methods are available for measuring goiter: Neck inspection and palpation, and thyroid ultrasonography. By palpation, athyroid is considered goitrous when each lateral lobe has a volume greater than the terminal phalanx of the thumbs of the subject being examined. In the classification system of WHO (37), grade 0 is defined as a thyroid that is not palpable or visible, grade 1 is a goiter that is palpable but not visible when the neck is in the normal position (i.e., the thyroid is not visibly enlarged), and grade 2 goiter is a thyroid that is clearly visible when the neck is in a normal position. Goiter surveys are usually done in school age children. However, palpation of goiter in areas of mild iodine deficiency has poor sensitivity and specificity; in such areas, measurement of thyroid volume by ultrasound is preferable. Thyroid ultrasound is noninvasive, quickly done (2 to 3 minutes per subject) and feasible even in remote areas using portable equipment. However, interpretation of thyroid volume data requires valid references from iodine-sufficient children. In a recent multicenter study, thyroid volume was measured in 6- to 12-year-old children living in areas of long-term iodine sufficiency on five continents. Age-specific and body surface area–specific 97th percentiles for thyroid volume were calculated for boys and girls (49). Goiter can be classified according to these international reference criteria, but they are only applicable if thyroid volume is determined by a standard method (37). Thyroid ultrasound is subjective and requires judgment and experience. Differences in technique can produce interobserver errors in thyroid volume as high as 26% (50).
In areas of endemic goiter, although thyroid size predictably decreases in response to increases in iodine intake, thyroid size may not return to normal for months or years after correction of iodine deficiency (51,52). During this transition period, the goiter rate is difficult to interpret, because it reflects both a population’s history of iodine nutrition and its present status. Enlarged thyroids in school-age children who were iodine deficient during the first years of life may not regress completely after iodine repletion (51). If true, this suggests that to achieve a goiter rate of <5% in children may require that they grow up under conditions of iodine sufficiency. A sustained salt iodization program will decrease the goiter rate to <5% in school-age children and this indicates disappearance of iodine deficiency as a significant public health problem (37). WHO recommends the total goiter rate be used to define severity of iodine deficiency in populations using the following criteria: <5%, iodine sufficiency; 5.0% to 19.9%, mild deficiency; 20.0% to 29.9%, moderate deficiency; and >30%, severe deficiency (37).
Thyroid Stimulating Hormone and Thyroid Hormones
Circulating TSH is an indirect indicator of iodine nutrition because its level in blood is determined mainly by the level of circulating thyroid hormone, which in turn reflects iodine
intake. However, in older children and adults, although serum TSH may be variably increased by iodine deficiency, values often remain within the normal range. An elevated TSH is therefore an insensitive indicator of iodine nutrition in adults. It may, however, be a sensitive indicator of iodine status in the newborn period (53,54), as discussed later in this chapter. Similarly, thyroid hormone concentrations are generally poor indicators of iodine status in children and adults, except in severe deficiency, when hypothyroidism develops. In mild-to-moderately deficient populations, serum T3 increases or remains unchanged, and serum T4 may decrease, but these changes are often within the normal range, and the overlap with iodine-sufficient populations is large. Thus, thyroid hormone concentrations are an insensitive measure of iodine nutrition (37,55).
intake. However, in older children and adults, although serum TSH may be variably increased by iodine deficiency, values often remain within the normal range. An elevated TSH is therefore an insensitive indicator of iodine nutrition in adults. It may, however, be a sensitive indicator of iodine status in the newborn period (53,54), as discussed later in this chapter. Similarly, thyroid hormone concentrations are generally poor indicators of iodine status in children and adults, except in severe deficiency, when hypothyroidism develops. In mild-to-moderately deficient populations, serum T3 increases or remains unchanged, and serum T4 may decrease, but these changes are often within the normal range, and the overlap with iodine-sufficient populations is large. Thus, thyroid hormone concentrations are an insensitive measure of iodine nutrition (37,55).
Thyroglobulin
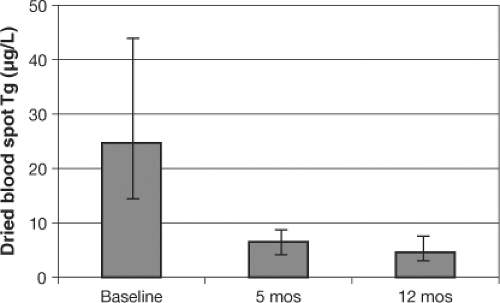
Thyroglobulin (Tg) is synthesized only in the thyroid, and is the most abundant intrathyroidal protein. In iodine sufficiency, small amounts of Tg are secreted into the circulation, and serum Tg is normally <10 μg/L (56). In areas of endemic goiter, serum Tg increases due to greater thyroid cell mass and TSH stimulation. Serum Tg is well correlated with the severity of iodine deficiency as measured by UIC (57). Intervention studies examining the potential of Tg as an indicator of response to iodized oil and potassium iodide have shown that Tg concentrations fall rapidly with iodine repletion, and that Tg is a more sensitive indicator of iodine repletion than TSH or T4 (46,58). Commercial Tg assays measure serum concentrations, but iodine surveys are often done in remote areas where venipuncture, centrifugation, and frozen sample transport are difficult. Thus, a new assay for Tg has been developed for dried blood spots taken by a finger prick (40,59,60), simplifying collection and transport. In prospective studies, dried blood spot Tg has been shown to be a sensitive measure of iodine status and reflects improved thyroid function within several months after iodine repletion (Fig. 11D.4) (59). Several questions need to be resolved before Tg can be widely adopted as an indicator of iodine status. One question is the need for concurrent measurement of anti-Tg antibodies to avoid potential underestimation of Tg; it is unclear how prevalent anti-Tg antibodies are in iodine deficiency, or whether they are precipitated by iodine prophylaxis (61,62). Another potential limitation is large inter-assay variability and poor reproducibility, even with the use of standardization (56). This has made it difficult to establish normal ranges and/or cutoffs to distinguish severity of iodine deficiency. However, an international reference range and a reference standard for DBS Tg in iodine-sufficient schoolchildren (4 to 40 μg/L) has recently been made available (60). The use of Tg to assess population response to introduction of iodized salt has been demonstrated in a national study in Denmark, where correction of mild-to-moderate iodine deficiency decreased the prevalence of elevated Tg (>40 μg/L) from 11.3% to 3.7% (63).
Assessing Iodine Status in Pregnancy, Lactation, and Infancy
The median UIC is recommended by WHO/ICCIDD/UNICEF (37) for assessing iodine nutrition in pregnant women. The expected UIC in μg/L can be extrapolated from a recommended daily iodine intake assuming median 24-hour urine volumes of 0.9 mL/hr/kg for girls aged 7 to 15 years and of ≈1.5 L for adult women (64,65), and assuming a mean iodine bioavailability of 92% (using the formula presented above). The recommended daily iodine intakes for pregnancy of 220 to 250 μg (8,37) would correspond to a UIC of ≈135 to 150 μg/L. Pregnancy may occur in adolescence, particularly in developing countries; in a 15-year-old girl weighing ≈50 kg, daily iodine intake of 220 and 250 μg would correspond to a UIC of ≈185 to 215 μg/L. However, during pregnancy this estimation of intake from UIC may be less valid due to an increase in glomerular filtration rate and, possibly, RIC. If RIC increases in pregnancy, the daily iodine intake extrapolated from the UIC in pregnancy would be lower than that in nonpregnancy. However, the evidence for an increase in RIC and a decrease in plasma inorganic iodide concentration during pregnancy is equivocal. One study (66) suggested an increase in RIC using an indirect method, while Liberman et al. (67) directly measured plasma inorganic iodide and reported no significant difference comparing pre- and postpartum values from 16 women in an area of high iodine intake. One iodine balance study (32) also found no differences in UIC pre- and postpartum. Thus, it is unclear if pregnancy per se significantly increases UIC. Considering this uncertainty, a recent WHO expert group recommended the median UIC that indicates adequate iodine intake during pregnancy to be 150 to 249 μg/L (37,68) (Table 11D.2). However, WHO emphasized that the data on which the recommendation is based are limited (68).
Using a median cutoff of 150 μg/L, several recent studies have found suboptimal iodine status in pregnant women from areas with only partial household coverage with iodized salt, including Italy, India, Thailand, and the United States (69,70,71,72). Traditionally, the median UIC in school-aged children is recommended for assessment of iodine nutrition in populations. If the median UIC is adequate in children, it is usually assumed that iodine intakes are also adequate in the remaining population,
including pregnant women. However, a recent Thai study in families eating from the same household food basket found that the median UIC in schoolchildren was 200 μg/L, while the median UIC in their pregnant mothers was only 108 μg/L (71). Thus, the median UIC in school-aged children may not always be a good surrogate for monitoring iodine status in pregnancy, and it may be necessary to directly monitor pregnant women. For the breastfeeding mother, although the iodine requirement is high (200 to 290 μg/day), after accounting for iodine losses into breast milk, the median UIC in lactating women that indicates adequate iodine nutrition is the same as that of nonpregnant, non-lactating women, that is, 100 to 199 μg/L (37).
including pregnant women. However, a recent Thai study in families eating from the same household food basket found that the median UIC in schoolchildren was 200 μg/L, while the median UIC in their pregnant mothers was only 108 μg/L (71). Thus, the median UIC in school-aged children may not always be a good surrogate for monitoring iodine status in pregnancy, and it may be necessary to directly monitor pregnant women. For the breastfeeding mother, although the iodine requirement is high (200 to 290 μg/day), after accounting for iodine losses into breast milk, the median UIC in lactating women that indicates adequate iodine nutrition is the same as that of nonpregnant, non-lactating women, that is, 100 to 199 μg/L (37).
Because the mammary gland is able to concentrate iodine, iodine supply to the newborn via the breast milk may be maintained even in the face of maternal iodine deficiency (73,74). This may help explain why, in areas of iodine deficiency, BMICs are often greater than expected based on the UIC of the lactating mother (74,75,76). For example, lactating women in the United States with a median UIC of 114 μg/L had a median BMIC of 155 μg/L (range, 3 to 1,968 μg/L) (75). However, a recent controlled trial in iodine deficient New Zealand women reported a ≈40% decrease in BMIC over 6 months of breastfeeding. In this study, daily supplementation with 75 or 150 μg iodine increased the BMIC but was insufficient to ensure adequate iodine status in women or their infants (77). Based on the balance studies of Delange et al., the full-term infant’s requirement for iodine is ≈7 μg/kg (40). On the basis of mean breast milk excretion of 0.78 L in the first 6 months of infancy (8), and assuming that the iodine in breast milk is 95% absorbed, a BMIC ≥80 μg/L would likely cover the infant’s iodine requirement (≈50 μg/day) until weaning foods are begun.
WHO recommendations state that a median UIC ≥100 μg/L in infants is sufficient (37). At the same time, they recommend a high iodine intake of 90 μg/day during infancy (37) and suggest extrapolating from this to a median UIC assuming a urine volume of 300 to 500 mL/day, but this would produce a higher cutoff of ≥180 μg/L. To clarify this, UIC were recently measured in a representative national sample of healthy, term, iodine-sufficient, euthyroid, breastfeeding Swiss infants (78). Median UIC was 77 (95% CI; 73,78) μg/L, and extrapolating from this median UIC assuming a urine volume of 300 to 500 mL/day suggests the mean daily iodine intake in iodine-sufficient Swiss newborns in the first week is 30 to 50 μg/day. This estimated iodine intake is consistent with estimated infant requirement of 40 μg iodine/day extrapolated from the relative energy requirements of adults in the 1989 U.S. RDAs (79).
Infants during the weaning period may be at risk of iodine deficiency, because iodized salt (and, in industrialized countries, iodine in milk) contribute little dietary iodine during this period. To fill this gap, iodine in infant formula milk and complementary foods is likely important (80). The challenge to the assessment of iodine intakes in infancy has been sample collection, but a simple collection system that may facilitate the use of UIC as an indicator of iodine status in newborns is available (78). Using this method, a recent Swiss study has suggested that even in countries where iodized salt programs supply sufficient iodine to older children and pregnant women, weaning infants, particularly those not receiving commercial infant foods containing iodine, may be at risk of inadequate iodine intakes (81).
TSH screening in newborns may also be useful in assessing iodine status in late pregnancy and the newborn period (37,54,82). TSH is used in many countries for routine newborn screening to detect congenital hypothyroidism. If already in place, such screening offers a sensitive indicator of iodine nutrition (37). Newborn TSH is an important measure because it reflects iodine status during a period when the developing brain is particularly sensitive to iodine deficiency. Compared to the adult, the newborn thyroid contains less iodine but has higher rates of iodine turnover. Particularly when iodine supply is low, maintaining high iodine turnover requires increased TSH stimulation. Serum TSH concentrations are therefore increased in iodine-deficient infants for the first few weeks of life. As demonstrated in national data from Switzerland (54), if <3% of newborn TSH values are above 5 μ/L in whole blood collected 3 to 4 days after birth, this suggests iodine sufficiency in the population (37). This cutoff needs confirmation in other iodine-sufficient countries with newborn screening programs.
Table 11D.3 the Iodine Deficiency Disorders, By age Group | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Effects of deficiency through the lifecycle
Iodine deficiency has multiple adverse effects on growth and development in humans. These are collectively termed the iodine deficiency disorders (IDDs) (Table 11D.3), and are one of the most important and common human diseases (4,83). They result from inadequate thyroid hormone production due to lack of sufficient iodine.
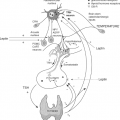
Thyroidal Adaptation to Iodine Deficiency
The thyroid adapts to low intakes of dietary iodine by marked modification of its activity. In most adults, if iodine intake falls below ≈100 μg/day, TSH secretion is augmented. This
increases plasma inorganic iodide clearance by the thyroid through stimulation of NIS expression. TSH exerts its action at the transcription level of the NIS gene through a thyroid-specific enhancer that contains binding sites for the transcription factor Pax8 and a cAMP response element–like sequence (84). There is a clear inverse relation between dietary iodine supply and thyroidal uptake of radioiodide. However, in areas of endemic goiter, a generalized increase in TSH is seen in populations only when iodine deficiency is severe. In areas of milder iodine deficiency, TSH is usually only increased in a minority of subjects, often the youngest. Thus, it is possible that thyroid sensitivity to TSH (rather than the TSH level itself) varies with iodide supply.
increases plasma inorganic iodide clearance by the thyroid through stimulation of NIS expression. TSH exerts its action at the transcription level of the NIS gene through a thyroid-specific enhancer that contains binding sites for the transcription factor Pax8 and a cAMP response element–like sequence (84). There is a clear inverse relation between dietary iodine supply and thyroidal uptake of radioiodide. However, in areas of endemic goiter, a generalized increase in TSH is seen in populations only when iodine deficiency is severe. In areas of milder iodine deficiency, TSH is usually only increased in a minority of subjects, often the youngest. Thus, it is possible that thyroid sensitivity to TSH (rather than the TSH level itself) varies with iodide supply.
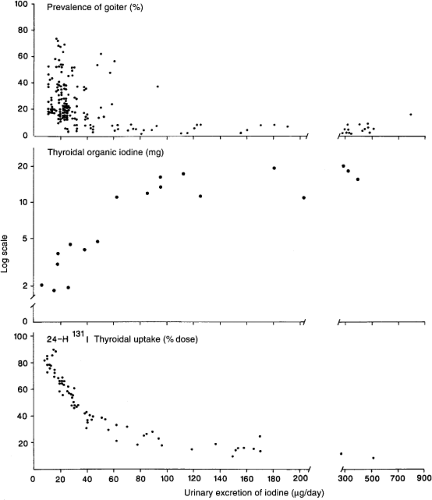
As the thyroid clears a greater fraction of circulating iodide, there is a progressive reduction in renal iodide excretion, and the UIC falls. Nevertheless, in most healthy adults, as long as daily iodine intake is at least ≈60 μg, despite a decrease in circulating plasma inorganic iodine and UIC, absolute uptake of iodine by the thyroid and thyroidal iodine stores remain adequate. Below this threshold, despite maximal fractional clearance of circulating iodine by the thyroid, absolute uptake falls, the iodine content of the thyroid is depleted, and many individuals develop goiter (Fig. 11D.5).
In iodine deficiency, goiters are initially characterized by diffuse, homogeneous enlargement, but over time, nodules often develop (Fig. 11D.6). While iodine deficiency produces diffuse goiter in all age groups, it also increases risk for multinodular toxic goiter, mainly in women older than 50 years (85). Many thyroid nodules derive from a somatic mutation and are of monoclonal origin (86); the mutations appear to be more likely in nodules under the influence of a growth promoter, such as iodine deficiency. There are no gross or microscopic features that distinguish the thyroid of endemic goiter from changes that appear in simple and sporadic goiter. In mild iodine deficiency, there is usually only a slight enlargement of the thyroid and the characteristic microscopic findings are hyperplasia with abundant parenchyma, high follicular epithelium, and rare colloid. In chronic severe deficiency, repeated episodes of hyperplasia followed by involution and atrophy result in a thyroid with a mixture of nodules, zones of hyperplasia, and involuting, degenerative, and repair elements. Scanning with radioiodide or pertechnetate shows a mottled distribution of the isotope. In areas known to be iodine deficient, goiter is usually assumed to be due to iodine deficiency, but the clinician must always be wary of missing individual patients with goiter due to other causes, such as thyroiditis, thyrotoxicosis, or thyroid carcinoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree