Abstract
Distant metastasis is the leading cause of tumor-related death from breast cancer. Hematogenous dissemination of tumor cells from the primary tumor to distant sites cannot be detected by standard imaging methods. There has been growing interest in the role of circulating tumor cells (CTCs) in the peripheral blood, which may serve as a marker of occult systemic micrometastatic disease. The detection and characterization of CTCs has provided important insight into early development of metastasis, as well as prognostic and predictive information in metastatic and early breast cancer. In this review, we describe the biology, detection strategies, and clinical application of CTCs. The genetic and molecular characterization of CTCs has great potential to improve the understanding of tumor biology and to optimize personalized cancer treatment.
Keywords
breast cancer, micrometastasis, circulating tumor cells, liquid biopsy
Breast cancer is the most common cancer type diagnosed among women in the United States with an estimated 232,000 new cases and 40,290 deaths in 2015; one in eight women will develop breast cancer during her lifetime. The leading cause of death is complications from distant metastases. To date, surgical resection of the primary tumor followed by adjuvant systemic therapy remains the standard of care for early-stage breast cancer. Despite successful primary treatment leading to a significant decrease in breast cancer–related mortality, approximately 30% of women initially diagnosed with early-stage cancer will develop metastatic disease. These relapses, many of which occur years after completion of adjuvant therapy, are often due to systemic micrometastatic tumor spread. Undetected micrometastasis can contribute to failure of primary treatment for breast cancer.
Single cancer cells may be shed by the primary tumor early in the course of disease, disperse throughout the body hematogenously and serve as a precursor for future metastatic growth at secondary sites. The importance of hematogenous dissemination of malignant cells from solid tumors was first recognized in the 19th century. In 1869, Thomas Ashworth described the presence of tumor cells in the peripheral circulation. Stephen Paget developed the “seed and soil” hypothesis in 1889, describing the interaction between tumor cells and the microenvironment of secondary homing sites. Unfortunately, the hematogenous spread of tumor cells from the primary tumor to distant sites cannot be detected by standard imaging methods. Therefore it is important to find new biomarkers that may effectively detect early development of systemic micrometastasis.
Detection of disseminated tumor cells (DTCs) in the bone marrow and of circulating tumor cells (CTCs) in the peripheral blood has become an important focus of translational research in breast cancer. CTCs are rare cancer cells that are released from tumors into the bloodstream and are thought to play a key role in cancer metastasis. The bone marrow is a common homing organ for DTCs derived from epithelial tumors of different organs including the breast and may serve as a reservoir for DTCs with the ability to enter other distant organs. Analysis of bone marrow aspirates from breast cancer patients has provided important information on the prognostic relevance of DTCs. In 2005 a large meta-analysis of 4703 patients with early breast cancer (stages I–III) enrolled in nine clinical studies showed that bone marrow micrometastasis was detected in 30.6% of patients at the time of initial diagnosis. Compared with women without bone marrow micrometastasis, patients with bone marrow micrometastasis had larger tumors, tumors with higher histologic grade, and were more often hormone receptor (HR)-negative and had frequent lymph node metastases ( p < .001 for all variables). The presence of micrometastasis was found to be a significant independent prognostic factor for worse breast cancer–specific survival (BCSS) and overall survival (OS) ( p < .001 for both outcomes).
A disadvantage of bone marrow sampling is the invasiveness of the procedure, and subsequent studies have focused on the evaluation of easily accessible CTCs in the peripheral blood. CTCs may be considered a surrogate marker for micrometastasis and can provide important prognostic and predictive information. In this chapter, we discuss the biological properties, detection methods, and prognostic relevance of CTCs, as well as their promising role in predicting and monitoring response to therapy in patients with early and metastatic breast cancer.
Gene Expression Profiling of Breast Cancer Cells
According to the traditional theory of carcinogenesis, metastasis arises from a small subpopulation of primary tumor cells that occur during later stages of tumor development. This model predicts that multiple genetic and epigenetic changes underlie the initiation of tumor invasiveness and subsequent multistep progression to metastasis. As increasing information has become available on gene expression profiling studies of primary breast cancers, this theory has been challenged by another model in which the tendency to metastasize is determined by a poor prognosis gene expression signature present within the primary tumor. In other words, cancer cells in a primary tumor may already possess a metastatic phenotype, and dissemination starts early in tumor development.
The theory of early metastasis is supported by gene expression studies, which reveal that patients with poorer prognosis can be identified before manifestation of overt metastases. A study used gene expression profiling by DNA microarray analysis of primary breast tumors from 117 patients to predict clinical outcome by identifying a gene expression signature that was strongly predictive of short interval to distant metastases in patients without tumor involvement in local lymph nodes at time of diagnosis. The poor prognosis signature consisted of genes regulating the cell cycle, invasion, metastasis, and angiogenesis. Another study demonstrated that dissemination of tumor cells in preclinical models of breast cancer, as well as from ductal carcinoma in situ in women, could occur in preinvasive stages of tumor progression.
Additional genetic or epigenetic events and release from dormancy are critical for the metastatic growth of early-disseminated cancer cells. One study isolated single disseminated cancer cells from the bone marrow of breast cancer patients (n = 371) and performed single-cell comparative genomic hybridization. The patients either underwent curative resection of the primary tumor (M0) or had overt metastases (M1); the disseminated cells were compared with the matched primary tumor. Disseminated cells from M0 patients had significantly fewer chromosomal aberrations compared with primary tumors or cells from M1 patients ( p < .008 and p < .0001, respectively). A possible explanation may be that the disseminated cancer cells may have separated from the primary tumor at an early stage and evolved independently, suggesting an earlier dissemination.
The hypothesis that cells of primary tumors have metastatic capacity has also been supported by gene expression analyses that identified signaling pathways involved in dissemination of cancer cells to distant organs. A study showed that primary hematogenous dissemination of breast tumor cells is a selective process associated with a specific molecular signature. Expression analysis with DNA microarray showed distinct molecular profiles between primary tumors from patients with bone marrow micrometastasis compared with patients without. The differentially expressed genes in those with micrometastasis—in particular, dysregulation of RAS and the hypoxia-inducible factor 1α pathway (HIF-1α) —were involved in extracellular matrix remodeling, cell adhesion, and signal transduction. RAS activation is known to increase cellular proliferation, and HIF-1α is involved in hypoxia-related processes (e.g., angiogenesis, cellular metabolism, and proliferation) associated with metastasis. In addition, carboxypeptidase N (CPN) is a metallopeptidase that plays an important role in regulating vasoactive peptide hormones, cytokines, and growth factors by cleaving their C-terminal basic residues. Li and colleagues showed that the circulating peptides generated by CPN in the breast tumor microenvironment can serve as a biomarker of early disease onset and progression.
Cancer Stem Cells
The biology of DTCs and CTCs remains not well understood. Characteristics include nucleated cells with expression of cytokeratin (CK) and absence of the leukocyte marker, CD45. A subpopulation of CTCs—cancer stem cells—has been shown to harbor tumorigenic potential. The cancer stem cell theory describes the small population of tumor cells that are capable of quiescence, self-renewal, sustaining tumor formation, and differentiation into heterogeneous population of cancer cells. Cancer stem cells are also often resistant to conventional treatments, such as chemotherapy and radiotherapy. Breast cancer stem cells have mostly been associated with a CD44 + CD24 −/low phenotype or by expression of aldehyde dehydrogenase 1 (ALDH1). Cells with this phenotype have been shown to be multipotent and retain tumorigenic activity. CD44 is involved in cell-to-cell interactions, cell adhesion, and migration. CD24 is expressed in early stages of B-cell development and on neutrophils. ALDH is a detoxifying enzyme responsible for oxidation of intracellular aldehydes and is involved in early differentiation of stem cells through oxidizing retinol to retinoic acid.
Esteva and colleagues investigated the relationship between serum concentration of CD44 and clinicopathologic features—in particular, human epidermal growth factor receptor 2 (HER2)—in 110 patients (of which 56 patients were HER2 positive) with breast cancer at a single institution. Serum samples were collected before definitive surgery or before initiation of neoadjuvant chemotherapy (if indicated) for those with stage I to III breast cancer and before initiation of systemic therapy in patients with stage IV breast cancer. Serum CD44 concentration correlated with tumor stage ( p = .0308), and serum CD44 levels were significantly higher in stage IV patients with liver metastases ( p = .0211) than in those with distant metastases to other sites. The OS rate did not differ between patients with high CD44 concentration and patients with low concentration. However, serum CD44 concentration significantly predicted OS for patients with HER2-positive breast cancer, but not for patients with HER2-negative breast cancer, suggesting a role for serum CD44 as a prognostic marker in this subtype.
Many early-disseminated cancer cells detected in the bone marrow of breast cancer patients possess a cancer stem cell phenotype. Balic and colleagues evaluated bone marrow specimens from early breast cancer patients for cancer stem cells by immunohistochemistry and found that the majority of DTCs in the bone marrow (71%) had a putative stem cell phenotype (CD44 + CD24 − ), compared with primary tumors where this phenotype represented a minor population (10%–20%). Ginestier and colleagues showed that ALDH1 is a common functional marker of both normal and malignant mammary stem cells. In breast carcinomas, cells with high ALDH1 activity contain the tumorigenic cell fraction, which is capable of self-renewal and the ability to generate tumors that recapitulate heterogeneity of the parental tumor. In a series of 577 breast carcinomas, high expression of ALDH1 in the tumors was associated with poor clinical outcome. The identification of normal and malignant stem/progenitor cells by the same ALDH1 marker lend support to the cancer stem cell hypothesis and offer new possibilities for studying mammary stem cells and their role in tumorigenesis.
Methods for Analysis of CTCs
The capability to detect CTCs in the peripheral blood of breast cancer patients is promising, with many technologies developed in recent years. However, CTCs are rare and present in very low concentrations in the blood, typically 1 per 10 6 to 10 8 mononuclear cells, therefore their isolation presents a technical challenge. CTCs can survive in a dormant state in the peripheral blood for many years, and overall, only a small fraction ever gives rise to distant metastases. A preclinical model showed that 2.5% of CTCs formed micrometastasis, most of which subsequently disappeared over time, and 0.01% of CTCs eventually formed macrometastases. Because of their rarity, the identification and characterization of CTCs require highly sensitive and specific methods, which comprise an essential combination of enrichment (isolation) and detection (identification) procedures ( Fig. 66.1 ).
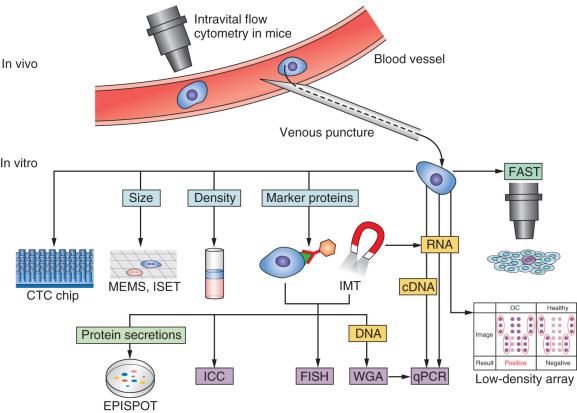
CTC Enrichment
CTC enrichment strategies are based on technologies that can distinguish CTCs among the surrounding hematopoietic cells according to their physical (e.g., size, density, electrical charge, cell deformity) and biological (e.g., cell surface protein expression, viability) characteristics. Advantages and disadvantages of the main techniques are summarized in Table 66.1 .
| Technique | Advantages | Disadvantages |
|---|---|---|
| CellSearch system | FDA approved High sensitivity Visual confirmation of CTCs Clinical relevance Semiautomated system | CTCs may be lost during enrichment that lack EpCAM expression (false negative) CTC determination is subjective Limited number of markers |
| Microfiltration (e.g., ISET) | Simple, fast, and inexpensive Can be used for EpCAM-negative CTCs | Smaller CTCs may be missed (low specificity) |
| Density gradient centrifugation (e.g., Ficoll, OncoQuick) | Simple, fast, and inexpensive Can be used for EpCAM-negative CTCs | Low specificity Low sample purity Cross-contamination of different layers (OncoQuick avoids this by incorporating a porous barrier) |
| CTC-chip | Visual confirmation of CTCs High sensitivity Additional molecular and genetic analysis possible | Dependent on EpCAM-positivity CTC determination is subjective |
| Immunocytochemistry | Morphologic analysis and quantification of CTCs Facilitates classical cytopathological review | CTC determination is subjective Time-consuming |
| Epithelial ImmunoSPOT (EPISPOT) assay | High sensitivity and high specificity Detects only viable cells | CTCs are not collected and subsequent cellular analysis cannot be performed Not an automated system |
| AdnaTest (RT-PCR) | High sensitivity Detects only viable cells | EpCAM and MUC1 dependent No morphologic analysis No quantification |
| CTCscope (RT-PCR) | High sensitivity Detects only viable cells | No morphologic analysis No visualization or quantification of CTCs |
Physical Properties
A method for CTC separation based on physical properties (i.e., label-independent technologies) includes size separation through special filters (e.g., isolation by size of epithelial tumor cells, or ISET). A limitation of filtration by size may be loss of smaller CTCs or clotting of filter pores by leukocytes. Another technique is based on density gradient separation (e.g., Ficoll, OncoQuick). Other methods based on physical properties of CTCs include a biochip that uses differences in size and deformability of cancer cells compared with blood cells, a photoacoustic flow cytometer, a microfluidics device that combines dielectrophoresis (DEP) and multiorifice flow fractionation cell separation techniques, and a DEP field-flow fractionation method that isolates viable CTCs due to differences in response to DEP.
Biological Properties
CTC separation methods based on biological properties (i.e., label-dependent technologies) primarily use immunologic techniques with antibodies directed against either tumor-associated antigens (positive selection) or CD45 (negative selection). Immunomagnetic assays target an antigen by an antibody coupled to a magnetic bead, and the antigen-antibody complex is subsequently isolated from the solution by exposure to a magnetic field. The majority of positive selection technologies are carried out with antibodies against the epithelial cell adhesion molecule (EpCAM), which is frequently overexpressed by breast cancer and is absent from hematologic cells.
Enrichment of CTCs by immunomagnetic capture has been the most successful and widely used approach to date, and among EpCAM-based technologies, the US Food and Drug Administration (FDA) has approved the CellSearch (Veridex) platform and the Ariol system. The current gold standard remains the CellSearch system, which combines semiautomated enrichment of EpCAM-positive cells using magnetic nanoparticles and characterization of CTCs by immunofluorescent staining of CK 8, 18, and 19, as well as the absence of CD45. The system is based on enumeration of epithelial cells, which are separated from blood by antibody-coated magnetic beads and identified by fluorescently labeled antibodies against CK with a fluorescent nuclear stain.
The process for validation and qualification of CTC assays has been described by the Cancer Steering Committee of the National Institutes of Health Biomarkers Consortium. CellSearch is the only CTC enumeration system to have been fully validated for reproducibility and performance characteristics. It is used to aid in prognosis of patients with metastatic breast, colorectal, and prostate cancer. The limitation of CellSearch, however, is that not all CTCs express EpCAM on their cell membrane or the expression may be weak.
Furthermore, cells undergoing epithelial to mesenchymal transition (EMT) may be missed during analysis. During this morphologic process, cells lose their epithelial characteristics and acquire a mesenchymal phenotype, endowing the cells with invasive properties and potential for metastasis. This cellular phenotype can also cause increased resistance to common chemotherapies. EMT is most evident in both the triple-negative breast cancer (TNBC) subtype and HER2-positive tumors and is less frequent in HR-positive tumors. Giordano and colleagues studied epithelial to mesenchymal transition-inducing transcription factors (EMT-TF) ( TWIST1, SNAIL1, ZEB1, and TG2 ) and cancer stem cell features in 28 patients with HER2-positive metastatic breast cancer. At least one EMT-TF mRNA was elevated in the CTCs of 88% of patients. TWIST1 and SNAIL1 transcripts were elevated in the CD326 + cell fractions, and SNAIL1 and ZEB1 transcripts were elevated in the CD45 − cell fractions. Patients with EMT-TFs in their CTCs had more ALDH + CD133 + cancer stem cells.
Promising novel EpCAM-based enrichment technologies include microfluidic devices, notably the CTC chip, Herringbone chip, iChip, and IsoFlux. In 2007, Nagrath and colleagues initially described the CTC chip, a microchip technology on a microfluidic platform that separates CTCs from whole blood using microposts coated with an antibody against EpCAM under controlled laminar-flow conditions. In a study of 116 patients, the CTC-chip technology successfully identified CTCs in the peripheral blood of 115 (99%) patients with metastatic breast, colon, lung, pancreatic, and prostate cancer. The CTC-iChip is capable of sorting rare CTCs from whole blood at a rate of 10 million cells per second by using tumor antigen-independent microfluidic technology. The MagSweeper (Illumina) is another automated device that positively enriches CTCs using a magnetic rod stirred through a blood sample that is prelabeled with anti-EpCAM antibody-coated magnetic beads.
If a subset of CTCs undergoes EMT in which epithelial markers are downregulated, technologies reliant on EpCAM expression for CTC capture may fail to enrich an important subpopulation of cells. Increasing attention has therefore been dedicated to technology platforms that use marker-independent enrichment methods. Capturing CTCs without expression of EpCAM has involved antibodies against stem cell antigens and other epithelial cell surface antigens (e.g., HER2, epidermal growth factor receptor, and mucin-1) and mesenchymal antigens.
CTC Detection
After enrichment, a substantial number of leukocytes still remain in the CTC fraction. CTCs need to be identified at the single-cell level by a method that can distinguish them from normal blood cells. Detection can be performed through cytometric strategies or nucleic acid–based techniques. Advances in the development of immunocytochemical and molecular assays enable the identification of individual disseminated tumor cells.
Protein-Based Strategies
Among cytometric techniques, classic immunocytochemistry is the most widely used immunologic approach. Immunocytochemical detection assays involve monoclonal antibodies that bind to tumor-associated or histogenic markers expressed on disseminated tumor cells but that are absent on the surrounding normal cells. The CellSearch system and many other CTC assays use the same identification step: cells are fluorescently stained for CK (positive marker), CD45 (negative marker), and a nuclear dye (4′,6-diamidino-2-phenylindole, or DAPI). Through multicolor image analysis with a fluorescence microscope, CTCs are defined as CK + /CD45 − /DAPI + cells. However, EpCAM-based methods do not recognize whether detected CTCs are viable or apoptotic cells, with only viable cells being able to contribute to metastasis. For detection of only viable CTCs, the newer functional Epithelial ImmunoSPOT (EPISPOT) assay can be added to any enrichment step. The EPISPOT assay can determine cell protein secretion frequency and has been used to analyze peripheral blood and bone marrow samples in breast, colon, and prostate cancer. High-speed automated digital microscopy using fiber-optic array scanning technology has also been developed to detect CTCs that have been labeled by antibodies with fluorescent conjugates.
Nucleic Acid–Based Strategies
Reverse transcription PCR (RT-PCR) assays that target specific mRNAs produced by viable CTCs have become the most widely used alternative to immunocytochemical assays. To detect most CTCs in breast cancer, a multimarker approach uses several cancer-related genes or epithelial markers (e.g., CK19, HER2, EpCAM, MUC1, CK18, mammaglobin, and c-MET). CK19 is one of the most frequently used mRNA markers in trials. A study examined the detection of CK19 mRNA-positive cells by RT-PCR in the peripheral blood of a cohort of 148 patients with stage I and II breast cancer before the initiation of adjuvant systemic therapy. Patients with CK19 mRNA-positive cells in the peripheral blood had a significantly reduced disease-free interval ( p = .0007) and decreased OS ( p = .01) compared with patients without detection.
The nucleic acid–based approach offers the highest sensitivity for CTC detection, although the specificity may decline if there is a high resemblance between mRNA markers of CTCs and those shed by normal blood cells, bone marrow cells, and other nontumor cells. Despite high sensitivity, this approach can only determine whether a cell sample is positive for the specific marker and does not allow for cytomorphologic analysis or direct enumeration of CTCs. A commercially available RNA-based CTC assay is the AdnaTest (AdnaGen), in which CTCs are enriched by immunomagnetic beads labeled with antibodies to EpCAM and MUC1. After enrichment, the mRNA of three markers (EpCAM, MUC1, and HER2) is amplified by multiplex PCR. It is a highly sensitive test with a detection limit of two tumor cells. The concordance rate of the CellSearch system and the AdnaTest has been reported to range between 70% and 90%. Another promising technology is the measurement of single RNA molecules with the RNAscope technology used by CTCscope (Advanced Cell Diagnostics) for the detection of single CTCs from metastatic breast cancer patients. This method requires minimal enrichment and is able to exclude apoptotic cells because they do not produce mRNA molecules.
Clinical Applications of CTCs
Metastatic Breast Cancer
CTCs can be detected in the peripheral blood of approximately 40% to 80% of patients with metastatic breast cancer. The CellSearch system is the most commonly used method for analysis with a cutoff for positivity of five or more CTCs per 7.5 mL of blood. Several trials have demonstrated the independent prognostic significance of CTCs in metastatic disease. In 2004, the seminal prospective study by Cristofanilli and colleagues showed that among 177 patients with measurable metastatic breast cancer who had at least five CTCs per 7.5 mL of blood detected by CellSearch at baseline before treatment, there was significantly shorter progression-free survival (PFS) (2.7 months vs. 7.0 months; p < .001) and OS (10.1 months vs. >18 months; p < .001) compared with patients with fewer than five CTCs per 7.5 mL. This difference between the groups persisted at the first follow-up (3–5 weeks) after initiation of treatment. Enumeration of CTCs both before and several weeks after initiating treatment was informative because maintaining or decreasing the number of CTCs to fewer than five indicated a treatment response and was predictive of improved PFS and OS. This pivotal study showed that the number of CTCs is an independent predictor of PFS and OS and subsequently lead to FDA approval of CellSearch in 2004 for prognosis and monitoring of treatment effectiveness for metastatic breast cancer.
Hayes and colleagues further expanded on the initial results of this cohort by demonstrating that CTC analysis at each consecutive follow-up time point (baseline, 3–5, 6–8, 9–15, and 15–20 weeks) during therapy predicted PFS and OS. For patients with five or more CTCs per 7.5 mL, median PFS from each time point was significantly reduced compared with those with fewer than five CTCs, respectively. Median OS for patients with fewer than five CTCs from the five time points was greater than 18.5 months. For patients with five or more CTCs, median OS from these same time points was significantly shorter: 10.9, 6.3, 6.3, 6.6, and 6.7 months, respectively. In addition, patients who converted from elevated CTCs to nonelevated levels demonstrated PFS and OS similar to those with CTCs that were never elevated.
A pooled analysis of 1944 patients with metastatic breast cancer across 17 European centers confirmed the independent prognostic role of CTC count by CellSearch method on PFS and OS in metastatic breast cancer patients. Patients who had a CTC count of five or more CTCs per 7.5 mL blood at baseline had decreased PFS (hazard ratio [HR] 1.92, 95% confidence interval [CI], 1.73–2.14; p < .0001) and OS (HR 2.78, 95% CI, 2.42–3.19; p < .0001) compared with patients with fewer than five CTCs per 7.5 mL. At both 3 to 5 weeks and 6 to 8 weeks after the start of treatment, increased CTC counts were significantly associated with shorter PFS and OS. The data provided strong evidence that CTC enumeration improved the prognostication of metastatic breast cancer when added to clinicopathologic prognostic models (e.g., tumor histologic subtype, histologic grade, number of prior lines of chemotherapy and hormonal therapy, presence of liver or visceral metastasis). In contrast, measurement of serum tumor markers—carcinoembryonic antigen (CEA) and cancer antigen (CA) 15-3—did not contribute significant information.
Other studies have shown that monitoring of CTC levels in the metastatic setting has been predictive of treatment efficacy. A strong association was demonstrated between CTC levels and radiographic disease progression in patients receiving chemotherapy or endocrine therapy for metastatic breast cancer. Reduced CTC counts at weeks 3 to 5 on treatment correlated with radiographic response, and patients with fewer than five CTCs at weeks 3 to 5 and weeks 7 to 9 had an improvement in PFS. This supported the clinical utility of serial CTC enumeration in conjunction with standard radiographic imaging to improve the ability to accurately assess treatment benefit and to expedite identification of effective therapies for individual patients.
Early detection of disease progression with CTC count while on treatment could potentially allow for switching from less effective therapies to alternative regimens. In the prospective Southwest Oncology Group (SWOG) S0500 trial, patients receiving first-line chemotherapy for metastatic breast cancer were randomly assigned to continue current chemotherapy or start a new treatment regimen if they had five or more CTCs per 7.5 mL at baseline and after the first treatment cycle of 21 days. The results, however, showed that for patients with persistently elevated CTCs at 21 days whose therapy was then changed to an alternative chemotherapy, both PFS and OS were not improved. The authors suggested that failure of treatment to reduce CTCs within the first cycle of starting first-line chemotherapy signifies a poor prognosis and may indicate resistance to chemotherapy.
After the SWOG S0500 trial, the ongoing CirCe01 trial ( clinicaltrials.gov identifier NCT01349842) is studying whether patients whose CTC count does not decrease after the first treatment cycle benefit from a regimen switch. A total of 304 metastatic breast cancer patients with high CTC count before the start of third-line therapy will be randomized between a CTC-driven arm and a standard arm (i.e., chemotherapy management according to usual clinical and radiologic criteria). Recently the nonrandomized run-in phase of the CirCe01 trial was reported, which was designed to evaluate CTC changes and thresholds for other prognostic scores and establish CTC thresholds to be used in the randomized part of the study. CTC count by CellSearch and other prognostic parameters were assessed in 56 metastatic breast cancer patients before the first cycle of third-line chemotherapy. Early changes in CTC count correlated with treatment outcome. Independent prognostic markers in multivariate analysis were as follows: five or more CTCs per 7.5 mL, poor performance status, low serum albumin, and TNBC subtype. For patients with five or more CTC per 7.5 mL at baseline, a composite criteria of fewer than five CTC per 7.5 mL or a relative decrease of 70% or more of the baseline CTC count demonstrated improved prognostication for PFS ( p = .002). Another study showed that analysis of CTCs before the second cycle of chemotherapy is an early and strong predictor of treatment outcome in metastatic breast cancer. Several ongoing interventional studies evaluating the clinical utility of CTCs are described in Table 66.2 .