Dermatologic complications of cancer chemotherapy
Anisha B. Patel, MD  Madeleine M. Duvic, MD
Madeleine M. Duvic, MD
Overview
Dermatologic complications of cancer chemotherapy have become increasingly significant, especially with the continued development of new targeted antineoplastic agents. The frequency of mucocutaneous complications in cancer chemotherapy is often a reflection of the increased proliferative nature of affected tissues, such as the mucous membranes, skin, hair, and nails, which renders them particularly susceptible to the actions of chemotherapeutic drugs. This chapter reviews the specific side effects associated with targeted therapies and the more classic side effects of cytotoxic chemotherapies and their associated drugs.
Diagnosis of cutaneous reactions in the cancer patient is complicated by the degree of their malignancy, concomitant diseases, polypharmacy, and immunosuppression. With the advances in bone marrow transplantation, graft versus host disease (GVHD), opportunistic infections, and malignancies are also being seen more frequently and may mimic and complicate the diagnosis of chemotherapy-induced reactions. The major cutaneous reactions and a variety of miscellaneous reactions are discussed in this chapter and are listed in Table 1. As seen in Table 2, these reactions occur in varying degrees of frequency and severity among the classes of chemotherapeutic drugs. Although dermatologic complications are rarely fatal, it is important to recognize potential reactions as they may result in significant morbidity, chemotherapy cessation or dose reduction, cosmetic disfigurement, and psychological distress. Proper treatment of potentially dose limiting cutaneous toxicity may also allow ideal schedules of chemotherapy administration and optimization of response.
Table 1 Immunologically mediated drug hypersensitivity reactions
| IgE-dependent drug reactions (formerly type I) | L-asparaginase, paclitaxel, docetaxel, teniposide, cisplatin (intravesical) | Urticaria Angioedema Anaphylaxis |
| Cytotoxic drug-induced reactions (antibody against a fixed antigen; formerly type II) | Petechiae secondary to drug-induced thrombocytopenia | |
| Immune complex-dependent drug reactions (formerly type III) | Vasculitis Serum sickness Urticaria (certain types) | |
| Delayed-type, cell-mediated drug reactions (formerly type IV) versus undefined | Procarbazine Cytarabine Nucleoside analogues (both can also have IgE-dependent and immune-complex-mediated drug reactions) | Exanthematous/morbilliform eruption Fixed drug eruption Lichenoid drug reaction SJS/TEN AGEP DRESS |
| No reported immunologically mediated reactions | Nitrosoureas Vinca alkaloids Altretamine Dactinomycin |
Source: Adapted from Ref. 1.
Table 2 Major cutaneous reactions associated with chemotherapy
| Drug hypersensitivity reactions (morbilliform, erythema multiforme/Stevens Johnsons/toxic epidermal necrolysis) |
| Mucosal reactions (stomatitis, aphthae) |
| Nail reactions (hyperpigmentation, onycholysis, paronychia) |
| Extravasation reactions (irritant, vesicant) |
| Pigmentary changes (hyperpigmentation, vitiligo) |
| Radiation-associated reactions (radiation enhancement, radiation recall, photosensitivity) |
| Alopecia (anagen effluvium, scarring alopecia) |
| Acral reactions (acral erythema/toxic erythema of chemotherapy, hand–foot skin reaction) |
| Neutrophilic dermatoses (Sweet’s syndrome, erythema nodosum) |
| Cutaneous eruption of lymphocyte recovery |
| Neoplasms (keratoses, squamous cell carcinoma, lentigines, melanoma) |
Drug hypersensitivity reactions
“Traditional” drug reactions have been categorized into immunologic and nonimmunologic or toxic. Of the immunologic drug reactions, there are four subtypes, formerly types I–IV, that are outlined in Table 3. The most common reactions are delayed-type, T-cell-mediated drug reactions and include the morbilliform or exanthematous drug eruption. They clinically present as erythematous macules and thin papules on the trunk spreading to the extremities and are usually asymptomatic. When the rash is painful, the differential diagnosis includes erythema multiforme (EM), Stevens Johnsons syndrome (SJS), and toxic epidermal necrolysis (TEN). EM is characterized by targetoid erythematous papules and plaques that tend to start on the extremities, can involve the palms and soles, and, when advanced, form central bullae and spread to the oral and genital mucosa. SJS and TEN, however, start with centrally distributed dusky papules and plaques that coalesce and vesiculate and have severe mucosal involvement. SJS involves <10% of body surface area and TEN involves >30% of body surface area. The mortality rate of SJS is 1–5% and that of TEN is 25–35%.1
Table 3 Most common mucocutaneous reactions of the major classes of chemotherapeutic drugs
| Alkylating agents | Antibiotics |
| Hyperpigmentation | Alopecia |
| Hypersensitivity | Stomatitis |
| Chemical cellulitis | |
| Hyperpigmentation | |
| Radiation-associated reactions | |
| Vinca alkaloids | Antimetabolites |
| Alopecia | Acral erythema |
| Chemical cellulitis | Alopecia |
| Inflammation of keratosis | Hyperpigmentation |
| Neutrophilic eccrine hidradenitis | Radiation-associated reactions |
Drug reaction with eosinophilia and systemic (DRESS) and acute generalized exanthematous pustulosis (AGEP) have unclear pathogeneses. The cutaneous findings in DRESS are not specific; however, the peripheral edema, lymphadenopathy, and liver transaminitis are characteristic. Finally, AGEP presents with abruptly appearing sheets of cutaneous pustules. It usually begins on the face or intertriginous areas, perhaps with burning and itching. It can be accompanied by fever, neutrophilia, and eosinophilia.2 Ninety percent of cases are drug induced, mostly with antibiotics such as β-lactams, cephalosporins, fluconazole, nystatin, and terbinafine.1 Other reported triggers include the histone deacetylase inhibitor bryostatin,3 imatinib, mercury, thallium, iohexol, patch testing, pseudoephedrine, diltiazem, furosemide, and viral infections.4 These two reactions are less common than the exanthematous eruption and can be more severe.
Targeted cancer therapeutics
These drugs started to emerge in the mid-1990s and we are still delineating all of the side effects. Although the systemic toxicities are decreased, many of the signaling mediators targeted also affect the epithelium, and these effects are much more specific than previous chemotherapy cutaneous reactions. This change is reflected in the literature of even the clinical trials, where adverse events were previously described as a “rash” or “lesion,” the descriptions have become more specific, which makes it easier to anticipate and track different reactions.
Numerous targeted therapies are available; however, only those with specific cutaneous reactions occurring at high incidences are discussed below.
Epidermal grown factor receptor (EGFR) inhibitors
Epidermal growth factor receptor (EGFR) has been recognized as a significant regulator of cancer cell proliferation, apoptosis, angiogenesis, and metastasis. Ligand binding to the receptor causes receptor dimerization, which activates the intracellular tyrosine kinase domain.5 EGFR also plays a significant role in normal skin homeostasis.6 Activation of EGFR in epidermal keratinocytes promotes cell cycle progression, differentiation, and migration, which are all critical for normal skin function and wound healing.7 The most common cutaneous side effects for EGFR inhibitors are an acneiform eruption, paronychia, xerosis, eczema, mucositis, and geographic tongue. Acne folliculitis appears on the face and upper trunk 8–10 days after treatment initiation. In phase 1 trials, erlotinib at the maximally tolerated dose induced a pustular acneiform eruption in 50% of cases during the second week of therapy.8 The eruption can be extremely prurituc and EGFR inhibitors have cutaneous side effects that lead to dose alteration in >75% of patients.9 The presence and severity of acne folliculitis has been correlated with tumor response and survival.10 This reaction has been reported with cetuximab, panitumumab, nimotuzumab, erlotinib, and gefitinib.11–13 Oral tetracyclines combined with topical steroids are the gold standard treatment. Retinoids and topical antibiotics have not been proven useful in these patients as the etiology is not follicular plugging and rupture, but epithelial dysregulation.11 Staphylococcus colonization can worsen the eruption and cultures and antibiotics are recommended if lesions are pustular or crusted (Figure 1).
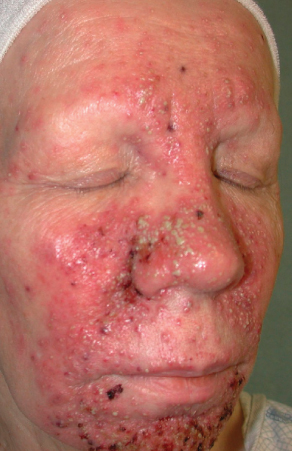
Figure 1 Severe acneiform eruption of the face associated with cetuximab therapy.
The human epidermal growth factor receptor (HER)1/2 blockers have the same side effects as EGFR inhibitors, but are milder. Trastuzumab, lapatinib, dacomitinib, and afatinib all have had reported acneiform eruptions.14–17 Similarly, the vascular endothelial growth factor (VEGF) inhibitors have overlap between the EGFR inhibitors, with mucositis and geographic tongue, and the multikinase inhibitors, with hand–foot skin reaction (HFSR), which is discussed below.18
BCR-ABL tyrosine kinase inhibitors
Imatinib mesylate targets the BCR–ABL gene and has been used in the treatment of chronic myeloid leukemia and acute lymphoblastic leukemia. It has been shown to frequently cause dose-dependent cutaneous reactions, including facial edema, morbilliform eruption, urticaria, eczematous dermatitis, and AGEP.19, 20 One patient developed an eczematous rash with histologic features of mycosis fungoides.21
Second- and third-generation Bcr–Abl-specific TKIs, dasatinib, nilotinib, and ponatinib have been associated with follicular lichenoid eruptions of the scalp, face, and body that can be pruritic and lead to scarring alopecia.22 This alopecia is irreversible, even upon dose cessation.
Multikinase inhibitors
Sunitinib and sorafenib are the multikinase inhibitors targeting VEGF receptor, platelet-derived growth factor receptor (PDGFR), c-Kit, and FLT-3. Sorafenib also inhibits RAF kinase. They were developed for advanced renal cell carcinoma but have also been used for hepatocellular carcinoma, gastrointestinal stromal tumors, and thyroid cancer. They are most associated with the HFSR as well as mucositis, alopecia, xerosis, and xerostomia. However, because they overlap with multiple groups of targeted therapies, the cutaneous squamous cell carcinoma (SCC) of BRAF inhibitors and the acneiform eruption of VEGF inhibitors can be seen as well.23
HFSR appears within 2–4 weeks of starting the therapy and is present in one-fifth to one-third of patients, with sorafenib having a slightly higher incidence. Patients develop a focal keratoderma at points of friction and pressure, which can vesiculate, leading to painful blisters (Figure 2). The risk of developing HSFR depends on which drug the patient is on and which cancer type is being treated. The original proposed mechanism was that the VEGFR blocking capabilities of these drugs caused the patients to have a poor response to damage caused by pressure and trauma.24, 25 Most recently, the Fas/Fas ligand response was implicated, proven by blocking the reaction by administering anti-Fas ligand antibody. These are the same mediators of Stevens Johnsons and TEN.26
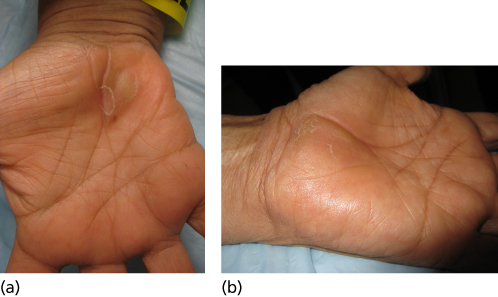
Figure 2 Hand–foot skin reaction with sorafenib therapy.
Although more common in multikinase inhibitors such as sorafenib and sunitinib, these lesions have been reported with BRAF inhibitors.27 These are distinct from the more common hand–foot syndrome/acral erythema (AE)/toxic erythema of chemotherapy seen with cytotoxic chemotherapies. HFSR does not have the diffuse erythema and edema of hand–foot syndrome and also has a longer latency period before appearing (2–4 weeks). HFSR usually self-resolves with continued treatment with the multikinase inhibitors.28
mTOR inhibitors
Overlapping with the EGFR signaling pathway is the PI3K/AKT pathway, which activates mammalian target of rapamycin (mTOR). This molecule is associated with cell growth and angiogenesis and sirolimus or rapamycin was the original drug in this category, followed by everolimus and temsirolimus. All are associated with the papulopustular rash of EGFR inhibitors, which has an incidence of 45.8% in the most recently developed drug, temsirolimus. They all also can induce the more classic morbilliform drug eruption and oral mucositis. As opposed to cytotoxic chemotherapies, individual deeper oral ulcerations more similar to aphthous stomatitis are present as well. Finally, everolimus and temsirolimus also had a population with eczematous dermatitides.29
BRAF inhibitors
BRAF inhibitors, first introduced in metastatic melanoma patients, have significant cutaneous side effects that include inflammatory, follicular, and neoplastic eruptions. These side effects lead to dose cessation or reduction in <10% of patients.30 Of the inflammatory cutaneous toxicities, neutrophilic dermatoses, including acute febrile neutrophilic dermatosis (Sweet’s syndrome) and neutrophilic panniculitis, have been attributed to the use of BRAF inhibitors.30–36
Two patients with the erythematous pseudovesicular papules and plaques of Sweet’s syndrome of the trunk and extremities presenting with systemic symptoms of fever and arthralgias have been reported.36, 37 The patients with neutrophilic panniculitis presented with tender, erythematous nodules of the legs and occasionally arms, and had histology consistent with a neutrophilic lobular panniculitis.31–34 Vitiligo,38 cutaneous sarcoidosis,39 Grover’s disease,40 and hidradenitis suppurativa41 have been reported less commonly.
Patients on BRAF inhibitors also have increased radiation sensitivity to UV (ultraviolet) light and radiation therapy with quicker and more severe sunburns and acute radiation dermatitis, respectively.42, 43 Further, cases of radiation recall dermatitis have been reported as well.44, 45
Epidermal and follicular dysregulation contribute significantly to BRAF inhibitor cutaneous side effects. Palmoplantar hyperkeratosis or keratoderma is a thickening of the epidermis without inflammation presenting as thick yellow plaques of the palms and soles similar to a large callus. Most commonly, the keratoderma is seen on the feet in pressure points, without vesiculation.46 A superficial keratotic plugging of the follicle results in a keratosis pilaris-like eruption. This is seen frequently on the trunk and extremities and is more often asymptomatic than pruritic. It was noted in 5–9% of patients in phase 2 and 3 trials,47, 48 although this may be underreported.27, 49, 50
Neoplastic lesions cause the highest morbidity in these patients. Actinic keratoses (AKs) represent precancerous epithelial lesions typically associated with chronic sun damage. The incidence of AKs is 6–16% in vemurafenib patients47–49 and 5–10% in dabrafenib patients.46, 51–53 Wart-like keratoses are papillated, hyperkeratotic, well-demarcated papules that are often inflamed and appear in an eruptive nature during BRAF inhibitor therapy. They appear about 3–4 months after therapy.53 These lesions are not true verruca as human papilloma virus testing has been negative in multiple reports.54, 55 Prompt treatment of both types of lesions with cryotherapy, photodynamic therapy, curettage, and topical 5-fluorouracil (5-FU) helps prevent SCC formation (Figure 3).
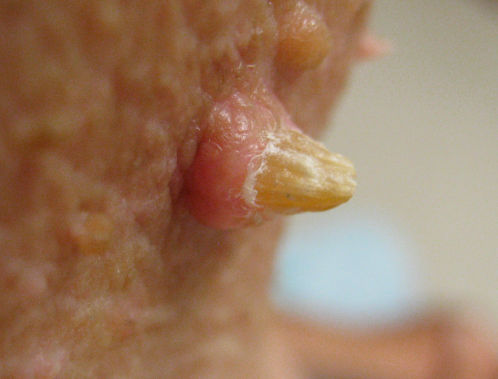
Figure 3 Squamous cell carcinoma with vemurafenib therapy.
Patients with SCC usually present with dome-shaped, well-demarcated, hyperkeratotic, erythematous papules, and nodules. They are quickly growing and more prevalent in older patients with chronic sun damage.46
The incidence of SCC is 4–31% in vemurafenib patients47, 48, 56 and 6–11% in debrafenib patients.51, 52, 57, 58 Sosman et al. showed that they are predominantly well-differentiated or keratoacanthoma-type SCC, which are less aggressive than the normal array of sun-induced SCC. The median time to occurrence is 8 weeks. HRAS upregulation has been implicated in a portion of BRAF-induced SCC causing a paradoxical upregulation of the MAP kinase pathway.47
Patients have been reported to have involution of nevi as well as new and darkening nevi. The new nevi have shown wild-type BRAF, lack the V600E mutation, and appear in 8–14 weeks.59 These lesions have been biopsied as common nevi, dysplastic nevi, and new primary cutaneous melanomas. Five of 464 patients in the phase 2 and 3 clinical trials with vemurafenib had a new melanoma.60
MEK inhibitors
Selumetinib and trametinib, two new MEK inhibitors, just downstream of BRAF have similar side effects to the EGFR inhibitors.61 Interestingly, the addition of a MEK inhibitor to a BRAF inhibitor decreases the squamous proliferations seen with the BRAF inhibitor alone, possibly addressing the HRAS mutation as well.
Immunomodulators
With advances in biotechnology, there have been increased developments of cytokines and immunotherapeutic agents, which target cancer at the cellular level. This class of drugs is less specific than the targeted therapies described above and works by enhancing the inflammatory response to metastatic and hematologic tumors. The cutaneous side effects are less specific than those described above, and generally encompass reactive inflammatory processes.
Immune check point inhibitors
Immunomodulatory drugs used for melanoma include ipilimumab and nivolumab or pembrolizumab. Ipilimumab is a monoclonal antibody targeting CTLA4, which inhibits binding of the costimulatory molecule, CD28. Blocking CTLA4 allows unopposed activation of cytotoxic T cells and stimulates the immune response to metastatic melanoma. The main side effects are morbilliform or eczematous eruptions.62 Similarly, pembrolizumab blocks PD-1, which, when bound to its ligand, decreases the cytotoxic effects of T cells. This molecule is upregulated in tumor cells and is thought to be a more specific target than CTLA4. The cutaneous side effects are similar to ipilimumab, but also include vitiligo.63
Cytokines
Roles have already been established for interleukin 2 (IL-2) as alternative treatment for advanced metastatic melanoma and renal cell cancer and for interferon-α (IFN-α) as standard treatment for chronic myelogenous leukemia, hairy-cell leukemia, cutaneous T-cell lymphoma, melanoma, and Kaposi sarcoma (KS). In addition to significant toxicities (Table 4) such as capillary leak syndrome, there is a 72% incidence of cutaneous reactions reported with IL-2.64 Commonly, a pruritic diffuse erythroderma occurs 1–3 days after administration and resolves with desquamation 2 days after cessation of therapy (Figure 4).64 This reaction is clinically similar to toxic shock syndrome and has been associated with staphylococcal sepsis in some patients. Intra-arterial IL-2 also causes hypersensitivity to iodine-containing contrast dyes in up to 30% of patients.65 Of potential importance, one study of IL-2 for metastatic melanoma has reported a possible correlation between the development of vitiligo and good prognosis.66 Although IFN-α is relatively less toxic than IL-2, several cutaneous reactions have been reported in the literature. Perhaps one-third of patients will develop a local injection-site reaction. In a study of 1000 patients receiving IFN-α, alopecia and herpes labialis exacerbation were common with 10% and 5% incidence, respectively.65, 67 Similar to nonmodified recombinant IFN-α, pegylated IFN-α has been shown to cause local cutaneous ulcerations at sites of subcutaneous injection.68 Both IFN-α and IL-2 also induce and/or exacerbate seborrheic dermatitis and psoriasis.65
Table 4 Cytokine reactions
| Other INF-α reactions | Other IL-2 reactions |
| Eosinophilic fasciitis | Erosions in surgical scars |
| Exacerbation of herpes labialis | Hypersensitivity to iodine contrast dye |
| Increased growth of eyelashes | Linear IgA bullous dermatosis |
| Necrotizing vasculitis | Pemphigus vulgaris (de novo, recurrent) |
| Paraneoplastic pemphigus | Poly/dermatomyositis exacerbation |
| Psoriasis exacerbation and de novo | Psoriasis exacerbation |
| Thyroiditis | Staphylococcal infections |
| TEN-like bullous desquamation | |
| Vitiligo |
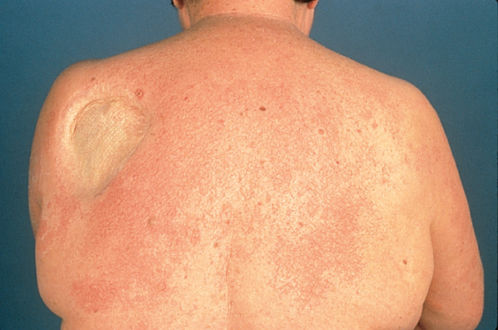
Figure 4 Erythematous rash associated with IL-2 therapy in a melanoma patient.
Alopecia
Alopecia is the most common dermatologic complication associated with chemotherapy. While most drug-induced alopecias involve a telogen effluvium pattern by inducing normal hairs to synchronize their cycles, the anagen effluvium pattern of hair loss is the most common type of alopecia produced by chemotherapeutic agents (Table 5), with the exception of IL-2 and IFN-α therapy. In chemotherapy, anagen effluvium is caused by the abrupt cessation of the high mitotic activity of hair matrix cells in the anagen phase of hair follicles.70 Anagen effluvium manifests within 1–2 weeks after the beginning of chemotherapy but is most noticeable 1–2 months later.71 Hair regrowth can usually be expected 5 months after the end of chemotherapy, although hair color and texture may change.69 Permanent alopecia has been reported with busulfan/cyclophosphamide therapy.72
Table 5 Chemotherapeutic agents associated with alopecia
| Most common or severe | Least common or severe | ||
| Bleomycin | Ifosfamide | Amsacrine | Melphalan |
| Cisplatin | Interferon-a | Busulfan | Mercaptopurine |
| Cyclophosphamide | Irinotecan | Carboplatin | Methotrexate |
| Cytarabine | Mechlorethamine | Carmustine | Mitomycin |
| Dacarbazine | Nitrosoureas | Chlorambucil epirubicin | Procarbazine |
| Dactinomycin | Paclitaxel | Gemcitabine | Teniposide |
| Daunorubicin | Thiotepa | Hydroxyurea | Vinorelbine |
| Docetaxel | Topotecan | ||
| Doxorubicin | Vinblastine | ||
| Etoposide | Vincristine | ||
| Fluorouracil | Vindesine | ||
| Idarubicin | |||
Source: Adapted from Ref. 69.
Hair loss often has emotional impact on patients receiving chemotherapy. Unfortunately, there are currently no widely accepted methods of prevention and treatment for alopecia, although scalp hypothermia, minoxidil, vitamin D3, cyclosporine, and topical doxorubicin monoclonal antibody have been studied.73, 74
Stomatitis
Stomatitis and other oral complications of cancer chemotherapy are discussed in 136 and 137.
Nail reactions
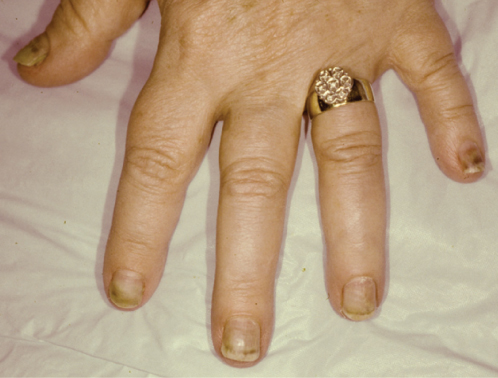
Hyperpigmentation is the most common nail abnormality encountered in patients receiving chemotherapy, particularly in dark-skinned patients.75 Hyperpigmentation due to chemotherapy-induced melanocyte stimulation should be distinguished from yellow nail syndrome (YNS). YNS nails have increased transverse curvature, absent lunulae, and no cuticle. Suggested etiologies include paraneoplastic process, AIDS (acquired immune deficiency syndrome) association, and drug induction (Figure 5).76

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








