Chapter 12 Delavirdine
STRUCTURE
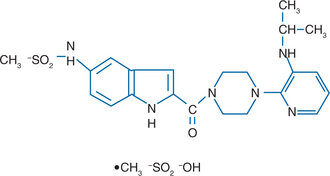
Delavirdine (Rescriptor, previously U-90152) (Fig. 12-1) belongs to the bisheteroarylpiperazine (BHAP) class of non-nucleoside reverse transcriptase inhibitors (NNRTIs).1–3
MECHANISMS OF ACTION AND IN VITRO ACTIVITY
Delavirdine, like other NNRTIs, inhibits reverse transcriptase (RT) by binding to a hydrophobic pocket in the p66 subunit of HIV-1 RT near the catalytic site of the enzyme. The delavirdine molecule is larger than other NNRTIs, such as nevirapine and efavirenz. The average median inhibitory concentration of delavirdine against a panel of laboratory and clinical HIV-1 isolates was 0.066 μM (range <0.005 to 0.690 μM); it does not inhibit HIV-2.2
PHARMACOKINETICS
Delavirdine is rapidly absorbed after oral administration; its absorption is reduced by gastric hypoacidity.4 The steady-state pharmacokinetics of delavirdine are nonlinear, leading to substantial prolongation of its apparent half-life with increasing delavirdine doses.5,6 Delavirdine is bound extensively to plasma proteins, primarily albumin.5,7 There is a relatively large intersubject variability in steady-state delavirdine levels.6 The major metabolic pathway of delavirdine is N-dealkylation, which is mediated by cytochrome P450 3A (CYP3A). Delavirdine inhibits CYP3A activity, thereby inhibiting its own metabolism.5 Delavirdine is excreted primarily as dealkyl delavirdine and pyridine-cleaved delavirdine in both urine and feces.4
DRUG INTERACTIONS
Delavirdine has important interactions with currently available protease inhibitors (PIs), which are metabolized primarily by CYP3A4 (Table 12-1). The PIs also inhibit CYP3A4 to different degrees, so these drugs have the potential to affect delavirdine metabolism. One potential advantage of delavirdine’s interactions with PIs is that it may increase the levels of these drugs, allowing reduced dosing or increased regimen potency. However, such interactions can be difficult to predict, without detailed pharmacokinetic studies, and such studies have not been performed for all currently available PIs. Delavirdine significantly increased trough levels and area under the curve (AUC) of indinavir.8–10 The recommended dose of indinavir when given in combination with delavirdine is therefore 600 mg PO tid, instead of 800 mg PO tid. Delavirdine increased the trough concentration and AUC of ritonavir, nelfinavir, and amprenavir.11–13 It is not known whether delavirdine has similar interactions with fosamprenavir. Studies of delavirdine and saquinavir suggest that a twice-daily regimen, and a lower daily dose of saquinavir can be used.4,14,15 The effects of delavirdine on the metabolism of the fixed dose of lopinavir/ritonavir have not been formally studied, although a small study of three HIV-1 infected patients found increases in lopinavir exposure after delavirdine addition, albeit with wide interpatient variability in the magnitude of these increases.16
Table 12-1 Important Drug Interactions with Delavirdine
| Type of Interaction with Delavirdine |
|---|
| Inhibition of Delavirdine Absorption |
| Didanosine Antacids |
| Acceleration of Delavirdine Metabolism |
| Carbamazepine Phenobarbital Phenytoin Rifampin Rifabutin |
| Inhibition of Metabolism by Delavirdine |
| Indinavir Saquinavir Nelfinavir Lopinavir Ritonavir Amprenavir Terfenadine Astemizole Clarithromycin Dapsone Rifabutin Ergot derivatives Alprazolam Midazolam Triazolam Dihydropyridines (e.g., nifedipine) Cisapride Quinidine Warfarin Sildenafil |
Delavirdine also has important interactions with other drugs metabolized by cytochrome P450. Rifampin increased the oral clearance of delavirdine approximately 27-fold, leading to negligible concentrations of delavirdine in patients who received both drugs.17 Co-administration of delavirdine and rifampin is therefore not recommended. Rifabutin increased oral clearance of delavirdine approximately fivefold, resulting in lower steady-state plasma delavirdine concentrations,18 but delavirdine doses in excess of 600 mg tid were able to overcome this interaction.19 Delavirdine inhibits rifabutin clearance and substantially increases rifabutin exposure.19 Anticonvulsants such as phenobarbital, phenytoin, and carbamazepine induce CYP3A4 and therefore could potentially increase the metabolism of delavirdine. Limited unpublished data indicate that trough plasma delavirdine concentrations are reduced by concomitant administration of these agents.4 Co-administration of delavirdine with these anticonvulsants is therefore not recommended.
A pharmacokinetic study of delavirdine and didanosine in nine HIV-infected individuals found that, under steady-state conditions, the AUCs of both drugs were reduced ∼20% when the drugs were administered simultaneously rather than 1 h apart.4,20 This effect is thought to be due to the buffering effect of the formulation of didanosine that was used, and it is not known if the same effect would be seen with nonbuffered preparations of didanosine (see Chapter 16).
TOXICITY
The most frequent side effect of delavirdine is a maculopapular rash (Table 12-2). The rash characteristically occurs within 1–3 weeks after initiation of therapy and often resolves despite continuation of the drug.21–23 The rash, which is uncommon after a month of therapy unless a prolonged interruption of treatment has intervened, is erythematous, maculopapular, and often pruritic, and involves the upper body and proximal arms, with decreasing intensity on the neck, face, trunk, and limbs.24 It can be managed successfully in more than 90% of patients. Serious delavirdine-related skin rashes, such as erythema multiforme and Stevens–Johnson syndrome, are rare (∼0.4%) and usually resolve after discontinuing the drug.
Table 12-2 Toxicities Associated with Delavirdine
| Toxicity | Frequency | Comments |
|---|---|---|
| Rash | 18–36% | Rare after first month of therapy |
| May be more common with higher delavirdine trough levels21 | ||
| Severe rash necessitating discontinuation of drug is uncommon (5–10%) | ||
| Hepatic enzyme elevations | Uncommon | 4/30 Subjects receiving delavirdine + saquinavir had reversible increases in alanine transaminase and aspartate transaminase8 |
| Neutropenia | Uncommon | Observed in 4/24 subjects receiving delavirdine + nelfinavir12 |
Occasionally, elevations of liver enzymes are seen in delavirdine recipients, most often in those who received other antiretroviral agents (Table 12-2). Significant but reversible elevations in alanine transaminase and aspartate transaminase occurred in four of 30 subjects during treatment with the combination of saquinavir and delavirdine.8 Frank hepatitis has been rare. Neutropenia was observed in one small study of HIV-infected patients receiving nelfinavir and delavirdine, which resolved promptly after discontinuation of both drugs.12 Another study demonstrated an increase in both cholesterol and high density lipoprotein (HDL) in patients receiving delavirdine plus two nucleosides, with more dramatic increases in cholesterol and HDL in patients receiving delavirdine in combination with a PI.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree