Fig. 10.1
Combined analysis of overall survival in the EORTC 30947 and SWOG 8949 trials. Median overall survival is 7.8 months in the observation group (O=interferon monotherapy) versus 13.6 months in the nephrectomy group (N = nephrectomy + interferon) (Adapted with permission from Flanigan et al. [3])
10.4 Proposed Mechanisms of Action
There are multiple hypotheses behind the survival advantage associated with CN prior to immunotherapy. With specific relevance to immunotherapy, large bulky primary tumors may act as an immunologic sink, and thus, removal of the primary tumor and bulky retroperitoneal lymph nodes may allow for an increased effectiveness of immunotherapy [16, 18, 19]. The primary tumor may also produce numerous growth and angiogenic factors which may contribute to the development and viability of distant metastatic disease (VEGF, TGF-ß1, PDGF, IL-8, IL-10, and FGF) [20–22]. A novel and interesting hypothesis reported by Gatenby et al. proposed that removal of the kidney and subsequent metabolic acidosis (rather than cytoreduction through removal of the primary tumor) was responsible for the increase in OS seen in the SWOG 8949 trial. The exact mechanism by which CN adds to OS is currently unknown.
10.5 Contemporary Therapies
CN in the era of molecular targeted agents has not been prospectively evaluated but has been generally accepted based on the earlier studies when performed prior to cytokine-based therapy [23, 24]. The current 2015 NCCN guidelines recommend CN in properly selected patients prior to immunotherapy [25]. With regard to patients prior to contemporary systemic targeted therapy, the NCCN guidelines cite retrospective series showing CN continues to play a role in patients treated with VEGF-targeted agents while anticipating the results from contemporary randomized trials [4, 5].
The overwhelming majority (90+%) of patients enrolled in clinical trials of targeted therapies have by default undergone prior CN. The one exception has been in the Global ARCC study of the mammalian target of rapamycin (mTOR) inhibitor, temsirolimus [26]. ARCC was a phase 3 randomized trial of interferon alpha, temsirolimus, or a lower dose combination of both agents in patients with poor risk mRCC of any histology. The primary tumor was not removed in 33 % of the patents and 20 % had non-clear cell histology. Temsirolimus improved OS among patients with mRCC and poor prognosis features. A subsequent subset analysis of this trial explored the influence of nephrectomy and histology on overall and PFS [27]. The improvement in PFS and OS in patients treated with temsirolimus was seen in both clear and non-clear cell histologies, while nephrectomy status did not impact the PFS or OS. Most of these patients had poor prognostic features and would not have been ideal candidates for CN [28].
Multiple retrospective analyses of patients treated with targeted therapy have provided conflicting data on the benefit of CN. In a multi-institutional analysis, Choueiri et al. reported on the outcomes of 314 patients receiving targeted therapy for mRCC [29]. Favorable risk and younger patients were more likely to undergo CN. After adjusting for significant differences in baseline prognostic factors, patients undergoing CN had a significantly improved OS (HR 0.44; CI 0.32–0.59, p < 0.01). As would be expected, this survival advantage did not extend to patients classified as poor risk (similar to Global ARCC).
Heng et al. published a large multi-institutional data set assessing the benefit of CN from patients enrolled on the International Metastatic Renal Cell Carcinoma Database Consortium [30]. A total of 1,658 patients with a diagnosis of synchronous mRCC were included, with 982 of these patients undergoing CN and 676 not receiving surgery. As expected, baseline clinical prognostic factors varied between the groups with those undergoing CN having more favorable clinical features. After adjusting for the prognostic differences between the two groups, the authors reported results favoring CN with the median OS (20.6 vs. 9.5 months; p < 0.0001; Fig. 10.2) and PFS (7.6 vs. 4.5 months; p < 0.001). In this series, patients surviving less than 12 months did not appear to benefit from CN. This study, along with others, highlights the importance of selecting patients most likely to benefit from CN [31].
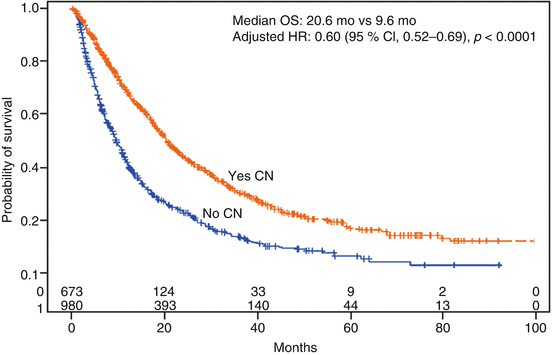

Fig. 10.2
Kaplan-Meier overall survival from the initiation of targeted therapy for 1,633 mRCC based on receiving a cytoreductive nephrectomy [30]
There are currently two phase III randomized trials attempting to provide insight into the role of CN in the era of targeted therapy and on the question of timing of nephrectomy with regard to systemic targeted therapies. The CARMENA trial is a randomized phase III trial comparing the first-line treatments of (1) CN followed by sunitinib to sunitinib monotherapy in clear cell RCC [4]. The anticipated enrollment is 576 patients with a primary end point of OS. This study will provide the only level I evidence assessing the role of CN in patients with mRCC treated with contemporary systemic targeted therapies. The second trial is being performed through the EORTC by randomizing patients to either (1) upfront CN followed by sunitinib versus (2) four 6-week cycles of sunitinib (4 + 2 schedule) followed by CN only in patients with non-progressive metastases [5]. The study is attempting to enroll 458 patients with the primary end point being PFS. Given the morbidity of CN and the considerable percentage of patients experiencing progression in the interval between surgery and the start of systemic therapy, this trial may provide evidence supporting the presurgical treatment of mRCC patients as a “litmus test” to further select candidates for CN. Completion of accrual and results from both of these trials are highly anticipated.
10.6 Patient Selection for CN
Based on the two randomized trials published in 2001, CN became the standard of care in patients with mRCC who are candidates for systemic immunotherapy. A very important caveat to the successful integration of surgery with systemic therapy is in defining the optimal patient selection criteria. CN can be associated with significant morbidity, which may preclude subsequent systemic therapy. In addition to complications and postoperative pain, some patient’s disease will rapidly progress while recovering from surgery and they subsequently may be unsuitable to receive systemic therapy. Reports from the immunotherapy era showed significant variation in the percentage of patients who were unable to receive postoperative systemic therapy (range 5.6–77 %) because of complications of surgery or rapid disease progression (Table 10.1) [3, 16, 32–36].
Table 10.1
Cytoreductive series from the immunotherapy era
Study | Year | Number of patients | Institution | Morbidity | Operative mortality | % inability to receive systemic therapy |
|---|---|---|---|---|---|---|
Walther et al. | 1993 | 93 | Single center | 13 % | 0 % | 40 % |
Rackley et al. | 1994 | 37 | Single center | 16 % | 2.7 % | 22 % |
Bennett et al. | 1995 | 30 | Single center | 50 % | 17 % | 77 % |
Franklin et al. | 1996 | 63 | Single center | 12.7 % | 0 % | 12 % |
Fallick et al. | 1997 | 28 | Single center | NR | 3.6 % | 7 % |
Levy et al. | 1998 | 66 | Single center | 35 % | 3 | 18.1 % |
Flannigan et al. (SWOG+EORTC) | 2004 | 331 | Multicenter | 23.4 % | 1.4 % | 5.6 % |
10.6.1 Predictive Variables
In one of the initial studies from the National Cancer Institute, Walther et al. reported on a series of 93 patients undergoing CRN with planned postoperative interleukin-2 therapy [16]. Forty percent of patients were not able to receive postoperative systemic therapy, most commonly due to rapid progression of systemic disease. Preoperative clinical factors and laboratory values were assessed in an attempt to identify factors associated with failure to receive subsequent therapy. The only significant predictor of not receiving subsequent therapy was having a preoperative ECOG PS >1 (p = 0.047).
In an attempt to mitigate the risks of CN, Fallick et al. used strict criteria to select patients for CN [35]. Patients being considered for CN had an ECOG PS of 0 or 1; predominant clear cell histology; greater than 75 % debulking of tumor burden technically feasible; absence of central nervous system, liver, or osseous metastases; and no major comorbid medical conditions. Over a 5-year time period, 85 patients with mRCC with their primary tumor in place were evaluated for CN. Patients in whom pretreatment biopsy revealed non-clear cell predominance were not considered for surgery. Only 33 % (28/85) met the eligibility criteria for CN. By utilizing these selection criteria, the operative outcomes and the ability to receive subsequent systemic therapy (93 %) were improved over prior series. Investigators at the Cleveland Clinic performed an independent analysis of metastatic burden in 46 patients undergoing CN [37]. In this contemporary series of patients undergoing CN, fractional percentage of tumor volume (FPTV) was shown to be associated with survival. In this cohort of patients treated only with targeted therapies, FPTV removed (<90 % versus ≥90 %) and preoperative corrected calcium were independent predictors of progression-free survival (PFS) (Fig. 10.3) [37].
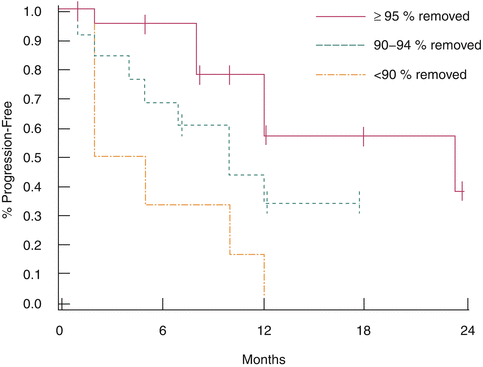
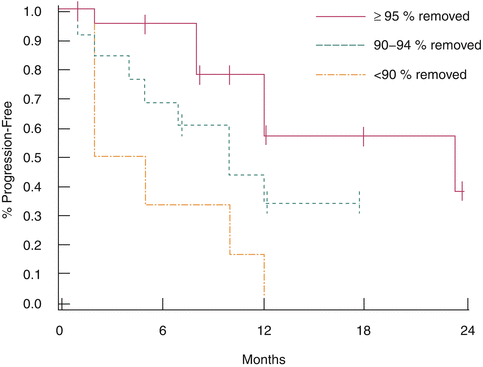
Fig. 10.3
Progression-free survival by fractional percentage tumor volume (Adapted with permission from Barbastefano et al. [37])
Published selection criteria for the two randomized trials were not as strict as those by Fallick et al. Eligibility for the SWOG 8949 and EORTC 30947 was identical [1–3]. All patients had a pre-randomization biopsy, adequate liver function (bilirubin <3× ULN), adequate renal function (Cr <3.0 mg/dl), ECOG PS of 0 or 1, and no prior malignancy within 5 years. Only 5.6 % of patients were unable to receive postoperative interferon alpha. In a later analysis of the SWOG 8949 data, Lara et al. analyzed predictive variables for OS after CN (Table 10.2) [38]. On multivariate analysis, patients with early progression (<90 days) and patients with an ECOG PS of 1 (versus 0) had significantly worse OS. Of course, early progression is not a preoperative variable, but perhaps identification of patients showing earlier signs of progression (SURTIME) would be desirable and aid in selection of patients for aggressive multimodal treatment through the use of CN.
Table 10.2
Multivariate analysis of predictors of overall survival after 90 days
Clinical variable | HR (95 % CI) | P value |
|---|---|---|
Progression by 90 days | 2.10 (1.50–2.92) | <0.0001 |
ECOG PS (1 versus 0) | 1.70 (1.26–2.31) | 0.0006 |
Lung metastasis (yes vs. no) | 0.81 (0.59–1.11) | 0.019 |
Alkaline phosphatasea | 1.24 (0.92–1.68) | 0.26 |
Hemoglobina | 0.84 (0.62–1.14) | 0.26 |
In 2006, a multidisciplinary panel used available data and the RAND/UCLA Appropriateness Method to develop recommendations regarding optimal patient selection [39]. Patients were classified as “good risk” surgical patients if the Eastern Cooperative Oncology Group (ECOG) performance status was 0 or 1 and major comorbid conditions were absent. Metastatic burden was classified as lung metastases only, limited metastases (low-volume lung or bone disease), or extensive burden (lung and bone metastases or any liver or CNS involvement). Symptoms were defined in relation to the primary tumor. The panel recommendations were as follows: for good surgical risk patients with planned postoperative immunotherapy, nephrectomy was rated appropriate in patients who had limited metastatic burden regardless of symptoms and in symptomatic patients regardless of metastatic burden. With regard to planned targeted therapy, the panel recommended only patients with the most favorable combination of surgical risk, metastatic burden, and symptoms undergo CN. The panel highlighted the limitations in defining the role of CN in patients for whom systemic targeted therapy is planned.
In addition to selection criteria established from single center retrospective series and the two randomized trials, many authors incorporate prognostic factors for OS when deciding appropriateness of CN. Whether these overall prognostic factors can be used to predict for early progression and thus be used as selection criteria for CN is unknown. Although the MSKCC risk stratification is one of the most widely accepted and validated set of prognostic factors in mRCC, this stratification system was not intended for use as a selection criterion for performing CN, but rather was established to provide prognostication for patients undergoing systemic therapy alone or after CN [40–42]. Utilizing these prognostic variables, patients are further categorized into favorable risk (0 risk factors), intermediate risk (1–2 risk factors), or poor risk (≥3 risk factors). A poor prognostic variable in the initial report was absence of a nephrectomy (presence of the primary tumor) [42]. Due to the rapid adoption of CN after the SWOG 8949 and EORTC 30947 publications, the subsequent MSKCC criteria replaced this variable with “time from diagnosis to treatment of less than 12 months” (Table 10.3) [41]. The original as well as subsequent modified risk stratification systems have also been shown to be useful for prognostication in contemporary cohorts of mRCC patients receiving targeted therapy [43–45].
Table 10.3
Poor prognostic factors for overall survival in patients with metastatic renal cell carcinoma
Study | Motzer et al. | Motzer et al. | Mekhail et al. | Motzer et al. | Heng et al. | Patil et al. |
|---|---|---|---|---|---|---|
Publication year | 1999 | 2002 | 2005 | 2009 | 2009 | 2011 |
Therapy | Multiple | Interferon alpha | Interferon alpha or cytotoxics | Interferon alpha or sunitinib | Sunitinib, sorafenib, bevacizumab | Interferon alpha or sunitinib |
Number (n) | 670 | 463 | 308 | 750 | 645 | 750 |
Risk criteria | ||||||
Favorable | 0 | 0 | 0–1a | 0 | 0 | n/a |
Intermediate | 1–2 | 1–2 | 2 | 1–2 | 1–2 | n/a |
Poor | >2 | >2 | >2 | >2 | >2 | n/a |
Prognostic factors | KPS <80 | KPS <80 | – | ECOG PS >1 | KPS <80 | ECOG PS >1 |
Low HGB | Low HGB | Low HGB | Low HGB | Low HGB | Low HGB | |
Corrected calcium ≥10 mg/dl | Corrected calcium ≥10 mg/dl | Corrected calcium ≥10 mg/dl | Corrected calcium ≥10 mg/dl | Corrected calcium ≥10 mg/dl | Corrected calcium ≥10 mg/dl | |
LDH >1.5× ULN | LDH >1.5× ULN | LDH >1.5× ULN | LDH >1.5× ULN | – | LDH >1.5× ULN | |
Absence of prior nephrectomy | Time from diagnosis to systemic therapy <12 months | Time from diagnosis to systemic therapy <12 months | Time from diagnosis to systemic therapy <12 months | Time from diagnosis to systemic therapy <12 months | Time from diagnosis to systemic therapy <12 months | |
>1 organ site with metastases | >1 organ site with metastases | Neutrophils > ULN | Osseous metastases | |||
Prior radiotherapy | Treatment with interferon (versus sunitinib) | Platelets > ULN | ||||
Retroperitoneal lymph node disease |
Several of these validated prognostic factors for OS after systemic therapy were either previously incorporated into patient selection criteria for CN or have been subsequently analyzed. Given the significant morbidity of CN, the indiscriminant use of CN is not advisable [22]. Kutikov et al. reported on the outcomes of 141 patients after CN treated between 1990 and 2008 [46]. Of those not receiving subsequent systemic therapy (30.5 %: 43/141), the most common reason was rapid disease progression (30.2 %). Patients not receiving systemic therapy had a trend toward lower survival although this was not statistically significant (p = 0.16). The risk of death after surgery correlated with the number of metastatic sites (p = 0.012), symptoms at presentation (p = 0.001), poor performance status (p = 0.001), high tumor grade (p = 0.006), and the presence of sarcomatoid features (p < 0.024).
Although there are significant practice variations among high-volume centers, the selection of patients for CN is based on a combination of prognostic factors for OS and predictors of surgical outcome after CN. In one of the largest series of its kind, Culp et al. attempted to identify preoperative clinical variables in a cohort of 566 patients undergoing CN and 115 receiving systemic therapy alone at the MD Anderson Cancer Center over a 15-year period (1991–2007) [31]. An extensive list of preoperative variables was analyzed which resulted in the identification of seven preoperative variables found to be significant negative predictors of overall survival (Table 10.4). The number of preoperative risk factors was correlated with OS and was inversely proportional to the median survival of patients who underwent CN. Patients who underwent CN with >3 preoperative risk factors did not appear to benefit from CN when compared to patients undergoing medical therapy alone (Fig. 10.4). Sarcomatoid dedifferentiation and Fuhrman grade 3 or 4 were also significant factors for OS, but in most cases these were not available preoperatively and thus were not included in the analysis of preoperative factors.
Table 10.4




Negative preoperative prognostic factors for overall survival after cytoreductive nephrectomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree