Interferons (INF): interferon-α, interferon-β, interferon-γ
Interleukins (IL): interleukin-2, interleukin-12, interleukin-21
Cytokine combination strategies
Cytokine combinations
Cytokines + cellular therapies (e.g., IL-2 and tumor-infiltrating lymphocytes)
Cytokines and chemotherapy or biologics (e.g., INF + bevacizumab)
Mini–allogeneic transplant approach
Reduced-intensity conditioning therapy followed by circulating hematopoietic progenitor cell transplantation
Tumor vaccines
Tumor cell-based vaccines
Gene-modified tumor cell vaccines
Dendritic cell-based vaccines
Heat shock protein-based vaccine
Antigenic peptide-based vaccines
Immune checkpoint inhibition
Anti-PD1 monoclonal antibodies (nivolumab)
Inhibition of cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)
Cytokines are non-antibody proteins used for cellular communication and can act as mediators or regulators of the immune system. Some of the most studied cytokines include interferon alpha (IFN-α) and interleukin-2 (IL-2). These cytokines have long been considered important factors in the activation and development of an immune response, including responses against tumor cells. These responses are believed to be mediated through enhanced T-cell, dendritic cell, and natural killer (NK)-cell activity directed against antigenic RCC cells. The discovery of methods to manufacture and purify cytokines through recombinant technology triggered a series of trials testing these agents in patients with advanced RCC.
15.2 Interferon
IFN-α is a cytokine that stimulates cytolytic activity and proliferation of NK cells, phagocytic functions and production of other cytokines by macrophages, and the expression of MHC molecules in most immune cells [3]. Another mechanism by which IFN-α operates is through regulation and proliferation of cytotoxic CD8+ T cells [4]. It is thought that IFN-α stimulates the proliferation, activation, and generation of CD8+ T cells leading to tumor cell destruction. In cancer, there is also dysregulation observed between T-helper (Th) 1 and Th2 CD4+cells, characterized by an imbalance in Th2 CD4+ cell production [5]. Th1 CD4+ cells mature to become macrophage-activating cells, whereas Th2 CD4+ cells turn into B cells. IFN-α can stimulate the expression of IL-12 receptors on Th1 cells leading to selective promotion of the Th1 response and also causing a suppression of IL-4 and IL-13 gene expression. This culminates in a subsequent dampening of the Th2 response [6]. This series of events is believed to lead to an enhancement in the activity of the cellular immune response wherein monocytes and macrophages exert a direct negative effect on tumor cell growth and proliferation via their phagocytic mechanisms. IFN-α also exerts its antitumor activity through its ability to upregulate MHC gene expression in tumor cells. Most tumor cells exhibit a partial or complete loss of MHC antigens on the cell surface [7]. This does not allow for dendritic cells – antigen-presenting cells (APCs) that are potent stimulators of IFN-α production – to recognize nonself antigens and to initiate the cytokine cascade. This can then lead to an indirect enhancement of the proliferation of tumor cells. Antitumor therapies that upregulate MHC gene expression in tumor cells, such as IFN-α, are thought to induce immunologic rejection of the tumor cells through the activation of APCs and cell-mediated cytotoxicity.
Three categories of interferons of relevance to RCC have been described: IFN-α, IFN-β, and IFN-γ. These IFN species vary according to the usual cell of derivation. IFN-α is mainly derived from white blood cells and IFN-β from fibroblasts, while IFN-γ is typically derived from T cells. As noted earlier, recombinant technology has allowed for the efficient manufacture of these molecules for human testing in clinical trials. The most active agent appears to be IFN-α, while IFN-β and IFN-γ appear to be of limited clinical utility. For example, in a phase II trial single-agent IFN-β serine in RCC, there was no signal of enhanced efficacy for IFN-β serine compared to historical data with IFN-α [8]. Furthermore, a placebo-controlled trial in metastatic RCC of IFN-γ 1b (dosed at 60 μg per square meter of body surface area subcutaneously once weekly) showed no significant differences between the groups in terms of response rates, time to disease progression, or overall survival. Thus, further clinical development of IFN-β and IFN-γ had been halted, while IFN-α was subsequently evaluated in a series of clinical trials.
Recently, there has been revival of interest in IFN-γ research. A study by Chen et al. draws attention to two issues limiting IFN-γ efficacy, which include previously exploiting only its immunomudulatory properties rather than its direct tumoricidal properties and its poor pharmacokinetics, which was improved by developing an antibody-cytokine conjugate. In this in vitro study, the investigators demonstrate that both human and murine IFN-y fused to an anti-CD70 antibody are able to induce RIP1-dependent necrosis in RCC cells in the presence of the proteasome inhibitor bortezomib [9]. Further studies evaluating IFN-γ are ongoing.
Wide ranges of dosing regimens and schedules for IFN-α have been employed across clinical trials. At this time, no one dose schedule has been definitively identified as the most optimal. A regimen of nine million units given by subcutaneous injection, three times a week for 12 weeks or to disease progression, has been widely used in the control arms of recently completed randomized phase III trials [10–14]. In 1990, IFN-α was approved for the treatment of metastatic renal cell carcinoma in Western Europe based on nonrandomized phase II studies. Notably, IFN-α has never received US Food and Drug Administration (FDA) approval for its use in advanced RCC (Fig. 15.1 shows the proposed 3D structure for the recombinant IFN-α2b molecule as depicted in RCSB Protein Data Bank at http://www.rcsb.org).
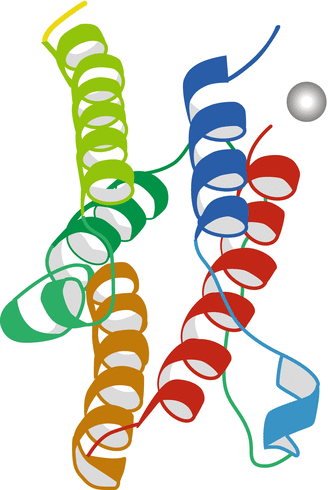

Fig. 15.1
Proposed three-dimensional structure of recombinant interferon alpha-2b (http://www.rcsb.org)
A number of randomized phase III studies have been completed using IFN-α in the setting of metastatic RCC; it must be noted that none of the trials were placebo controlled. One study compared IFN-α2b with medroxyprogesterone acetate (MPA) [15, 16]. Patients with mRCC were randomized to receive either subcutaneous IFN-α2b (three doses, five million units, five million units, and ten million units for the first week, and then ten million units three times per week for a further 11 weeks, with a total number of patients = 174) or oral MPA (300 mg once daily for 12 weeks, with a total number of patients = 176). A total of 111 patients died in the IFN-α2b group compared to 125 patients in the MPA group. There was a relative reduction in the risk of death by 28 % in the IFN-α group (hazard ratio 0.72 [95 % CI 0 · 55–0 · 94], p = 0 · 017). IFN-α2b gave an absolute improvement in 1-year survival of 12 % (MPA 31 % survival vs. IFN-α2b 43 %) and an improvement in median survival of 2.5 months (MPA 6 months vs. IFN-α2b 8.5 months). Side effects were more common with the IFN-α2b group and included moderate to severe lack of appetite, nausea, lack of energy, shivering, and dry mouth. Other studies compared IFN-α2a plus vinblastine with either vinblastine alone [16–18] or against MPA [19]. When IFN-α and vinblastine were compared to vinblastine alone, the interferon-containing arm was superior in terms of response rates (17 % vs. 3 %) and survival (67.6 vs. 37.8 weeks, p < 0.05). On the other hand, when the combination IFN-α2a and MPA was compared to MPA alone, there was a significant difference in response rate (21 % vs. 0 %), but not in overall survival (16 months vs. 10 months, p = 0.19).
This notion was confirmed in a 2005 Cochrane review of published randomized controlled trials employing IFN-α in advanced RCC [20]. Pooled results from four trials consisting of 644 patients suggested that IFN-α was superior to controls (odds ratio for death at 1 year was 0.56, 95 % CI 0.40–0.77), while the overall hazard ratio for death was 0.74 (95 % CI, 0.63–0.88). The pooled remission rate was 12.5 % for IFN-α versus 1.5 % for controls, with a pooled odds ratio of 7.6 (95 % CI 3.0–19.2). The weighted average improvement in survival was 3.8 months (11.4 vs. 7.6 months). Based on these results, IFN-α became a reasonable community standard for the systemic management of advanced RCC. Recently, the discovery of novel targeted agents has decreased the use of IFN-α with its application limited to combination therapy with biologic agents (discussed later in this chapter and in Chap. 17).
Observational case reports noted improved responses and survival when the primary tumor was removed surgically. This was the impetus for a randomized trial comparing IFN-α to nephrectomy followed by IFN-α in mRCC conducted by the Southwest Oncology Group (SWOG trial 8949). The results were noteworthy for a significant improvement in median overall survival in patients who had a nephrectomy prior to immunotherapy. The median overall survival in the group receiving IFN-α only was 8.1 months, while the median overall survival in the group of patients who received a nephrectomy followed by IFN-α was 11.1 months [21]. An updated analysis with a median follow-up of 9 years was conducted to evaluate predictors of overall survival. Patients randomized to nephrectomy continued to demonstrate improved overall survival (HR 0.74, 95 % CI 0.57–0.96, p = 0.022). Multivariate analysis showed that performance status 1 vs. 0 (HR 1.95, p < 0.0001), high alkaline phosphatase (HR 1.5, p = 0.002), and lung metastasis only (HR 0.73, p = 0.028) were overall survival predictors [22]. The findings seen in the SWOG 8949 were confirmed by another similar but much smaller randomized trial conducted by the European Organization for Research and Treatment of Cancer Genitourinary Group (EORTC 30947). This trial reported a significant increase in the time to progression (5 months vs. 3 months) and median survival duration (17 months vs. 7 months) in the group that underwent debulking nephrectomy followed by IFN-α when compared to IFN-α alone [23]. Furthermore, when both of these trials were combined in a meta-analysis conducted by the Cancer Care Ontario Program in Evidence-Based Care (CCO-PEBC), the overall median survival time for patients treated with nephrectomy and INF-α2b was 13.6 months compared with 7.8 months for patients treated with INF-α2b alone (p = 0.002). This represents a 31 % decrease in the risk of death in the surgical arm [24].
These data support the role for cytoreductive nephrectomy. Among the many caveats here are that some patients who undergo surgery may have resultant complications that either delay or make them ineligible to receive further systemic therapy. Nevertheless, IFN-α following debulking nephrectomy in patients fit enough to undergo the procedure should be considered as part of the standard treatment strategy in mRCC.
15.3 Interleukin-2
Interleukin-2 is an immune cytokine that is essential in the activation of a specific response to antigens by T cells, as well as crucial in triggering the innate immunity by stimulating several functions of NK cells and macrophages [25]. The actual mechanism by which IL-2 exerts its antitumor effects is unknown, but there are several hypotheses. Experiments in animal models showed that IL-2 can offset defective antigen recognition and overcome tolerance, thus suggesting its use as therapy to stimulate tumor destruction by T- or NK-cell activation while overcoming possible forms of tolerance or immunological ignorance which are known to occur toward tumor antigens [25]. In vitro studies with murine and human cells showed that IL-2 can activate lymphokine-activated killer (LAK) cells, a subpopulation of lymphocyte effectors that include NK, T, and NKT cells. These cells are endowed with the capacity of killing neoplastic cells in a MHC-unrestricted fashion. Clinical trials have noted a response in the tumor burden of patients treated with IL-2, but the mechanism of such clinical responses has not been clarified since accumulation of LAK cells in metastatic deposits (i.e., direct tumor kill) has not yet been demonstrated [25]. Thus, tumor shrinkage has been attributed to nonspecific cytotoxic activity of LAKs as well as to activation of tumor-specific T cells, but the release of tumor cytotoxic cytokines (e.g., TNF-α) by activated lymphocytes may also have contributed.
A total of 255 patients with metastatic RCC were entered onto seven phase II clinical trials and treated with high-dose IL-2 at either 600,000 or 720,000 international units per kg (IU/Kg) per dose intravenously every 8 h for up to 14 consecutive doses until maximally tolerated [26, 27]. A second identical cycle of treatment was scheduled following 5–9 days of rest. These courses could be repeated every 6–12 weeks in stable or responding patients for a total of three courses. The overall response rate was 14 % with 12 (5 %) complete responses and 24 (9 %) partial responses. The median response duration was 19 months for partial responders and had not been reached for complete responders. The median overall survival was 16.3 months [27]. These studies showed that patients who responded to IL-2 could attain a durable response and were living longer than historical controls that had received no therapy. The durability of response was confirmed elsewhere when 6 % of patients with metastatic renal cell cancer treated with high-dose IL-2 were found to be in complete remission from 4 to 10 years after treatment [28]. Based on the phase II single-arm studies discussed above, the FDA approved the dose of 600,000 IU/kg (high-dose IL-2) in 1992 for the treatment of metastatic RCC as front-line therapy.
High-dose IL-2 is associated with systemic toxicities and can affect every organ system in the body. Patients are generally admitted to an intensive care unit or similarly staffed unit for the administration of this drug. Prior to initiating therapy, one must make sure that the patient does not have significant cardiac, pulmonary, or renal disease. During a typical treatment course, patients will often experience the following symptoms occurring at different time points within the course. Within 2–3 h after the first or second dose of IL-2, patients often start experiencing fevers and chills. Around this same time, patients will also start experiencing mild hypotension and tachycardia that will progressively become more severe with each dose. They will typically establish a new baseline blood pressure around 20–30 mmHg below their usual blood pressure. Oliguria usually starts within the first 24 h, requiring small boluses of fluid to keep urine output greater than 20 ml/h. As the patient nears the end of the cycle, hypotension and oliguria can become refractory to judicious hydration (no more than 1–1.5 L per day) requiring pharmacologic intervention including dopamine and phenylephrine. Pulmonary congestion, increase in weight, and peripheral edema may then ensue due to fluid overload and as a manifestation of capillary leak. Nausea, vomiting, and diarrhea also occur closer to the completion of the cycle [29]. Neurologic, infectious, metabolic, and dermatologic effects can also be manifested; these are specified in more detail in Table 15.2. These symptoms are primarily thought to be due to capillary leak syndrome (CLS) and lymphoid infiltration within the organ systems. Proper management of the adverse events discussed above can limit toxicity and improve patient outcomes.
Table 15.2
Side effects and management of high-dose IL-2 administration
System | Adverse reaction | Treatment |
|---|---|---|
Cardiovascular | Hypotension due to capillary leak syndrome | Fluids (normal saline), limit to 1–1.5 L/day |
Add phenylephrine drip if refractory to fluids | ||
Sinus tachycardia due to hypotension | Increase time between doses of IL-2 | |
Atrial fibrillation or ventricular arrhythmia | Hold IL-2, evaluate for ventricular damage (ischemia), correct electrolytes and anemia, and use medications as needed. Restart IL-2 only if arrhythmia is easily corrected | |
Peripheral edema | Hold IL-2, watchful waiting as this will resolve over time or with the use of diuretics. Elevate extremity | |
Increased troponin or creatinine kinase | Hold IL-2; exercise ECHO before next dose of IL-2 to evaluate for myocardial dysfunction. If evidence of ischemia, stop IL-2 | |
Pulmonary | Hypoxia – fluid overload | Diuretics |
Tachypnea – due to hypoxia or metabolic acidosis | Diuretics if due to fluid overload | |
IV sodium bicarbonate | ||
Renal | Elevated creatinine with adequate urine output | Fluids (normal saline), limit to 1–1.5 L/day |
Add dopamine drip if unresponsive/unable to tolerate fluids | ||
If oliguria and/or elevated SCr, hold IL-2 | ||
Neurologic | Confusion, disorientation, hallucinations | Hold IL-2 until resolution; then rechallenge. If symptoms are recurrent, then hold treatment |
Metabolic | Metabolic acidosis | Bicarbonate infusion (100 meq/L) to keep serum bicarbonate level >18 meq/L |
Hypokalemia | Replace electrolytes as needed | |
Hypocalcemia | ||
Hypomagnesemia | ||
Systemic | Fevers and chills | Premedication with acetaminophen 650 mg po q4h and indomethacin 25 mg po q6h. An H2 blocker to protect the gastric mucosa should be utilized. Consider infectious etiology if first fever is over 24 h after therapy initiation |
Rigors | Meperidine 25–50 mg IV × 1 | |
Nausea and vomiting | Ondansetron 4 mg IV × 1 | |
Prochlorperazine 25 mg IV × 1 | ||
Skin | Dermatitis | Topical emollients and antihistamines. Avoid steroid- or alcohol-containing lotions |
Pruritus | Histamine antagonist (e.g., diphenhydramine) | |
Gastrointestinal | Diarrhea | Diphenoxylate or loperamide as needed |
Given the difficulty of administering high-dose IL-2, attempts were made to find a lower dose of IL-2 or an alternative administration schedule, whereby its antitumor activity would be preserved with diminished or mitigated side effects. A three-arm study sponsored by the National Cancer Institute compared high-dose IL-2 administered at 720,000 international units/kg to low-dose IL-2 dosed at 72,000 international units/kg to low-dose subcutaneous daily IL-2 [30]. Response rate was significantly higher with the high-dose compared with the low-dose IV and subcutaneous schedules (21 % vs. 13 % vs. 10 %, respectively). There were more adverse events in the high-dose IV therapy group, but no deaths were attributed to it. There was also a trend toward more durable responses with the high-dose IL-2 group. Overall, there was no difference in overall survival. Toxicities though were seen much less frequently in the low-dose arm, especially the major side effect of hypotension. Although, subcutaneous IL-2 did not have a significant response rate in this study, impressive response rates were seen in patients with metastatic RCC in other phase II trials [31–33]. This led to the popularization of this mode of therapy in European countries in the 1990s. There was however no definitive studies conducted to fully evaluate its utility and its place among the treatment options for metastatic RCC.
More recently, a systematic review evaluating patients with unresectable or mRCC, comparing treatment regimens containing IL-2 to those without, revealed that mortality at 1 year was not statistically significant between IL-2-based regimens and non-IL-2 controls [34]. The pooled response rates, however, were higher in patients receiving IL-2-based regimens (range, 9–39 %) compared with non-IL-2 controls (0–20 %). There was an increase in toxicity in the IL-2-based regimens compared to non-IL-2 controls; however, most patients tolerated treatment well. Of note, this review did not include any high-dose IL-2 trials, as there are no known randomized trials comparing high-dose IL-2 to non-IL-2 control or placebo (all prior studies were phase II single-arm studies).
Based on the data above, non-high-dose IL-2-containing regimens do not appear to provide superior treatment efficacy over non-IL-2-containing regimens and are associated with increased toxicity. High-dose IL-2 does provide higher response rates, albeit with higher toxicity, and can provide a small chance for a complete and durable remission and hence continues to play a role in the treatment of mRCC in the appropriate treatment population.
15.4 Interferon plus Interleukin-2 Combination(s)
Interferon alpha and interleukin-2 have been shown to have efficacy in the treatment of metastatic RCC; however, whether these two drugs given in combination would be more efficacious was the subject of intense investigation in the 1990s.
Phase II trials were first performed to assess combining these two agents in hopes of a synergistic response. One study evaluated high-dose IL-2 alone (1.33 mg/m2; approx. 600,000 IU/kg) versus non-high-dose IL-2 (0.8 mg/m2) in combination with IFN-α in patients with mRCC [35]. In this study, patients in both arms had responses to therapy, but the IL-2 alone arm (high-dose IL-2) was noted to have a higher objective and durable response rate. This study concluded that IL-2 alone, when given as a high-dose IV bolus, was active in metastatic RCC and that combining it with IFN-α was not as efficacious. A somewhat varying conclusion was noted from a publication around the same time that had tested alternate daily dosing of intravenous IL-2 and subcutaneous IFN-α [36]. In that study, 36 patients received 14 days of daily alternating treatments of IL-2 and IFN-α every 6 weeks for up to four cycles. Of the 30 patients who completed at least two cycles, there were nine objective responses, and seven of them had relapse-free survival times that were >6 months, the longest being 2 years. The toxicity was reported to be less, and these results led to a conclusion that the combination of IL-2 and IFN-α was active, rivaled responses of each agent alone from other phase I and II studies, and warranted further study. Other phase II studies were carried out in order to evaluate the use of subcutaneous IL-2 and IFN-α [37–39]. These studies noted encouraging responses with less toxicity, but results were discordant and did not provide definitive conclusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree