OUTLINE
Epidemiology of Cardiac Injury From COVID-19, 137
History of Cardiovascular Effects From SARS-CoV-1 and MERS-CoV, 138
Pathophysiology and Potential Mechanisms of Cardiovascular Injury From COVID-19, 138
Renin-Angiotensin System, 138
Direct Infection of the Myocardium, 139
Cytokine-Mediated Cardiac Injury, 140
COVID-19–Associated Thrombosis, 140
Clinical Manifestations and Complications, 143
Myocarditis, 143
Acute Coronary Syndrome, 143
Heart Failure, 144
Cardiac Arrhythmias, 144
Thromboembolic Events, 145
Cardiovascular Comorbidities and COVID-19, 145
Coronary Artery Disease, 145
Hypertension, 145
Management of COVID-19–Related Cardiac Complications, 146
Myocardial Injury and Acute Coronary Syndromes, 146
Myocarditis, 146
Heart Failure, 147
Hypertension, 148
Cardiac Arrhythmias, 148
Thromboembolic Syndromes, 149
Case Reports, 150
Case Report 6.1 COVID-19–Associated Myocarditis, 150
Case Report 6.2 COVID-19–Associated Coagulopathy With Stent Thrombosis, 151
Case Report 6.3 COVID-19–Associated Coagulopathy With Large Right Ventricular Thrombus, 152
COVID-19–Induced Cardiac Complications and Long-Term Sequelae, 152
Conclusion, 153
The heart, like the brain and liver, is a solitary vital organ. Anatomically and physiologically the heart plays a central role by pumping blood to various organs and tissues of the body, keeping them perfused and oxygenated and thereby maintaining their critical and life-sustaining functions. Disease processes involving the heart and causing cardiac injury are a matter of grave concern because of the potential for increased mortality and morbidity.
Epidemiology of Cardiac Injury From COVID-19
Precise estimates of the extent of cardiac injury and their ramifications in hospitalized patients with coronavirus disease 2019 (COVID-19) are difficult to determine. Thus the prevalence and clinical impact reported by various investigators vary depending on study sample characteristics and geography. Many of the early studies are reports from Wuhan and other regions of China because the initial outbreak occurred in the city of Wuhan and subsequently spread to different parts of Hubei and other provinces of China. Moreover, many of the patients hospitalized with COVID-19 had comorbidities of high blood pressure (BP) and cardiovascular disease (CVD) confounding precise estimates of new cardiac injury resulting from COVID-19. A study of 138 hospitalized patients with COVID-19 in Wuhan reported that 41 (31%) had high BP and 20 (15%) had CVD. During the course of hospitalization, 23 (17%) developed arrhythmia and 10 (7%) manifested acute cardiac injury. Another multicenter cohort study of 191 hospitalized patients with COVID-19 (135 from Jinyintan hospital and 56 from Wuhan pulmonary hospital) reported cardiovascular comorbidities of high BP in 58 patients (30%) and coronary artery disease (CAD) in 15 patients (8%). The study also reported acute cardiac injury in 33 patients (17%). Guo et al. in a study of 187 patients hospitalized with COVID-19 in Wuhan city reported comorbidities of high BP in 61 patients (33%), CAD in 21 patients (11%), and cardiomyopathy in 8 patients (4%). The study reported that 52 (28%) patients showed acute myocardial injury based on elevated troponin T level measurements. A multicenter study of 5700 patients with COVID-19 admitted to hospitals in New York City and nearby regions reported comorbidities of high BP in 3026 patients (57%), CAD in 595 patients (11%), and congestive heart failure (CHF) in 371 patients (7%). Based on elevated troponin levels, 801 patients (23%) showed evidence of acute myocardial injury.
In a study of 2736 patients admitted to the Mount Sinai Health Systems Hospitals in New York City with COVID-19, Lala et al. reported comorbidities of atrial fibrillation in 206 patients (8%), CAD in 453 patients (17%), heart failure (HF) in 276 patients (10%), and high BP in 1065 patients (39%). Based on elevated troponin concentrations, a total of 985 patients (36%) demonstrated the presence of cardiac injury.
From a meta-analysis of 20 studies with a pooled total of 6130 patients (range 21–2736), Fu et al. reported a prevalence of 22% for cardiac injury (95% confidence interval [CI], 16%–28%). In their review of 26 studies involving a large patient population of 11,685, Bavishi et al. reported a prevalence of 20% (range 5%–38%) for acute myocardial injury.
These studies provide a broad estimate in the range of 20% to 35% for acute myocardial injury in patients hospitalized with COVID-19.
History of Cardiovascular Effects From SARS-CoV-1 and MERS-CoV
The first SARS-CoV outbreak occurred in late 2002 and soon became a pandemic in early 2003, resulting in the death of more than 700 people, with a large cluster of fatalities reported from Hong Kong. This SARS-CoV-1 virus is thought to have originated in a single or multiple species of bats. A more recent coronavirus pathogen is the Middle Eastern respiratory syndrome (MERS) coronavirus (MERS-CoV), which first emerged in 2012 in Saudi Arabia and spread to many countries in the region. By 2018, MERS-CoV had infected more than 2000 people, causing 803 deaths, the majority of them in Saudi Arabia. Camels and bats are considered to be reservoirs of this pathogen.
Before the emergence of SARS, human coronaviruses typically caused only mild upper respiratory tract infections, resulting in the common cold. All this changed with the emergence of SARS-CoV, MERS-CoV, and the newest member SARS-CoV-2, the causative agent of the COVID-19 pandemic. Because the SARS-CoV-2 pandemic is still evolving, and there is considerable variance in testing protocols among the different countries, a precise mortality rate is hard to determine. Moreover, a majority of SARS-CoV-2 infections are thought to be asymptomatic or quite mild. Based on reported confirmed cases and deaths the world over, the mortality rate appears to be in the range of 2% to 3%. In comparison, the mortality rate for SARS-CoV-1 was 10% and that of MERS-CoV 37%.
A variety of thrombotic and hematological manifestations were reported with SARS-CoV-1 infection. They include pulmonary embolism (PE), deep vein thrombosis, venous thromboembolism (VTE), and pulmonary artery thrombosis.
MERS-CoV disease is also associated with thrombotic and hematological manifestations. Thrombocytopenia was reported in about a third of laboratory-confirmed MERS-CoV patients and MERS-CoV–induced disseminated intravascular coagulation (DIC) was also reported. Different types of cardiac manifestations such as acute myocarditis, acute myocardial infarction (AMI), and HF were described in patients hospitalized with SARS-CoV-1 and MERS-CoV. However, acute myocardial injury, including myocarditis, was not widespread with SARS-CoV-1 infections.
SARS-CoV-1 can infect different types of immune cells, including monocytes, macrophages, and activated T lymphocytes, but can undergo replication only in alveolar epithelial cells. MERS-CoV is more aggressive; it infects the immune cells and alveolar epithelial cells and replicates inside these host cell types and lyses them.
Pathophysiology and Potential Mechanisms of Cardiovascular Injury From COVID-19
SARS-CoV-2 is primarily a respiratory virus, and its predominant target is the lung. Like any major extrapulmonary organ, the heart and the general vasculature are also broadly susceptible in two ways: (1) direct infection of the cardiac tissue by the virus; and (2) indirect or systemic effects resulting from the host inflammatory response, endothelial activation, thrombotic changes, and coagulation dysfunction. , Fig. 6.1 provides an overview of these mechanisms of cardiac injury. The pathophysiology of cardiovascular injury is largely dependent on the renin-angiotensin system (RAS), which plays a key role in cardiovascular homeostasis.

Renin-Angiotensin System
The local RAS , plays a key role in mediating and channeling the mechanistic forces responsible for cardiovascular injury. The RAS system and more specifically the hormone angiotensin is responsible for the maintenance of the cardiovascular homeostasis. The classic and counterregulatory renin-angiotensin (RA) pathways play key regulatory roles in this modulation of cardiovascular physiology. The key components of the classic RA pathway are (1) angiotensinogen is converted to angiotensin-1 by renin and (2) angiotensin-1 is further processed by the angiotensin-converting enzyme (ACE) to angiotensin-2. Angiotensin-2 induces vasoconstriction by binding to angiotensin-1 receptors (AT1Rs) on the vascular smooth muscle cells and also by the release of aldosterone from the adrenal cortex. In addition to vasoconstriction, activation of AT1R results in sympathetic stimulation, cardiac hypertrophy, fibrosis, and inflammation.
The counterregulatory RA pathway has two salient components: (1) Angiotensin-1 is cleaved by angiotensin-converting enzyme 2 (ACE2) to generate angiotensin 1-9; (2) angiotensin 1-7 is formed by cleavage of angiotensin-2 by ACE2. Angiotensin 1-9 activates angiotensin-2 receptors (AT2R) to trigger nitric oxide production resulting in vasodilatation and concomitant lowering of BP. Angiotensin 1-7 binds to the proto-oncogene Mas receptor (MasR) causing dilatation of blood vessels and a reduction in BP. Both angiotensin 1-9 and angiotensin 1-7 peptides are cardioprotective and reduce inflammation, cardiac hypertrophy, and fibrosis. Both receptors AT1R and AT2R are expressed in the heart and blood vessels. Fig. 6.2 shows the regulatory and counterregulatory arms of the RAS.
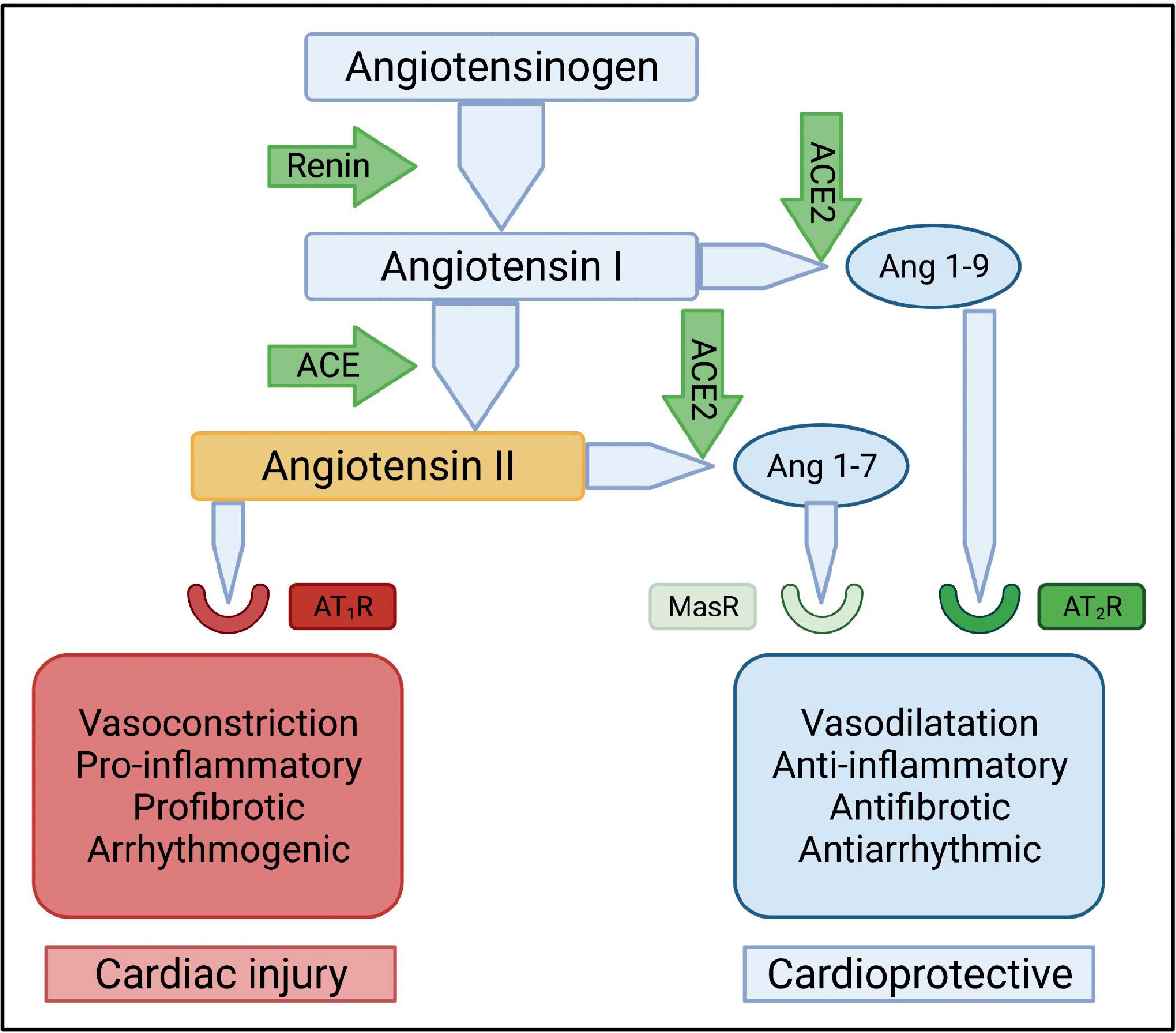
ACE2 is a key element of the counterregulatory arm of RAS and is an essential regulator of heart function. ACE2 is also the functional receptor for SARS-CoV-2; the SARS-CoV-2 spike protein binds to ACE2 for cellular entry of the virus. ACE2 has been shown to be upregulated in HF and ACE2 mRNA levels are elevated in myocardial infarction (MI). Inflammatory processes play a key role in the pathogenesis and natural history of many CVDs, including atherosclerosis, hypertension, cardiomyopathy, MI, and cardiac failure. , SARS-CoV-2 infection causes a functional depletion of ACE2 as the virus binds to ACE2 receptors for cellular entry. This causes downregulation of the counterregulatory arm of RAS and accumulation of angiotensin-2. Angiotensin-2 in turn binds to AT1R, triggering cardiac injury.
Direct Infection of the Myocardium
Direct entry of SARS-CoV-2 into cells is facilitated by the affinity of the viral spike protein S to the host cell ACE2 receptor, with the host protease transmembrane protease serine 2 (TMPRSS2), acting as the enabler. , Many organs and tissues of the body express both ACE2 and TMPRSS2, including the lungs, heart, gut, liver, kidney, neurons, joints, and eye, and this explains the tropism and potential of the virus to cause injury to these organs and tissues. Moreover, Chen et al. showed that although the expression levels of ACE2 are lower in the heart compared with the gut and kidney, the levels were higher in the heart than in the lungs, which act as the primary targets for SARS-CoV-2. Fig. 6.3 presents the ACE2 receptor expression profile in the cardiovascular system.
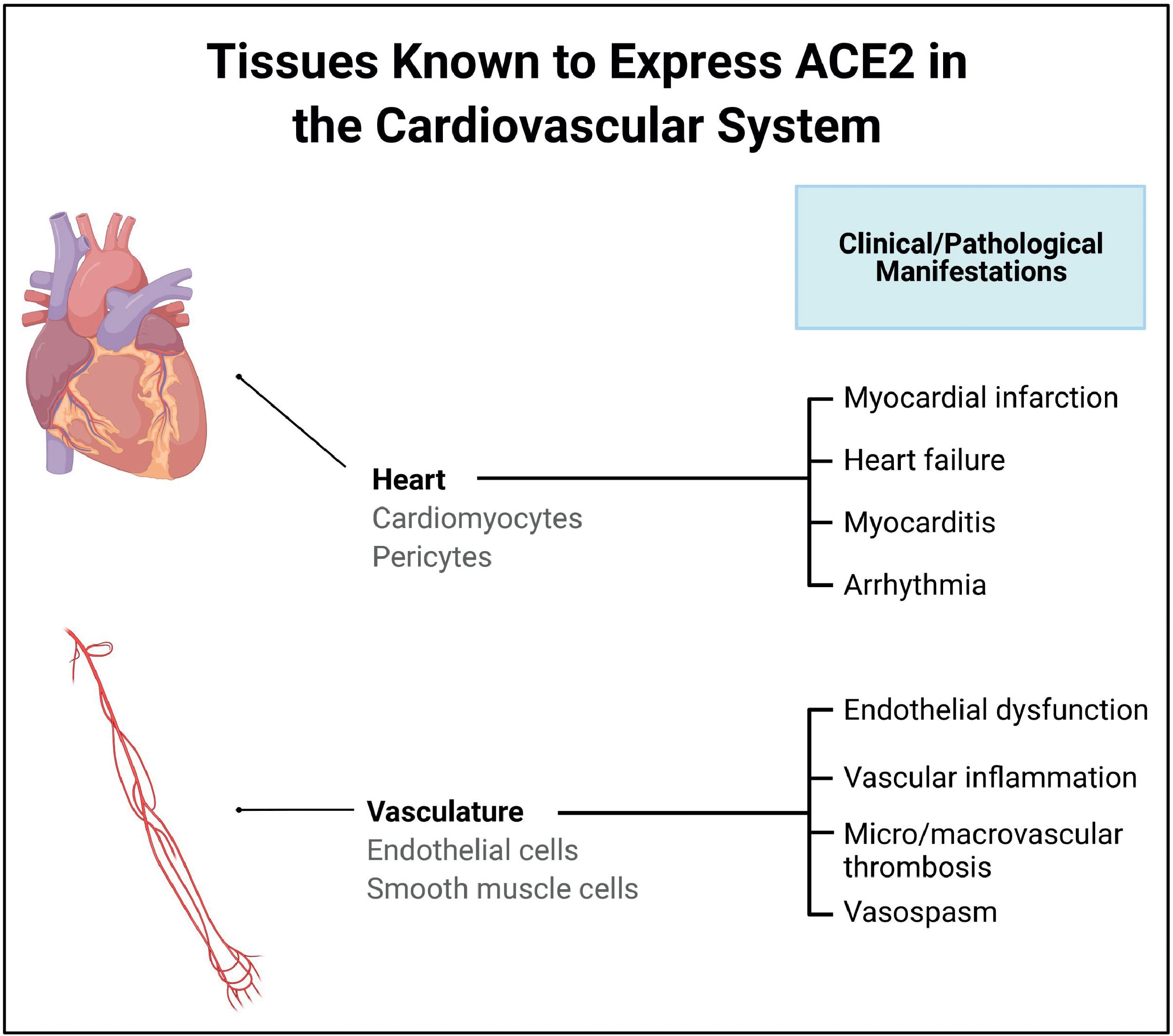
Different studies and case reports have demonstrated the presence of SARS-CoV-2 genomic RNA or viral particles morphologically identified as SARS-CoV-2 in myocardial tissue obtained from biopsy-confirmed myocarditis patients. , , Tavazzi et al. reported one of the earliest cases of biopsy-proven myocardial localization of viral particles with the morphology of a coronavirus in a COVID-19 patient who presented with cardiogenic shock. Escher et al. identified SARS-CoV-2 genomic RNA in 5 of 104 endomyocardial biopsy (EMB) samples of patients with suspected myocarditis or unexplained HF. A more recent study by Bearse et al. looked at 41 consecutive autopsies of patients who died from COVID-19 to understand the relationship of myocardial injury to SARS-CoV-2 infection. Based on in situ hybridization (ISH) and NanoString transcriptomic (NST) profiling, the researchers determined that endomyocardial infection by SARS-CoV-2 was present in 30 of 41 cases (73%). Cellular targets for SARS-CoV-2 include cardiomyocytes, pericytes, fibroblasts, and resident macrophages of the heart. Fig. 6.4 provides additional details of the mechanism of SARS-CoV-2 entry into the heart and blood vessels.
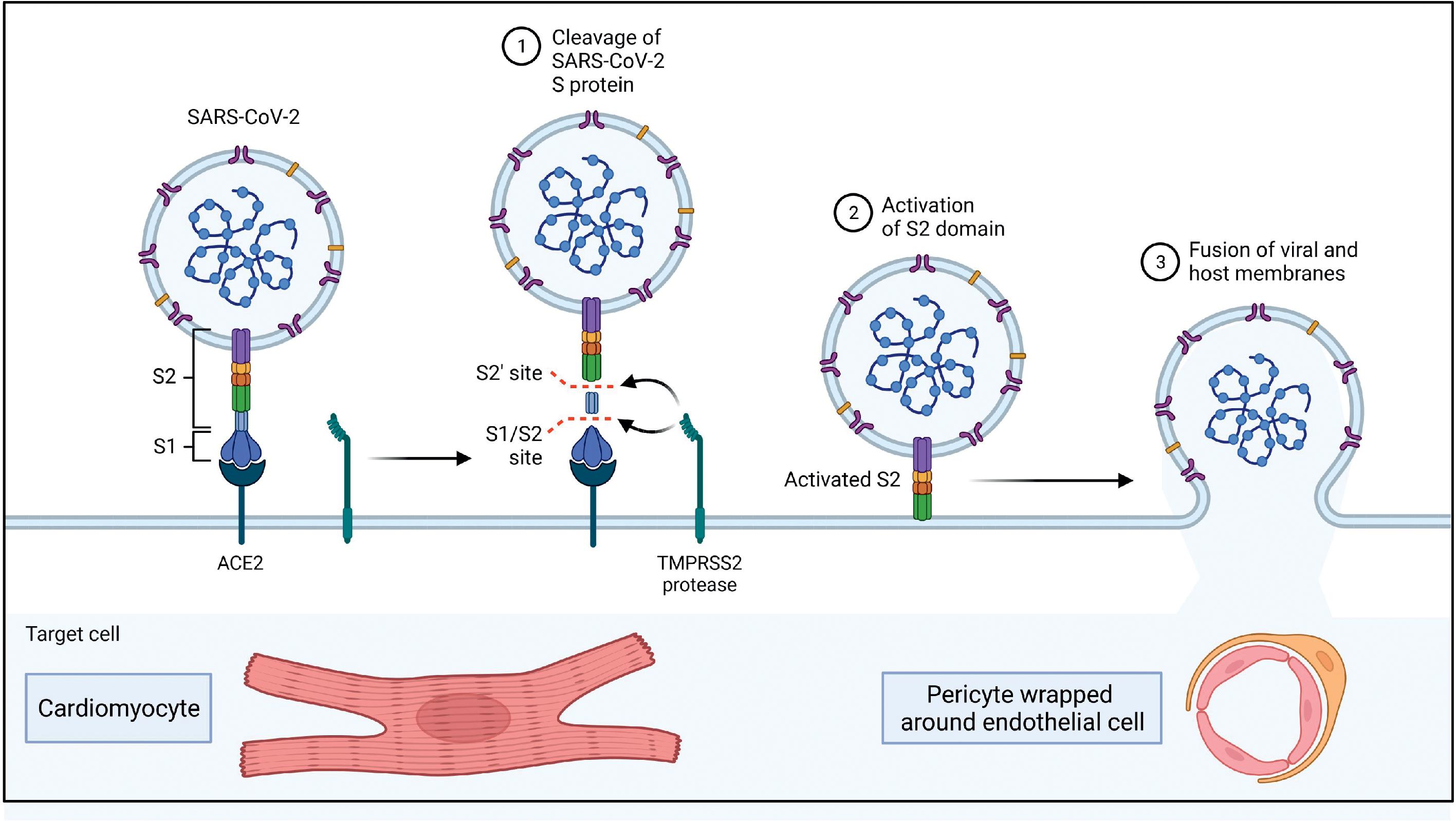
Based on ISH, NST profiling for virus positivity and histopathological findings of inflammatory infiltrates characteristic of myocyte injury for defining myocarditis, the researchers partitioned their study sample (n = 41) in three groups—virus-positive with myocarditis (n = 4), virus-positive without myocarditis (n = 26), and virus-negative without myocarditis (n = 11) to study the relationship between cardiac pathological changes and cardiac infection by the SARS-CoV-2 virus. Table 6.1 presents the details of the pathological findings in the various groups. Electrocardiographic (ECG) abnormalities in the form of atrial fibrillation, premature atrial complexes, prolongation of QTc, and nonspecific ST segment and T wave alterations were observed disproportionately in patients whose hearts were infected with SARS-CoV-2.
| Description of Observed Pathological Conditions | Virus(+) With Myocarditis (n = 4) | Virus(+) Without Myocarditis (n = 26) | Virus(–) Without Myocarditis (n = 11) |
|---|---|---|---|
| SARS-CoV-2+ (cells/cm 2 ) | 1.2 | 1.2 | 0.2 |
| Microthrombi present | 1 (25%) | 4 (15%) | 0 (0%) |
| CD68+ CD3+ CD4+ density | High | Low | Low |
| Focal pericarditis | 2 (50%) | 6 (23%) | 1 (9%) |
| Heart weight (grams) | 593 | 449 | 480 |
| Severe coronary artery disease | 1 (25%) | 5 (19%) | 3 (27%) |
Pellegrini et al. in their histopathological study of 40 patients hospitalized with COVID-19 who subsequently died, report that SARS-CoV-2 RNA was much more prevalent in the lung tissue (34/40 [85%]) compared with cardiac tissue (8/40 [20%])
Cytokine-Mediated Cardiac Injury
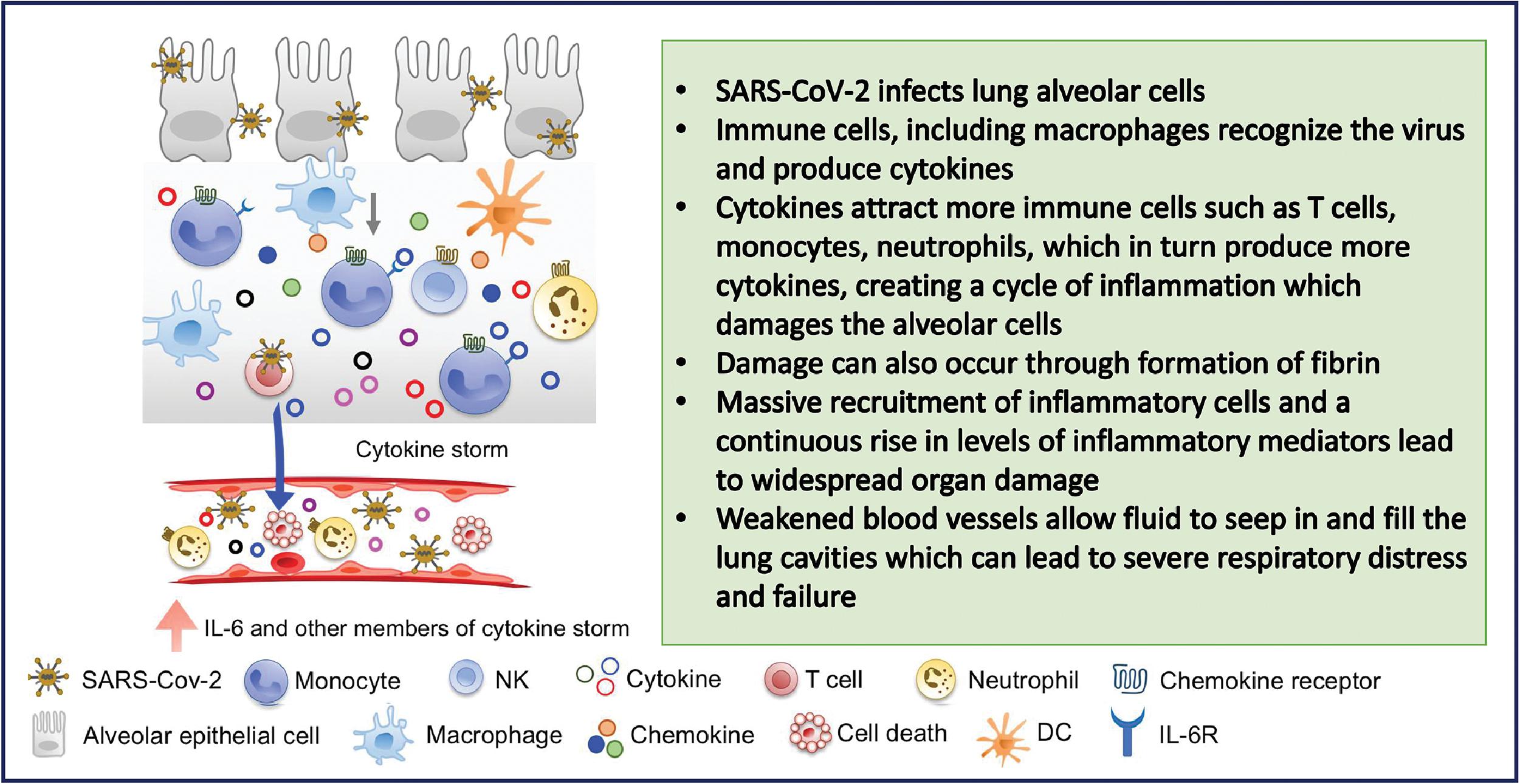
The systemic inflammatory response, and the resulting concomitant cytokine storm, is a major contributor to cardiac injury from SARS-CoV-2 infection. , , , , The aggravated inflammatory reaction resulting in increased cytokine production and release occurring in critically ill patients leads to the development of disseminated intravascular coagulopathy (DIC). In a study of 183 hospitalized patients with COVID-19 pneumonia, Tang et al. reported that DIC was observed in most of the 21 patients who did not survive. DIC could lead to microvascular thrombosis in coronary vessels resulting in myocardial injury. , Fig. 6.5 provides a summary of the cytokine storm phenomenon and its salient characteristics.
COVID-19–Associated Thrombosis
Critically ill hospitalized patients are at a high risk for thromboembolic complications. , In a cohort of 170 COVID-19 patients in intensive care in Boston area hospitals, more than 35% developed arterial or venous thromboemblism. COVID-19–associated coagulopathy (CAC) has been postulated as a distinct condition separate from DIC and coagulation abnormalities resulting from acute respiratory distress syndrome (ARDS). , , In the natural history of COVID-19, dysregulated coagulation has been documented in various studies as contributing to increased disease severity and higher mortality. , , The causative mechanisms of CAC are multifactorial. Direct invasion of the vascular endothelium by SARS-CoV-2, inflammatory cytokines such as interleukin-6 (IL-6), IL-8, and tumor necrosis factor-alpha, and coagulation factors such as factor VIII, von Willebrand factor, and angiopoietin-2 are all significant contributors to the prothrombotic state, resulting in thrombus formation in COVID-19. ,
DIC is characterized by a marked elevation in d -dimer and prothrombin time (PT) with concomitant reduction in fibrinogen and significant thrombocytopenia. In CAC, d -dimer and fibrinogen are elevated and the PT is not markedly increased. Thrombocytopenia is also not a characteristic feature of CAC. However, the CAC can evolve into a decompensated coagulopathy in critically ill COVID-19 patients. Fibrinolysis suppression leading to fibrinolytic shutdown also has been observed in severe COVID-19 complicated by CAC. , There seems to be significant overlap in the pathophysiology of DIC and CAC. However, arterial thrombosis and vascular complications are characteristic of CAC and microvascular thrombosis in coronary arteries is a distinct possibility.
Bois et al. in their histopathological study of 15 COVID-19 patients found nonocclusive fibrin microthrombi without ischemic injury in 12 cases, but its clinical significance is not clear. A larger histopathological study by Pellegrini et al. that studied the hearts of 40 patients who died from COVID-19 reported that 14 of 40 subjects (35%) had evidence of cardiac injury in the form of myocyte necrosis. Most of them, 11 of 14 (79%), demonstrated focal myocyte necrosis; the major cause of myocyte necrosis was cardiac microthrombi, which were observed in 9 of 14 (64%) hearts with myocyte necrosis. Both of these studies demonstrate the significance of microvascular thrombosis as a cause of cardiac injury in hospitalized patients with COVID-19.
Another potential pathophysiological mechanism of cardiac injury is the downregulation of ACE2 in SARS-CoV-2 infection. This was demonstrated in the mouse model by Oudit et al. As mentioned earlier, ACE2 has been shown to have a protective effect on the heart and blood vessels based on its antiinflammatory, antifibrotic, antioxidative, and vasodilatory properties. ACE2 converts angiotensin-2 to angiotensin 1-7, which reduces vasoconstriction mediated by the RAS. The downregulation of ACE2 causes angiotensin-2 to be upregulated, which results in an increased production of inflammatory cytokines , (see Fig. 6.2 ).
Clinical Manifestations and Complications
A spectrum of cardiovascular manifestations has been reported with SARS-CoV-2 infection. , , , , , , , The CVD presentations of COVID-19 include myocarditis, , acute coronary syndrome, , , HF, , , cardiac arrhythmias, , , and various types of vascular complications and thromboembolic events. , , , , , , , , , , , Fig. 6.6 depicts the spectrum of cardiovascular clinical manifestations and complications of COVID-19.

Myocarditis
Myocarditis can result from direct viral myocardial invasion or consequent to systemic inflammation. A clinical diagnosis of myocarditis is arrived at based on laboratory and imaging markers of myocyte involvement such as elevated troponin levels and cardiac magnetic resonance (CMR) imaging features. , , It is important to exclude CAD, and when CMR is not feasible, it is recommended that cardiac CT angiography may be performed. The clinical presentation of myocarditis can be variable from mild disease to fulminant myocarditis complicated by arrhythmias, HF, and cardiogenic shock. , Atrial and ventricular arrhythmias are quite common in the setting of myocarditis. , Various pathophysiological mechanisms have been postulated for the observed arrhythmicity in myocarditis. , Fig. 6.7 provides an illustration of these mechanisms leading to various types of arrhythmias in myocarditis. Myocarditis is an uncommon pathological diagnosis occurring in 5% to 10% of highly selected cases undergoing autopsy or EMB. , , Elevated troponin levels have been shown to be associated with higher mortality in the setting of COVID-19. , The sequelae of myocarditis such as low-grade inflammation and fibrosis play a major role in disease progression leading to HF.
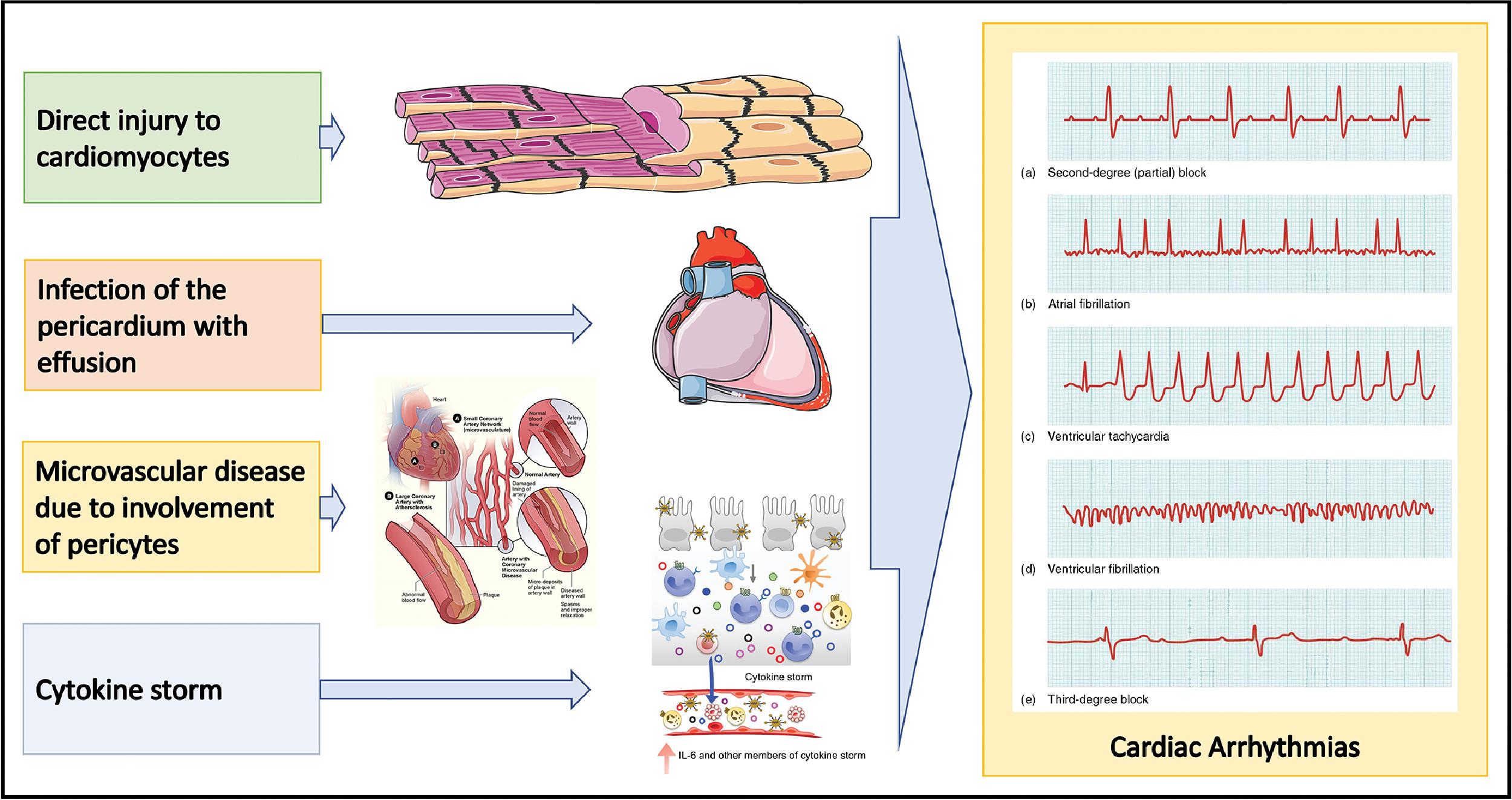
It is important to distinguish fulminant myocarditis from sepsis; other differential diagnoses include acute coronary syndrome and sepsis-related cardiomyopathy. Echocardiography, CMR, and cardiac computed tomography (CT) are useful in resolving the differential diagnosis; for a definitive diagnosis, EMB may be required.
Acute Coronary Syndrome
Severe systemic inflammation, increased levels of circulating cytokines, and a hypercoagulable state observed in COVID-19 have the potential for increased risk for AMI. Based on a study of 28 COVID-19 patients with ST-elevation myocardial infarction (STEMI) from the Lombardy region of Italy, Stefanini et al. reported that the first clinical manifestation of COVID-19 could be STEMI. Coronary angiography revealed a culprit lesion requiring revascularization in only 17 patients (61%), whereas the remaining 11 patients (39%) did not have obstructive CAD. Diaz-Arocutipa et al. performed a systematic review of 42 studies covering 161 COVID-19 patients with STEMI, which found obstructive CAD as the cause for ST elevation in 133 patients (83%). However, the mortality rate in the two groups, obstructive CAD, and nonobstructive CAD were similar (≈30%).
The causes of acute myocardial injury in patients without CAD in the context of COVID-19 include myocarditis, cytokine storm and stress cardiomyopathy; hypoxemia, microvascular dysfunction resulting from small vessel thrombosis, and endotheliitis also have been postulated as potential causes in this setting. ,
Heart Failure
HF occurs commonly in patients hospitalized with COVID-19. The prevalence is 25% among patients hospitalized with COVID-19 and about a third in patients whose condition is critical. , SARS-CoV-2 infection can also worsen preexisting cardiac failure, leading to a decompensated heart. Based on a retrospective study of more than 132,000 hospitalized patients with a history of HF over a period of 6 months, the mortality rate among the three categories of hospitalized patients were as follows: (1) Non-COVID non-HF, 4512 deaths (4.5%); (2) non-COVID acute HF, 617 deaths (2.6%); and (3) COVID-19, 2026 deaths (24.2%). Among hospitalized COVID-19 patients with a history of HF, with a high risk for complications, 1 in 4 patients died during the course of hospitalization. As discussed earlier, SARS-CoV-2 uses the ACE2 receptor for cellular entry. The ACE2 receptor is known to be upregulated in the failing heart, and this makes patients with a history of HF more susceptible to SARS-CoV-2 and potentially a more severe form of COVID-19. Fig. 6.8 illustrates the pathophysiological mechanisms contributing to severe disease in COVID-19 patients presenting with a history of HF.
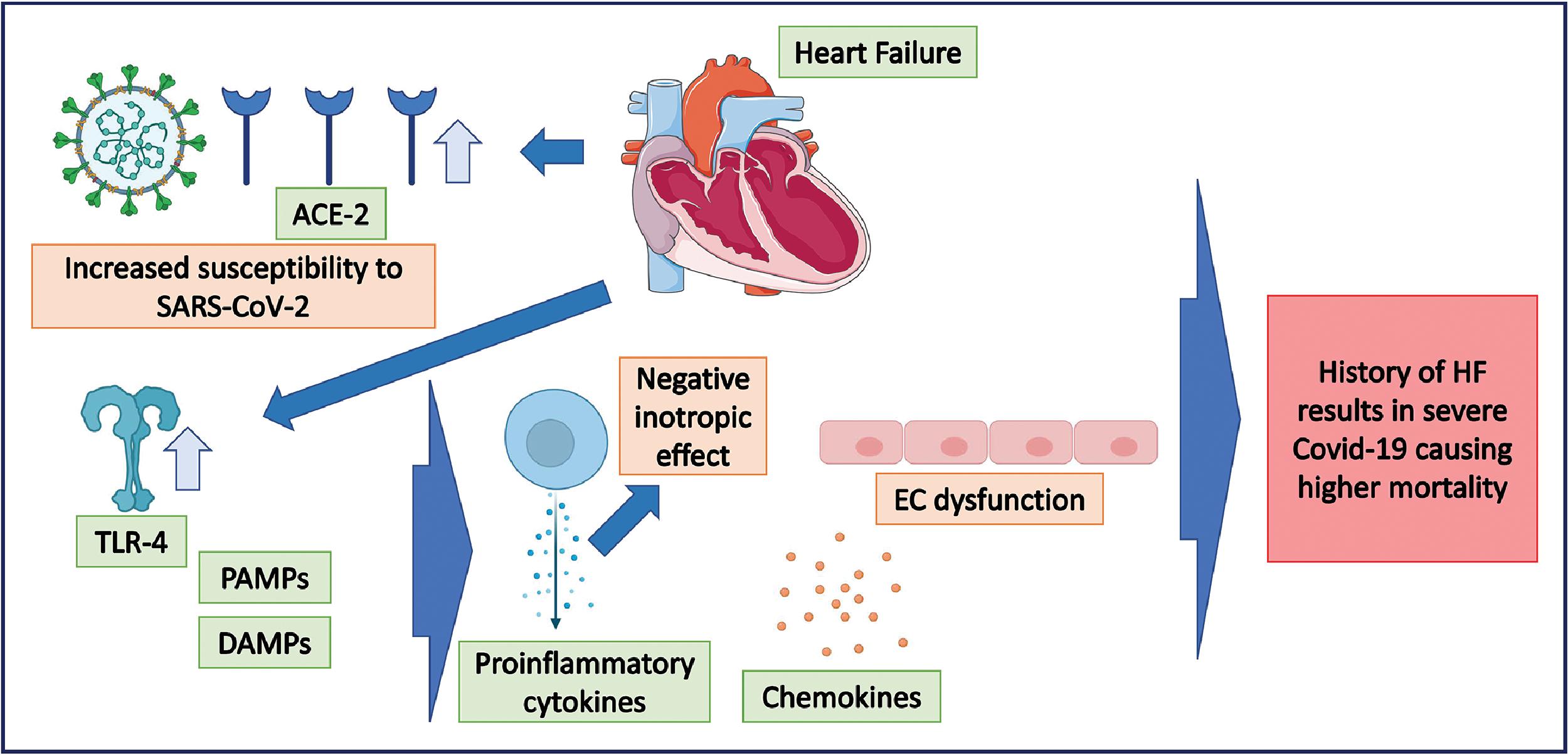
Cardiac Arrhythmias
Early reports of the incidence of arrhythmias occurring in hospitalized patients with COVID-19 in Wuhan varied from 6% for ventricular arrhythmias to 17% for all types of arrhythmias. Based on their study of 700 patients hospitalized with COVID-19 at the University of Pennsylvania Hospital, Bhatla et al. reported 53 arrhythmic events: 9 cardiac arrests, 25 atrial fibrillations, 9 clinically significant bradyarrhythmias, and 10 nonsustaining ventricular tachycardias. The cardiac arrests were independently associated with acute mortality whereas the other three types of arrhythmias did not independently contribute to increased mortality.
Based on a retrospective study of 4526 hospitalized patients worldwide with COVID-19, Coromilas et al. reported an incidence of arrhythmias in 827 (18%) patients, most without a history of arrhythmias. Atrial fibrillation, atrial flutter, and supraventricular tachycardia were observed in 82% of the patients, ventricular arrhythmias were seen in 21%, and 23% developed bradyarrhythmias. Presence of arrhythmias resulted in an adverse prognosis; approximately 40% had to be mechanically ventilated, and the mortality in the arrhythmia group was 50%.
Thromboembolic Events
VTE is common in hospitalized patients with COVID-19. Wichmann et al., in their postmortem study of 12 consecutive deceased patients who had been hospitalized with COVID-19, found a high incidence of VTE (7/12 [58%]). Piazza et al. observed an incidence of major arterial or venous thromboembolic event in in 60 of 170 (35%) patients with COVID-19 admitted to the intensive care unit (ICU), and in 6 of 229 (3%) in patients undergoing nonintensive care; DVT in 39 of 170 (23%) in the ICU setting and none in the non-ICU setting; PE in 3 of 170 (2%) in the ICU setting and 5 of 229 (2%) in the non-ICU setting. Ribes et al. in their review of thromboembolic events in COVID-19 reported a range of 25% to 69% for VTE in ICU setting, 15% to 53% for DVT in the non-ICU setting, 23% to 86% for DVT in the ICU setting, and an incidence of ≈20% for PE. In a multicenter cohort study of 1240 hospitalized patients with COVID-19, Fauvel et al. reported an incidence of PE in 103 cases (8.3%).
Although d -dimer is a nonspecific marker of inflammation, a high level of d -dimer has been shown to be associated with adverse outcomes of severe and critical illness and higher mortality in hospitalized patients with COVID-19. , , Prolonged PT also has been well-documented in hospitalized patients with COVID-19, , with worse prognosis and higher mortality associated with increase in PT. , The reduction in platelet count in hospitalized patients with COVID-19 has been mild to moderate but not clearly associated with severity of the disease. However, Zhou et al. reported that moderate thrombocytopenia was more frequent in nonsurvivors than among survivors.
Cardiovascular Comorbidities and COVID-19
The impact of cardiovascular comorbidities on COVID-19 resulting in adverse outcomes such as severe disease and increased mortality has been reported by various researchers. , , Nashiry et al. looked into the interactions of COVID-19 and cardiovascular comorbidities using a bioinformatics approach. The two major cardiovascular comorbidities resulting in adverse outcomes in patients hospitalized with COVID-19 are CAD and hypertension.
Coronary Artery Disease
Based on their study of 8438 patients hospitalized with COVID-19 in the New York City area, of whom 9% had CAD, Kuno et al. reported that patients with CAD had significantly higher rates of mechanical ventilation and mortality. The relative risk for CAD was markedly higher for both mechanical ventilation and death in patients 50 years and younger. Phelps et al., in their study of 4090 COVID-19–positive patients in Denmark, found that among patients who had severe disease or who died within 30 days of COVID-19 diagnosis, 20% had a history of CAD, and the representation of patients with a history of CAD in the nonsevere disease manifestation group was only 10%.
Hypertension
Although the risk for infection with SARS-CoV-2 remains the same for hypertensive and normotensive individuals, patients with hypertension are at higher risk for developing a more severe disease and increased mortality. However, Savoia et al. and Kreutz et al. argued that a direct role for hypertension as an independent risk factor for higher mortality in COVID-19 has not been clearly established. Because the prevalence of hypertension is higher in the older population, who have disproportionately suffered severe manifestations of COVID-19 resulting in increased mortality, the reported association between hypertension and severity of COVID-19 could be confounded by age and other comorbidities.
Management of COVID-19–Related Cardiac Complications
The available evidence needed to guide management is mostly limited given the relative brief experience since the onset of SARS-CoV-2. This has left the medical community with little high-quality evidence on management of this disease. This holds especially true for the wide range of cardiovascular manifestations and complications. Most of the cardiovascular management strategies are from published regional experiences and extrapolations from prior well-established experiences.
Myocardial Injury and Acute Coronary Syndromes
The assessment of troponin elevations with coexisting COVID-19 infection can be difficult in eliciting the cause. Myocardial injury defined as any cardiac troponin elevation is associated with significant morbidity and mortality. , Myocardial injury from COVID-19 is potentially from plaque rupture MI, inflammation, hypoxic injury, myocarditis, stress cardiomyopathy, microthrombi, or endothelial injury. , , Management of troponin elevations in association with COVID-19 is mostly extrapolated from pre–COVID-19 existing recommendations. , Acute myocardial injury should initially be assessed for clinical deterioration, hemodynamic instability, and arrhythmias. Imaging modalities should initially be performed with echocardiography and then consideration given for CMR imaging or CT scan based on clinical suspicion of the cause. , The majority of care is supportive in those with non–acute coronary syndrome troponin elevation. In general, supportive measures, including mechanical support, should be considered on a case-by-case basis.
Troponin elevations in COVID-19 patients can be related to plaque rupture that is consistent with an AMI. During the COVID-19 pandemic, patients presenting with an AMI should be treated as probable or possible COVID-19 positive. Those presenting with a probable STEMI should be treated with a primary percutaneous coronary intervention (PCI) strategy in the standard of care fashion. , In those with ST elevations on electrocardiography and unclear diagnosis of STEMI, additional testing, including point-of-care ultrasound to evaluate for regional wall motion abnormality or coronary CT angiography (CCTA) in cases with conflicting information, may have to be considered. The likelihood of having acute coronary syndromes versus nonacute coronary syndrome–associated troponin elevations should be weighed to help guide appropriate treatments. Non–ST-elevation myocardial infarct (NSTEMI) management should be guided by risk stratification and identification of high-risk features. A NSTEMI due to plaque rupture with high-risk features should be managed with an early invasive strategy with the intention of performing PCI; those without high-risk features should be carefully evaluated for alternative diagnoses. This should be undertaken along with management recommendations according to existing guidelines for noninvasive studies such as echocardiography or CCTA. ,
Myocarditis
COVID-19 is associated with myocarditis, which can range from mild disease to fulminant myocarditis. The mechanism, risk factors, and prevalence are not well understood. , , Myocarditis has a wide range of presentations but should be considered with new-onset HF, cardiogenic shock, and arrhythmias. , Troponin levels will be elevated and suggestive of myocardial injury but given the wide range of presentations, ischemia will often need to be ruled out first. The troponin elevation associated with COVID-19 infections can be difficult to discern, creating a challenging diagnostic dilemma. Fig. 6.9 is useful in the approach to this management issue.