Cancer affects all body systems, potentially leading to metabolic derangements, structural changes, and infection. Most individuals diagnosed with cancer will experience a complication at some time during their illness ( ). Clinicians need to be able to recognize such complications so as to rapidly diagnose, treat, and prevent chronic sequelae. Complications of cancer include acute or subacute changes, sometimes also referred to as oncologic emergencies, and chronic changes. These may be categorized by the systems affected—infectious, structural, metabolic, hematologic, vascular, and neurologic ( Table 20.1 ). Key complications and their pictorial descriptions are reviewed in this chapter.
| Metabolic |
| Hypercalcemia |
| Hyponatremia |
| Tumor lysis syndrome |
| Lactic acidosis |
| Adrenal insufficiency |
| Infectious |
| Neutropenic fever |
| Typhlitis (cecitis) |
| Hematologic |
| Hyperviscosity syndrome |
| Hyperleukocystosis |
| Hypercoagulable states |
| Nonbacterial thrombotic endocarditis |
| Pulmonary embolism |
| Deep venous thrombosis |
| Trousseau’s syndrome |
| Antiphospholipid syndrome |
| Sweet’s syndrome |
| Vascular/Structural |
| Superior vena cava syndrome |
| Malignant pericardial effusion |
| Hemorrhagic cystitis |
| Malignant effusions |
| Pleural effusion |
| Pericardial effusion |
| Peritoneal effusion (ascites) |
| Neurologic |
| Spinal cord compression |
| Intracranial metastases |
| Leptomeningeal carcinomatosis |
| Paraneoplastic Syndromes (see Chapter 5, Table 5.7 ) |
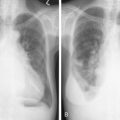
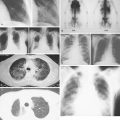
Infectious Complications
NEUTROPENIC FEVER
Infections in cancer account for a significant portion of morbidity and mortality, particularly in patients receiving chemotherapy. Neutropenic fever is defined as an oral temperature of 38°C (100.4°F) or greater in the setting of an absolute neutrophil count less than 1 × 10 9 per liter. It is more likely to occur with longer duration and depth of neutrophil nadir. Although the causative agents often elude diagnosis, enteric gram-negative bacilli are the most common organisms recovered from blood cultures. Factors favoring a lower risk of serious infection in the setting of neutropenic fever include absolute monocyte count of 1 × 10 9 per liter or more, normal chest radiograph, duration of neutropenia less than 7 days, no intravenous catheter site infection, and malignancy in remission ( Table 20.2 ). Some studies support the use of white blood cell growth factors to prevent or reduce the occurrence of neutropenic fever. Patients may present with symptoms supporting infection, although some patients are asymptomatic with the exception of a fever. Management comprises obtaining cultures of blood, urine, wounds, and cerebrospinal fluid (CSF) if applicable, as well as initiating broad-spectrum antibiotics. A fungal blood culture and antifungal medications are added if the patient does not defervesce within 24 hours of presentation. Patients with a total score of 21 or more on the Multinational Association for Supportive Care Cancer Scoring System have a low risk of serious complications from neutropenic fever ( Table 20.3 ).
| Absolute neutrophil count ≥1.0 × 10 9 cells/L |
| Absolute monocyte count ≥1.0 × 10 9 cells/L |
| Normal chest radiograph |
| Normal or only minimally abnormal renal and liver chemical test results |
| Duration of neutropenia <7 days |
| Resolution of neutropenia expected in <10 days |
| No intravenous catheter-site infection |
| Early evidence of bone marrow recovery |
| Malignancy in remission |
| Peak temperature of <39°C |
| No mental or neurologic changes |
| No appearance of illness |
| No abdominal pain |
| No comorbidity complications (e.g., shock, hypoxia, pneumonia, deep-organ infection, vomiting, or diarrhea) |
| Characteristic | Score |
|---|---|
| Burden of illness: no or mild symptoms | 5 |
| No hypotension | 5 |
| No chronic obstructive pulmonary disease | 4 |
| Solid tumor or no previous fungal infection | 4 |
| No dehydration | 3 |
| Burden of illness: moderate symptoms | 3 |
| Outpatient status | 3 |
| Age <60 years | 2 |
* Patients with a total score of ≥21 have a low risk of having serious medical complications. Points attributed to the variable “burden of illness” are not cumulative. Therefore, the maximum theoretical score is 26.
Metabolic Complications
HYPERCALCEMIA
Hypercalcemia occurs in up to 30% of patients during the course of their malignancy. Hypercalcemia is one of the complications of malignancy with skeletal involvement ( Table 20.4 ). Although it occurs predominantly in lung, breast, and multiple myeloma cancer, any malignancy can be associated with high levels of serum calcium ( ). Hypercalcemia is mediated by ectopic parathyroid hormone production (parathyroid hormone–related protein), generation of vitamin D analogues, or cytokine-induced local bone destruction. Parathyroid hormone–related protein, usually detected in cases of hypercalcemia associated with small cell lung cancer, mimics the action of parathyroid hormone, leading to bone resorption and distal tubular calcium resorption. In contrast, hypercalcemia associated with lymphoma is mediated by the generation of vitamin D analogues, such as calcitriol. The sequelae of hypercalcemia include constipation, psychosis, lethargy, excessive thirst and urination, hypovolemia, and renal failure. Treatment includes aggressive intravenous hydration and bisphosphonates to block osteoclastic bone resorption. Dialysis may also be required.
| Complication | No. (%) of Patients |
| Hypercalcemia of malignancy | 70 (19%) |
| Pathologic feature of a long bone | 68 (19%) |
| Spinal cord compression | 36 (10%) |
| Bone marrow failure/leukoerythroblastic anemia | 33 (9%) |
HYPONATREMIA
Hyponatremia may occur due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or salt-wasting nephropathy. SIADH is due to ectopic vasopressin production by tumor leading to renal retention of water and reduced aldosterone secretion associated with sodium loss in the urine and consequent reduced serum osmolarity. Chemotherapy, particularly high-dose cyclophosphamide and cisplatin, can also contribute to SIADH. Patients may present with euvolemia or hypovolemia. In hypovolemic states, oliguria and low urine sodium levels characterize SIADH. In contrast, nonoliguria and high urine sodium levels in the setting of hypovolemia characterize salt-wasting nephropathy. Salt-wasting nephropathy can occur as a result of chemotherapy, adrenal insufficiency, or cerebral salt wasting after intracranial surgery or subarachnoid hemorrhage. Treatment of hyponatremia includes water and salt restriction, hypertonic saline to slowly correct serum sodium levels, and administration of vasopressin receptor antagonists as indicated ( ).
TUMOR LYSIS SYNDROME
Tumor lysis syndrome occurs when intracellular materials are released from rapidly dying tumor cells leading to excess serum levels of uric acid, potassium, phosphorus, and reduced levels of calcium. The Cairo-Bishop definition of tumor lysis syndrome includes criteria for laboratory evaluation ( Table 20.5 ) and a grading classification ( Table 20.6 ). These metabolic derangements often occur within 5 days of chemotherapy or irradiation and can lead to hypovolemia, renal failure, seizures, and cardiac arrhythmias. Treatment involves frequent monitoring of serum laboratory values, aggressive intravenous hydration, alkalinization of the urine, and uric acid sequestrants (allopurinol or rasburicase).
| Laboratory tumor lysis syndrome |
| Uric acid ≥8 mg/dL (≥476 μmol/L) or 25% increase from baseline |
| Potassium ≥6.0 mEq/L (≥6 mmol/L) or 25% increase from baseline |
| Phosphorus ≥6.5 mg/dL (≥2.1 mmol/L) or 25% increase from baseline |
| Calcium ≤7 mg/dL (≤1.75 mmol/L) or 25% decrease from baseline |
| Clinical tumor lysis syndrome |
| Creatinine ≥1.5 times upper limit of normal |
| Cardiac arrhythmia or sudden death |
| Seizure |
| Grade 0 * | Grade I | Grade II | Grade III | Grade IV | Grade V | |
|---|---|---|---|---|---|---|
| LTLS | No | Yes | Yes | Yes | Yes | Yes |
| Creatinine † | ≤1.5 × ULN | 1.5 × ULN | >1.5–3.0 × ULN | >3.0–6.0 × ULN | >6 × ULN | Death ‡ |
| Cardiac arrhythmia † | None | Intervention not needed | Nonurgent intervention needed | Symptomatic and incompletely controlled medically or controlled with a device | Life-threatening (e.g., arrhythmia associated with CHF, hypotension, or shock) | Death ‡ |
| Seizures † | None | None | One brief, generalized seizure, seizures controlled with anticonvulsant drugs, or infrequent motor seizures | Seizures with impaired consciousness, poorly controlled seizures, generalized seizures despite medical interventions | Status epilepticus | Death ‡ |
† Not attributable to a therapeutic drug or an intervention.
‡ Attributable probably or definitively to clinical tumor lysis syndrome.
ADRENAL INSUFFICIENCY
Adrenal insufficiency results when mineralocorticoids and glucocorticoids stores are reduced in the adrenal gland (see Chapter 5 ). Secondary or tertiary adrenal insufficiency is the most common manifestation of adrenal failure. Primary adrenal failure (Addison’s disease) is uncommon, requiring over 90% of the adrenal gland to be replaced by tumor ( ). Megestrol acetate administration is the most frequent cause of secondary adrenal failure associated with cancer while chronic corticosteroid administration is frequently the cause of tertiary adrenal failure. Patients present with symptoms of decreased appetite, weight loss, fatigue, and nausea and vomiting, and signs of hyperpigmentation and postural hypotension. Adrenal insufficiency should be suspected in patients receiving megestrol acetate or chronic corticosteroid therapy, or in patients with documented metastatic disease to the adrenal glands. It can be diagnosed using a low-dose adrenocorticotrophic hormone stimulation test. Treatment is based on repletion of depleted mineralocorticoid and glucocorticoid stores.
LACTIC ACIDOSIS
Lactic acidosis is defined as accumulation of serum lactate in the setting of metabolic acidosis (serum pH <7.37). It occurs as a result of hypoperfusion or ischemic states as well as in association with predisposing diseases, including cancer. Patients present with signs and symptoms of poor organ perfusion (e.g., confusion, decreased urine output, hypotension, tachycardia). Treatment includes targeted therapy for the underlying disease (chemotherapy in the case of cancer) and volume replacement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree