Cancer chemotherapy is a major component of cancer therapy, along with surgery and irradiation. Classical cancer chemotherapy agents differ from most drugs in that they are intentionally cytotoxic to human cells. This aspect of cancer chemotherapeutic agents produces a narrow therapeutic index (desired vs. undesired) for most, but not all, agents in this class. The target of classical cancer chemotherapeutic agents is the proliferating cancer cell. While many normal tissues are nonproliferating, others are, and toxicity of this class tends to preferentially overlap proliferating tissues—hematopoietic, gastrointestinal mucosa, and skin. In addition, each agent often has specific organ toxicity related to its chemical class or unique mechanism of action.
The major groups of classical cancer chemotherapeutic agents are the direct-acting alkylating agents, the indirect-acting anthracyclines and topoisomerase inhibitors, the antimetabolites, the tubulin-binding agents, hormones, receptor-targeted agents, and a class of miscellaneous agents. Despite the disparate nature of this broad class of agents, some generalizations about the effects of chemotherapy are still possible.
Molecularly targeted cancer chemotherapeutic agents have become the standard of care in an increasing number of cancers. Molecularly targeted agents may be monoclonal antibodies or small-molecule competitive adenosine triphosphate kinase inhibitors. Monoclonal antibodies may be directed at ligands (i.e., bevacizumab) or receptors (i.e., trastuzumab). Toxicities are related either to the mechanism of action or, if the antibody is not fully human, to allergic reactions. Despite the best efforts of medicinal chemists, no small molecule is exclusively selective for its intended targets, and off-target as well as mechanistic toxicities occur. For more information readers are referred to detailed reports ( ).
Acute Hypersensitivity Reactions
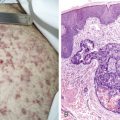
Acute hypersensitivity can occur with any drug. However, several cancer chemotherapeutic agents are derived from hydrophobic plant chemicals and must be solubilized with agents with a marked propensity for causing acute hypersensitivity reactions, especially histamine-mediated anaphylactic reactions, such as the polyethoxylated castor oil (Cremophor EL; BASF Corp., Mt. Olive, NJ) used with paclitaxel. Docetaxel has a lower incidence of this complication. The incidence of severe hypersensitivity reactions with paclitaxel may be up to 25% without ancillary measures. With antihistamine H1 and H2 blockade and corticosteroids the incidence falls to 2% to 3%. Hypersensitivity reactions occur in up to 40% of patients receiving single-agent l -asparaginase but only 20% when administered in combination therapy with glucocorticoids and 6-mercaptopurine, perhaps as a result of immunosuppression. The hypersensitivity usually occurs after several doses and in successive cycles. The reaction may be only urticaria (see Fig. 21.1 ) but may be severe with laryngospasm or, rarely, serum sickness. Fatal reactions occur less than 1% of the time. Changing the source of enzyme is the appropriate initial step. Two other proteins in clinical use, rituximab and trastuzumab, have a similar incidence of hypersensitivity reactions.
Certain drugs such as etoposide are associated with a greater incidence of reactions, but most are not true hypersensitivity reactions. The polysorbate 80 (Tween 80; ICI Americas, Inc., Bridgewater, N.J.) diluent in the clinical etoposide formulation produces hypotension, rash, and back pain. The platinum compounds carboplatin and cisplatin are associated with hypersensitivity reactions, particularly on subsequent cycles; most of these reactions are severe (75%). Hypersensitivity to platinum and related compounds is actually quite frequent, up to 14% in industrial workers, so such reactions in patients receiving these agents parenterally should not be surprising; they are often unappreciated in combination chemotherapy regimens, as with taxanes, and may be equally suppressed by the prophylactic regimens used. Liposomal-encapsulated anthracyclines are associated with an increased incidence of hypersensitivity compared with the parent drugs. Like the reaction to polyethoxylated castor oil and radiocontrast agents, the reaction is a “complement activation pseudoallergy.” Up to 45% of cancer patients show activation of the classical or alternative complement pathway, or both, although the incidence of clinical reactions is about 20%.
Monoclonal antibodies such as trastuzumab, rituximab, bevacizumab, and cetuximab have had enormous impact on cancer therapeutics. Monoclonal antibodies may be chimeric (a murine Fab binding site but human amino acid sequences elsewhere) or fully human. Allergic or hypersensitivity reactions are more frequent with chimeric proteins such as cetuximab (1% to 5% clinically significant) and are treated with antihistamines and steroids plus slowing of the infusion.
l -Asparaginase is a bacterial protein that frequently results in hypersensitivity reactions. These reactions are more frequent with interrupted schedules and with subsequent rechallenge. Changing the source from Escherichia coli to Erwinia is one accepted strategy if immunosuppression does not work.
Alopecia
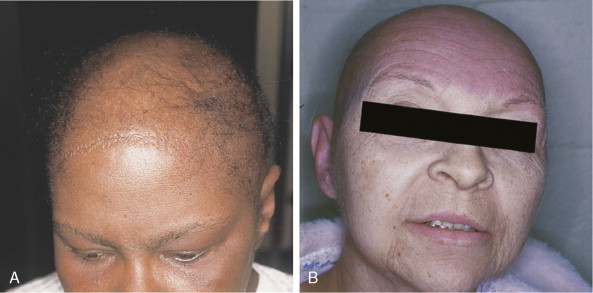
Many antineoplastic drugs can produce marked hair loss (see Fig. 21.2 ). This includes not only scalp hair but also facial, axillary, pubic, and all body hair. The germinating hair follicle has an approximately 24-hour doubling time. Cancer chemotherapy agents preferentially affect actively growing (anagen) hairs. The interruption of mitosis produces a structurally weakened hair prone to fracture easily from minimal trauma such as brushing. Since 80% to 90% of scalp hairs are in anagen phase, the degree of hair loss can be substantial. Hair loss, while often emotionally difficult for patients, is reversible, although hair may regrow more curly and of a slightly different color.
Stomatitis/Mucositis
The oral complications of cancer chemotherapy are many and frequently severe. The disruption of the protective mucosal barrier serves as a portal of entry for pathogens, which, especially when combined with chemotherapy-induced neutropenia, predisposes to local infection and systemic sepsis. Once established, these infections may be difficult to eradicate in immunocompromised patients. The most common infectious organisms are Candida albicans , herpes simplex virus, β-hemolytic streptococci, staphylococci, opportunistic gram-negative bacteria, and mouth anaerobes.
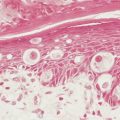
Several agents of the antimetabolite class of cancer chemotherapeutic agents, especially those that target pyrimidine biosynthesis such as methotrexate, 5-fluorouracil (5-FU), and cytosine arabinoside, and the anthracyline agents, such as doxorubicin and daunorubicin, are particularly toxic to the mucosal epithelium (see Fig. 21.3 ). These agents have a marked capacity to produce more severe injury in irradiated tissues, even if the irradiation is temporally remote. These agents produce marked ulceration and erosion of the mucosa. These lesions occur initially on those mucosal surfaces that abrade the teeth and gums, such as the sides of the tongue, the vermilion border of the lower lip, and the buccal mucosa. More advanced mucosal injury may occur on the hard and soft palate and the posterior oropharynx. These ulcerations cannot often be distinguished from those caused by infectious organisms. Appropriate tests must be performed to exclude viral, fungal, and bacterial causes or superinfection.
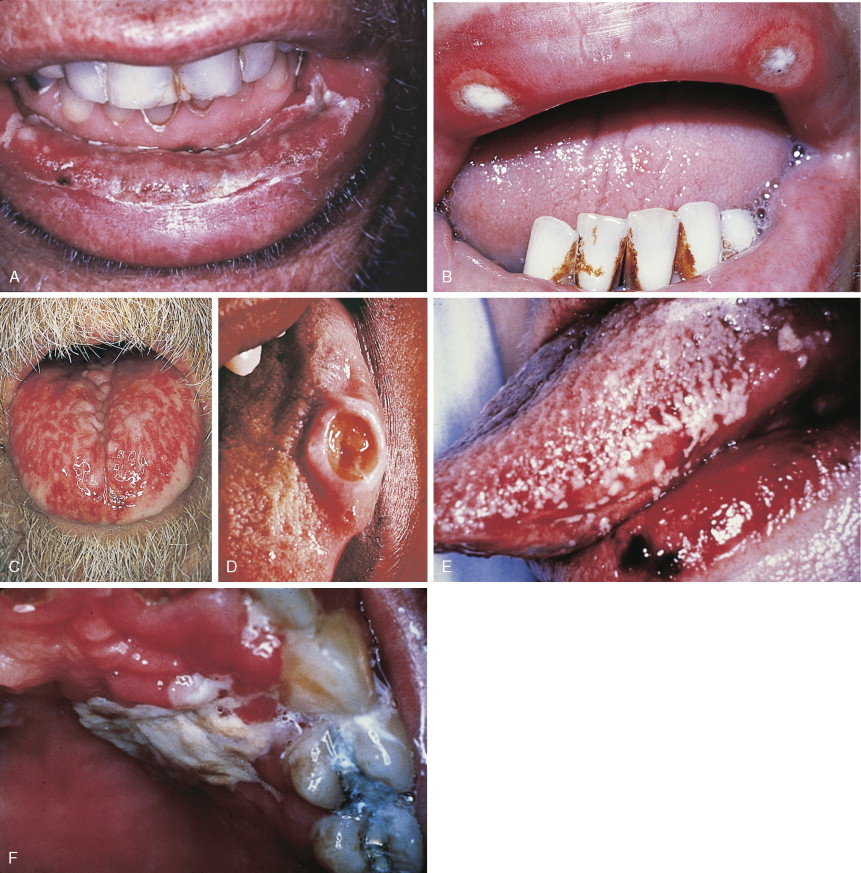
In addition to the risk of infection, the resultant pain makes patients unable to maintain adequate nutrition and hydration. This may compromise the capacity to complete a course of chemotherapy and require prolonged administration of parenteral fluids and even parenteral nutrition.
Stomatitis occurs with several kinase inhibitors, including sunitinib, erlotinib, and sorafenib. The incidence is 15% to 25% and is usually mild in severity.
Dermatitis, Skin Rashes, and Hyperpigmentation
Superficial manifestations of cancer chemotherapy agents are noted frequently by patients, although they are considered significant much less often by clinicians. The cosmetic changes may be disturbing to patients without requiring discontinuation of therapy.
Of the direct-acting alkylating agents, busulfan has been associated with a wide variety of specific and nonspecific cutaneous changes. Diffuse hyperpigmentation has been noted (see Fig. 21.4 ), which resolves with discontinuation of therapy. Systemic mechlorethamine (nitrogen mustard) has no cutaneous toxicity. However, when applied topically for cutaneous T-cell lymphomas, telangiectasias, hyperpigmentation, and allergic contact dermatitis may occur. The development of more effective, safer alternative agents has made busulfan and mechlorethamine of essentially historical interest only or for narrow indications (busulfan in allogeneic bone marrow transplant for hematologic malignancies). Cyclophosphamide, ifosfamide, and melphalan produce hyperpigmentation of nails, teeth, gingiva, and skin. The antimetabolites methotrexate and 5-FU are frequently associated with cutaneous reactions. In contrast, the purine antimetabolites 6-mercaptopurine, 6-thioguanine, cladribine, fludarabine, and pentostatin are devoid of cutaneous toxicity. Methotrexate, a folate antagonist, may cause reactivation of ultraviolet burns when given in close proximity to previous sun exposure. This is not prevented by leucovorin, a reduced folate that prevents the myelosuppression and stomatitis of high doses of methotrexate. Methotrexate should be given more than a week after a significant solar burn. It may cause stomatitis and cutaneous ulcerations at high dose, despite the use of leucovorin. Extensive epidermal necrolysis may occur and be fatal. Multiple areas of vesiculation and erosion over pressure areas have been noticed.
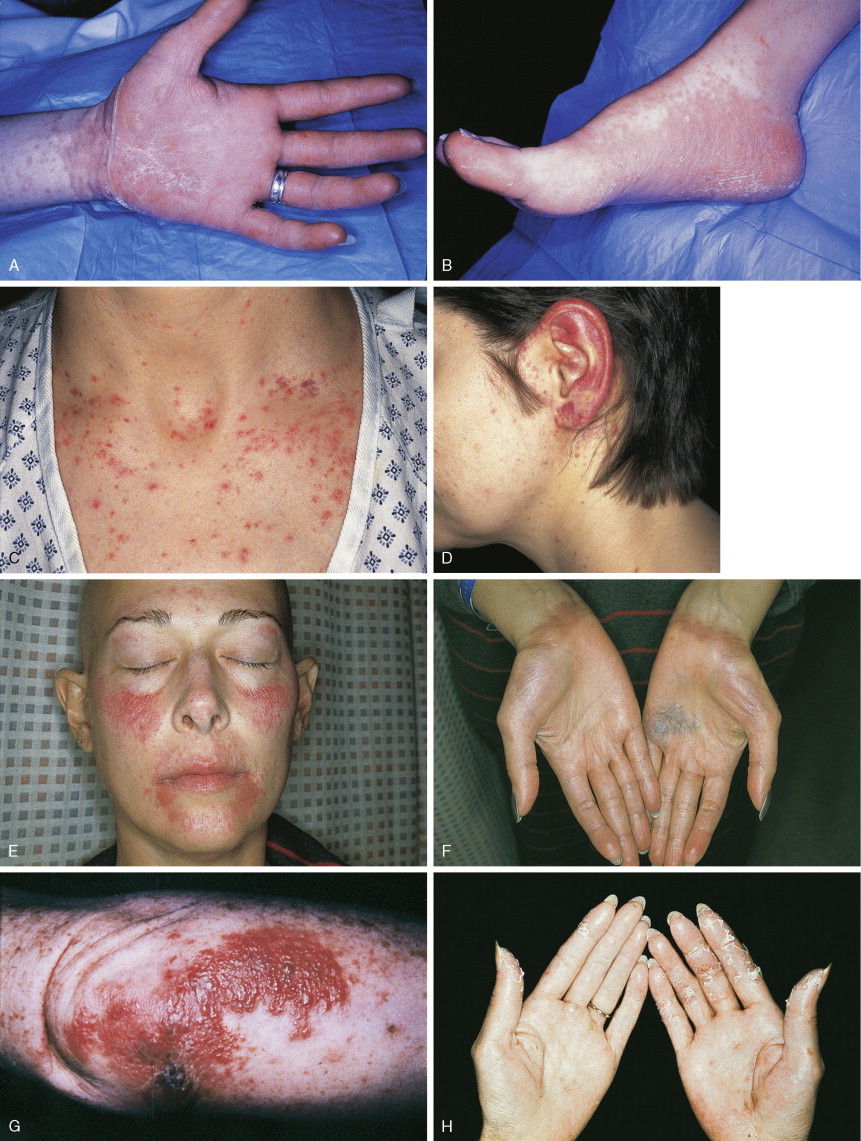
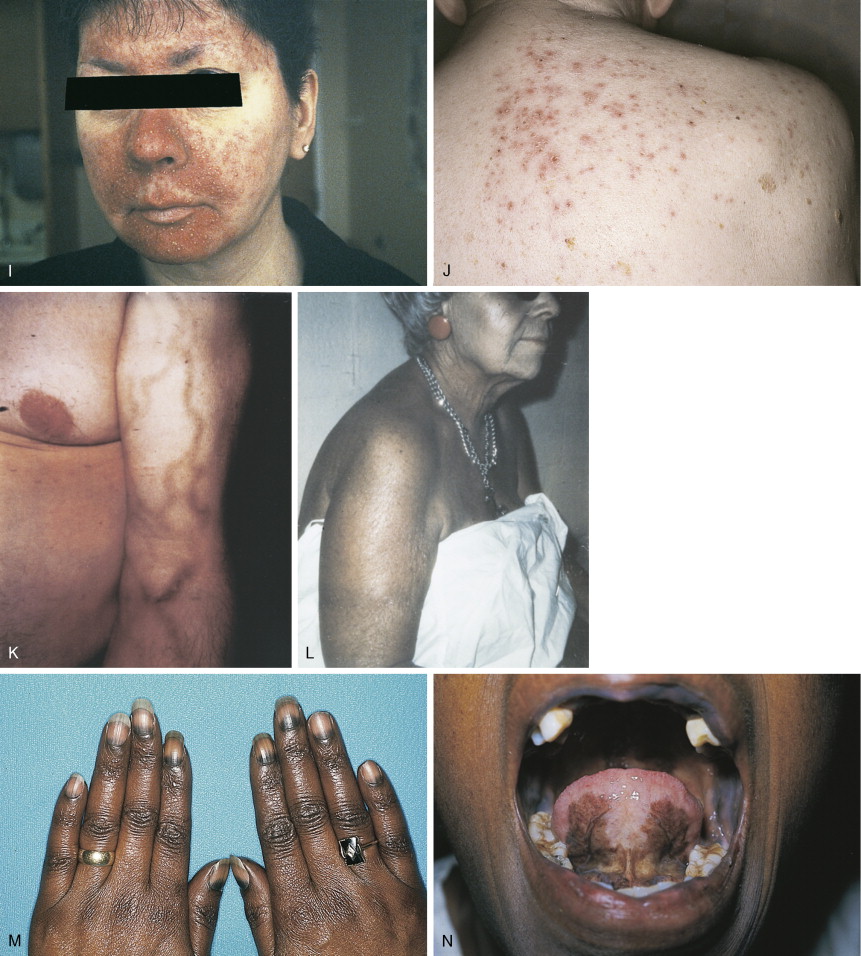
5-FU is an antimetabolite with steric properties similar to uracil. Like methotrexate, 5-FU produces increased sensitivity to ultraviolet-induced toxic reactions in a large number of patients, over 35% in one study. Enhanced sunburn erythema and increased posterythema hyperpigmentation characterize these reactions. A hyperpigmentation reaction over the veins in which the drug is administered may occur. This is probably hyperpigmentation secondary to chemical phlebitis due to chemotherapeutic agents in the superficial venous system. Nail and generalized skin hyperpigmentation have been reported with 5-FU. Occasionally, acute inflammation of existing actinic keratosis is seen in patients receiving 5-FU. This differs from a drug reaction in that it occurs in discrete inflamed regions only in sun-exposed areas, not in a generalized distribution. The end result is usually the disappearance of the actinic keratosis as a result of an inflammatory infiltration into the atypical epidermis and resultant removal of atypical cells. High doses of cytosine arabinoside may produce ocular toxicity through an ulcerating keratoconjunctivitis. This may be prevented by the prophylactic administration of steroid eyedrops. Excessive lacrimation may be noted with 5-FU therapy due to lacrimal duct stenosis. This is corrected by surgical dilatation of the duct.
The indirect-acting anticancer drugs may produce superficial cutaneous toxicity. The anthracyclines doxorubicin, daunorubicin, epirubicin, and idarubicin produce complete alopecia. Radiation recall reactions are frequent, even when the two modalities are separated by years. Skin, nail, and mucous membrane hyperpigmentation may be striking; these may be localized or general. Hyperpigmentation of the hands, feet, and face may occur in patients of African descent. Liposomal anthracyclines, such as Doxil (doxorubicin) and Daunosome (daunorubicin), may produce a severe erythromayalagia with palmar and plantar erythema and desquamation similar to 5-FU. Actinomycin D produces a characteristic skin eruption in many patients. Beginning 3–5 days after drug administration, patients develop facial erythema followed by papules, pustules, and plugged follicles similar to the open comedones of acne. This eruption is benign, self-limited, and not a reason to stop therapy. A similar acneiform skin rash occurs in patients taking the new oral epidermal growth factor receptor inhibitors such as gefitinib and erlotinib (see Fig. 21.4 ). In most patients the rash is mild and may regress with continued treatment. When severe the skin lesions will rapidly regress with discontinuation of the drug. Topical steroids and antibiotics may be indicated.
Bleomycin is actually a mixture of peptides isolated from Streptomyces verticullus . Its most common toxic effects involve the lungs and skin because of high concentrations in these organs due to the deficiency of the catabolic enzyme bleomycin hydrolase in these tissues. Cutaneous toxicity occurs in the majority of patients treated with bleomycin doses in excess of 200 mg. Bleomycin causes a morbilliform eruption 30 minutes to 3 hours after administration in approximately 10% of patients (see Fig. 21.4 ). It most likely represents a transient hypersensitivity response (it may be accompanied by fever). Linear or “flagellate” hyperpigmentation may occur on the trunk. This may likewise represent postinflammatory hyperpigmentation. Bleomycin may cause a scleroderma-like eruption of the skin. Infiltrative plaques, nodules, and linear bands of the hands have been described. Pathologic findings include dermal sclerosis and appendage entrapment similar to that seen in scleroderma. These changes are reversible when the drug is stopped.
Etoposide has relatively few cutaneous manifestations at standard doses (<600 mg/m 2 ). At higher doses (1800–4200 mg/m 2 ) a generalized pruritic, erythematous, maculopapular rash occurs in approximately 25% of patients. The most severe toxicity occurs at the highest doses. In these patients an intense, well-defined palmar erythema develops. Affected areas become edematous, red, and painful. Bullous formation and desquamation follow. The severity of the reaction is related to the dose. A short course (3–5 days) of corticosteroids controls the symptoms.
Cutaneous rashes are the most common toxicities encountered with gefitinib and erlotinib. The chimeric monoclonal antibodies cetuximab and panitumumab are associated with dermatologic toxicity. The severity and extent of the skin changes, including dry skin, desquamation, erythema, nail changes, and acneiform eruptions, vary from report to report, and no consistent grading system for incidence and severity is universally agreed upon. There is neutrophil and macrophage infiltration of the dermis and hair follicles, with thinning of the epidermis and stratum corneum. The incidence and severity is dose-dependent. Certain epidermal growth factor receptor polymorphisms increase the incidence of developing a rash. For erlotinib, cetuximab, and panitumumab, several studies support a positive correlation between development of a rash and response, and rash and survival ( ). Management is usually supportive with creams, including 1% clindamycin, 5% benzoyl peroxide, and systemic antibiotics when there is evidence of infection, including tetracycline and amoxicillin/clavulanate. These should be used only when necessary.
Sunitinib causes a yellowing of the skin in 10% of patients. This is not due to deposition of bilirubin in the skin, since there is no scleral icterus and there are no changes in serum bilirubin. Between 10% and 20% of patients experience loss of hair pigmentation in 2–3 weeks, which resolves in the same period of time after discontinuation of the drug ( ).
Skin Ulceration and Extravasation
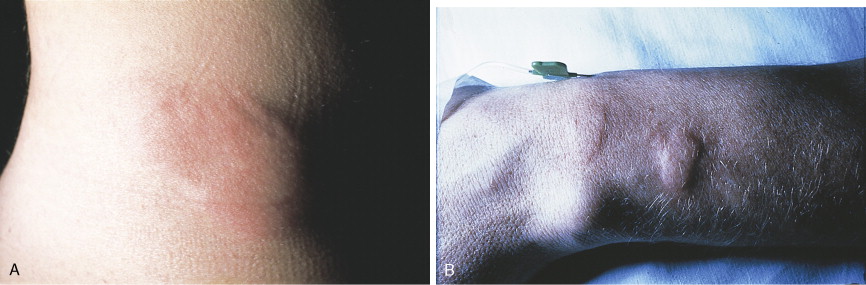
Vesicant reactions from extravasated cancer chemotherapeutic agents are one of the most debilitating complications seen with cancer therapy (see Fig. 21.5 ). The anthracyclines, especially doxorubicin, are particularly noted for an intense inflammatory chemical cellulitis caused by subcutaneous extravasation. This results in ulceration and necrosis of affected tissue. No local measures have proven unequivocally helpful once the accident has occurred. Doxorubicin should be stopped immediately but the intravenous line left in place. Dilution of doxorubicin with sodium bicarbonate and the local instillation of steroids before catheter withdrawal are standard measures, but their efficacy is uncertain. Rest and warm compresses are recommended. If healing does not proceed well, excision of the affected area and surgical grafting are recommended to avoid excess morbidity. Other agents with vesicant properties include the vinca alkaloids (vincristine, vinblastine, vinorelbine) and actinomycin. General recommendations for the administration of vesicant drugs include the use of veins as far away from the hands and joints as possible and placement of the intravenous line so that infusion occurs at a rapid rate and blood return is good. The use of venous access devices is accepted as appropriate in this situation unless contraindicated on specific clinical grounds.
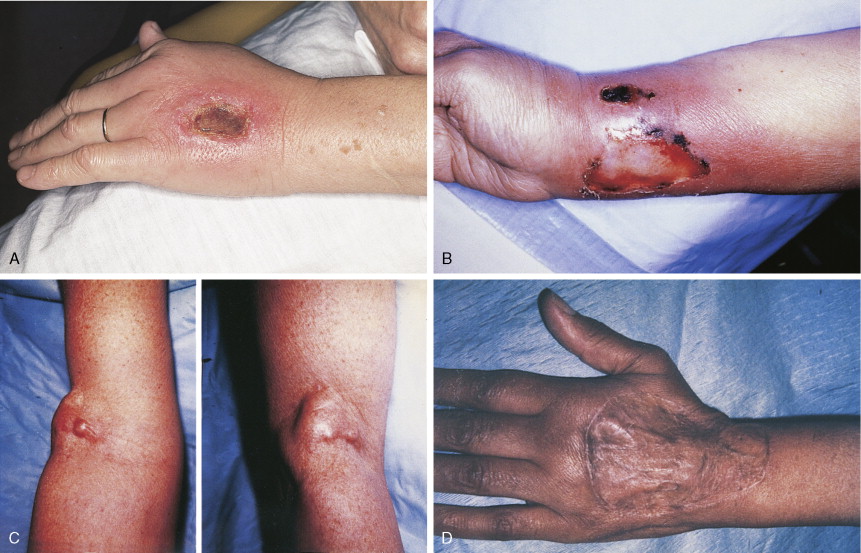
Generalized skin ulceration is an infrequent, albeit dramatic, occurrence. Mucocutaneous ulcerations are frequently noted with bleomycin. These begin as edema and erythema over pressure points such as the elbows, knees, and fingertips and in intertriginous areas such as the groin and axillae. These areas then proceed to shallow ulcerations. These ulcerations may also occur in the oral cavity. Biopsy shows epidermal degeneration and necrosis with dermal edema. Total epidermal necrosis can even be found without any dermal changes. This suggests that the epidermal toxicity is the primary event.
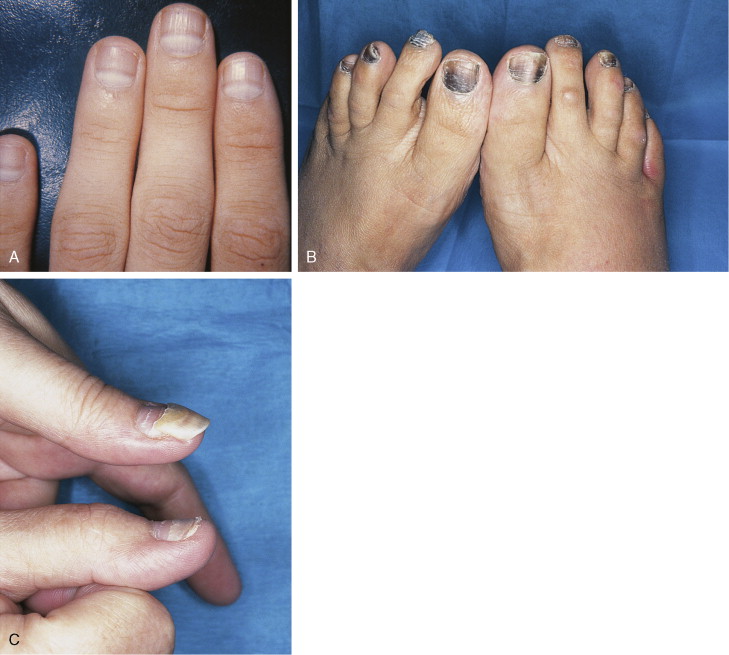
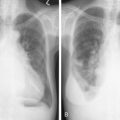
Nail Changes
Banding of the nails is the appearance of linear horizontal depressions in the nails that occur as a result of growth interruptions in the nail germinal cell layer by a cytostatic effect from the administration of cancer chemotherapy agents. These occur in other disease settings and are called Beau’s lines (see Fig. 21.6 ). The direct-acting alkylating agents cyclophosphamide, ifosfamide and melphalan may also produce hyperpigmentation of nails. The nails may show linear or transverse banding or hyperpigmentation. These changes begin proximally and progress distally and clear, proximally to distally, when the agents are discontinued. Similar effects are seen with the indirect-acting anthracyclines, such as doxorubicin, and bleomycin. The anthracyclines may cause hyperpigmentation of the hyponychia (the soft layer of skin beneath the nail), especially in dark-skinned persons.