Fig. 21.1
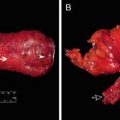
Wound infection in a patient who underwent open low anterior resection with loop ileostomy for rectal cancer after pelvic radiation therapy. She had a long history of smoking as well. This wound healed by secondary intention over a period of 3 weeks
The perineal wound resulting from abdominoperineal resection (APR ) poses a particular challenge, with a significant proportion of patients suffering from wound complications [16]. The risk is especially high in patients who undergo extralevator abdominoperineal excision (ELAPE), with a 44% risk of wound complication and 26% risk of perineal hernia [17]. Patients who underwent neoadjuvant pelvic radiation are also at higher risk for perineal wound complications, especially if the perineal wound is closed primarily [18–21]. In addition to the morbidity caused by perineal wound complications, a recent study found that perineal wound dehiscence was also associated with decreased survival after APR, with a difference of 10 months [22].
Primary closure of perineal wounds has been questioned due to the high morbidity of these wounds, especially with the larger defects resulting from ELAPE. A prospective randomized controlled trial of primary perineal wound closure vs closure with a pedicled vertical rectus abdominis myocutaneous (VRAM ) flap demonstrated a lower rate of wound complications in the latter group [23] (Fig. 21.2). Other retrospective studies have supported this finding [24, 25]. The gluteus maximus and gracilis are other options for reconstruction of the perineal wound. More recently, pelvic floor reconstruction with the use of biologic mesh has been introduced as an alternative for tissue flaps—preliminary studies suggest that they have equivalent outcomes [26]. Perineal wounds that have been closed primarily had improved healing with the use of an omental flap to fill the pelvis [27–29].
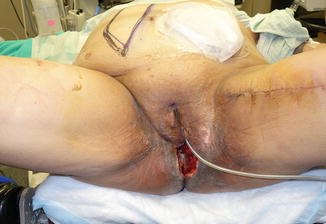

Fig. 21.2
Perineal wound dehiscence in a patient who underwent laparoscopic abdominoperineal resection with posterior vaginectomy and reconstruction of the perineal defect with a gracilis flap for a locally advanced anal squamous cell carcinoma that persisted after chemoradiation therapy
Pelvic Abscess
While incisional SSI rates have improved, organ space SSIs often manifesting as pelvic abscesses remain a significant cause of morbidity. Postoperative pelvic abscesses occur in 10–30% of rectal cancer patients and are more common in patients undergoing surgery for that indication than for ulcerative colitis or diverticular disease [30, 31]. Pelvic abscesses may be due to anastomotic leak, but may also be due to infected hematomas in the pelvis in the absence of anastomotic leak. The risk of pelvic abscess without an anastomotic leak is higher in patients who had neoadjuvant pelvic radiation therapy [32] (Fig. 21.3).
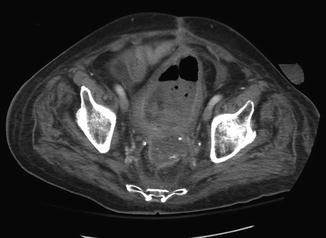

Fig. 21.3
Pelvic abscess in a patient who underwent low anterior resection with end colostomy after neoadjuvant pelvic radiation for rectal cancer. His course was complicated by massive bleeding from the rectum several weeks after his initial operation, for which he was transferred to our institution. Angiography demonstrated extravasation from a branch of the internal iliac artery; embolization successfully controlled the bleeding. He developed this pelvic abscess, which was due to a pelvic hematoma and dehiscence of the rectal stump staple line. Ultimately, he underwent completion proctectomy
Clinical signs and symptoms of postoperative pelvic abscess include: fever, leukocytosis, prolonged ileus, urinary retention, diarrhea, and pain. Patients with a perineal wound infection that fails to heal with local wound care may have a deeper pelvic abscess that is necessitating through the perineal wound. Computed tomography (CT) scanning will confirm the diagnosis with the appearance of a fluid collection with rim enhancement. Abscesses smaller than 3 cm can be managed medically with broad-spectrum antibiotics. Larger collections can usually be drained percutaneously under image guidance. Patients with perineal wounds who have pelvic abscesses may undergo image-guided or surgical drainage through the perineal wound. Patients with large abscesses that are not amenable to percutaneous drainage may require operative washout and drain placement; reoperation greater than a week after the initial operation is ill-advised unless there is a pressing clinical need and no other treatment options.
Anastomotic Leak
Anastomotic leak is one of the most dreaded complications of colorectal surgery. The incidence of clinically significant leakage after colorectal anastomosis ranges from 3% to 21% and is associated with a 6% to 30% mortality rate [31–33]. Risk factors for anastomotic leak include: more distal anastomoses [34, 35], neoadjuvant pelvic radiation therapy, male gender [36], prolonged operative time, intraoperative spillage, advanced tumor stage, perioperative transfusion requirement, and multiple firings of the linear stapler across the rectal stump [37–39].
Intraoperative placement of pelvic drains is a common practice for rectal resections involving a low colorectal anastomosis but prevents neither the formation of pelvic abscesses nor the development of anastomotic leak [40]. It is unclear whether a protective stoma affects the risk of anastomotic leak, with some studies suggesting that it decreases the leak rate and others arguing that the presence of a stoma does not decrease the leak rate but rather mitigates the septic complications of an anastomotic leak [41, 42]. Thus, the need to reoperate is lower, as is the mortality rate from the complication [39, 43, 44]. A well-accepted rule of thumb is to proximally divert colorectal anastomoses at 6 cm or less from the anal verge with a covering loop ileostomy. A loop ileostomy is preferred to loop colostomy for its ease of care for the patient and simpler operation at closure. While the effect of mechanical bowel prep on SSI in colorectal surgery is controversial, it should be considered in the case of total mesorectal excision (TME) with a low pelvic anastomosis and loop ileostomy [10]. Finally, perioperative supplemental oxygen administration with 80% FiO2 compared to 30% FiO2 appeared to decrease the risk of low colorectal anastomotic leak in a randomized controlled trial [45].
Diagnosis of Anastomotic Leak
While leaks classically present between 5 and 7 days after the operation, up to half of all leaks may present after the patient has been discharged, with over 12% occurring over a month after surgery [46]. While early leaks present with varying levels of severity from an ileus or fever to peritonitis and sepsis, late leaks tend to present insidiously with pelvic pain, failure to thrive, and other nonspecific symptoms that can be attributable to other postoperative complications.
When there is clinical suspicion of a leak, radiologic examination can aid in confirming the diagnosis. CT scanning, with or without enteric or rectal contrast, is a valuable tool to evaluate for the presence of free air, fluid collections, and gas-containing collections suggestive of an anastomotic leak. The size and location of a fluid collection will help direct subsequent management. Another imaging option is a water-soluble contrast enema to determine whether there is extravasation of intraluminal contents.
Management of Anastomotic Leak
The management of an anastomotic leak depends on whether the leak is contained or not. Uncontained leaks in which feculent or purulent fluid spreads throughout the abdominal cavity may cause peritonitis and septic shock and is a clear indication for an emergent laparotomy with abdominal washout and either takedown of the anastomosis with creation of an end colostomy or pelvic drainage and proximal fecal diversion. These intraoperative decisions depend on many factors including the condition of the patient, accessibility and visibility of the anastomosis, and location and size of the anastomotic defect. In the situation of an anastomotic leak requiring operative washout, resection of the anastomosis with creation of a new anastomosis is not advisable. If the decision is made to take down the leaking anastomosis to create an end colostomy, one must pay particular attention to the management of the rectal stump to reduce the risk of a rectal stump dehiscence, which can cause chronic pelvic sepsis. Oversewing of the rectal stump staple line, drain placement over the rectal stump, and rectal tube placement may all help in reducing the risk of rectal stump dehiscence.
Contained leaks result in limited extravasation of intraluminal contents. Small abscesses (<3 cm) can be successfully treated nonoperatively with broad-spectrum antibiotics. Larger abscesses (>3 cm) may require percutaneous drainage in addition to broad-spectrum antibiotics. Contained leaks managed in this manner usually heal without surgical intervention (Fig. 21.4). A colorectal anastomotic leak that fails to heal may develop into a colocutaneous fistula or a sinus tract that causes recurrent pelvic abscesses (Fig. 21.5a, b). Reoperation to address a chronic anastomotic fistula should be deferred for at least six months if possible to allow for spontaneous closure of the leak and fistula tract and resolution of inflammatory adhesions in the pelvis. Resection of the anastomosis and redo colorectal or coloanal anastomosis is sometimes possible, although often completion proctectomy with permanent end colostomy is necessary or preferable.
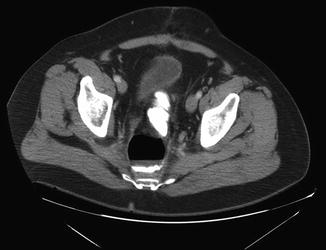
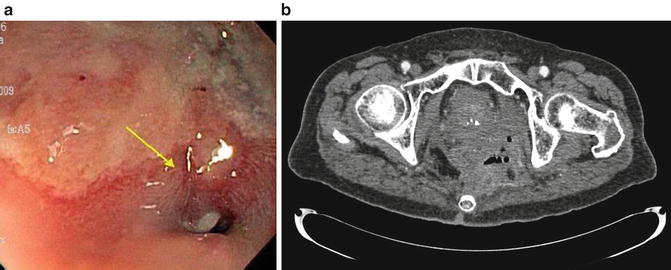

Fig. 21.4
Presacral collection containing gas and fluid, including rectal contrast, in a patient who underwent low anterior resection for a mid-rectal tumor after neoadjuvant pelvic radiation therapy. This patient was managed with percutaneous drainage of the presacral abscess and ultimate resolution of the anastomotic leak without reoperation

Fig. 21.5
(a) Fistula opening at a colorectal anastomosis in a patient with a chronic anastomotic leak after low anterior resection for rectal cancer. (b) The chronic anastomotic leak led to recurrent pelvic abscesses requiring percutaneous drainage. The patient finally underwent completion proctectomy with end colostomy to address this problem
Outcomes After Anastomotic Leak
Anastomotic leak after rectal resection is associated with poorer quality of life and functional outcomes [47, 48]. Patients who experienced anastomotic leak after proctectomy with colorectal anastomosis had more frequent bowel movements during the day and night, worse control of solid stool, and increased pad use [49]. One explanation of the reason for poor function after anastomotic leak is that the chronic inflammation induces pelvic fibrosis, which in turn reduces the compliance of the rectum and colon.
Anastomotic leaks are also associated with an increased risk of mortality compared with patients without a leak, with decreased overall 5-year survival (44–53% versus 64%) and cancer-specific 5-year survival (42% vs 67%) [50, 51]. Patients with anastomotic leaks were also found to have increased local recurrence and systemic recurrence rates [51–55].
Hemorrhagic Complications
Anastomotic Bleed
Minor anastomotic bleeding that does not require transfusion or intervention is common and is typically self-limited, resolving within 24–48 h. Major anastomotic bleeding which requires transfusion and intervention is relatively uncommon, with rates under 1% reported [56, 57]. Due to the small numbers of reported cases, risk factors for anastomotic bleeds have not been identified. Common intraoperative techniques to reduce the risk for anastomotic bleeding include inspection of the staple line, suture ligation of bleeding points along the transverse staple line prior to creating the anastomosis, and suture reinforcement of the anastomosis. Endoscopic evaluation of the anastomosis is also commonly performed not only to confirm that the anastomosis is intact but also to check for major intraluminal bleeding. If there is intraoperative evidence of significant intraluminal bleeding from the anastomosis, oversewing of the anastomosis is usually successful in establishing hemostasis.
Major anastomotic hemorrhage in the postoperative period requires resuscitation and correction of coagulopathy to establish hemodynamic stability. For ongoing bleeding, endoscopic management and angiographic embolization are options to consider prior to operative intervention. Most patients can be successfully treated with endoscopic electrocautery, epinephrine injection, or placement of hemostatic clips. If the bleeding anastomosis is not successfully controlled by endoscopic means, angiography and embolization or surgical control of the anastomosis can be considered. Angiography and embolization increases the risk of ischemia to the anastomosis, and thus the risk for anastomotic leak or stricture [58].
Intraoperative Presacral Venous Plexus Hemorrhage
Presacral venous bleeding can be a dangerous intraoperative complication of rectal surgery. The posterior rectal dissection should remain in the avascular plane between the fascia propria of the rectum and the presacral fascia. Dissection inside this plane may cause bleeding from the mesorectum and compromise the oncologic integrity of the resection. Dissection outside this plane can cause lacerations of the lower presacral venous plexus or the sacral basivertebral veins, leading to massive venous hemorrhage that can be quite difficult to control. It is imperative to stay in the 1–2 mm just outside the fascia propria of the rectum. To do this one must understand the rectum moves cranially away from the sacrum/coccyx as it passes ventrally to exit the pelvic floor. Proper retraction and direct visualization decrease the chance of entering the wrong plane.
The first steps in management of presacral venous hemorrhage should be application of direct pressure, resuscitation, and consideration of calling for experienced assistance. Blood products should be brought to the operating room. The pressure should be maintained continuously for 30 min. Implementation of this strategy not only gives the anesthesiologist time to resuscitate the patient but also may lead to resolution of venous bleeding or at least significantly decrease the rate of bleeding to allow for definitive surgical hemostasis. Adequate exposure is critical, underscoring the importance of an experienced assistant. Conventional hemostatic techniques such as suture ligation are often ineffective and may even worsen the hemorrhage, as the veins are thin walled and are likely to retract [59]. Direct electrocautery is best applied slightly cephalad to the points of bleeding. If this is ineffective, then the use of a muscle flap to apply indirect high-energy electrocoagulation can be quite effective in controlling presacral venous hemorrhage. A 1 to 2-cm square piece of rectus abdominis muscle is harvested and placed over the area of bleeding in the pelvis, and high-energy electrocautery applied to the muscle flap, which will coagulate the retracted bleeding veins by welding the muscle tissue into the sacrum [60, 61]. Another well-established technique for control of presacral venous hemorrhage is the use of sterile thumbtacks or occluder pins pushed directly into the presacral plexus to tamponade the bleeding [62, 63]. These techniques are particularly effective when the veins have retracted into the venous plexus within the sacrum.
Hemostatic agents may also be useful in control of pelvic venous bleeding. Topical hemostatic products such as FloSeal (Baxter, USA) and Surgicel (Ethicon, USA) in combination with pelvic packing were shown to be successful, as was the use of medical glue in combination with a hemostatic gauze applied to the presacral plexus [64–66]. Another hybrid approach using a hemostatic sponge affixed to the presacral fascia with a laparoscopic helical tacker was also shown to be effective [67].
Finally, in the most extreme circumstances, intractable bleeding may require a “damage control” strategy with pelvic packing and temporary abdominal closure followed by resuscitation, stabilization, and correction of coagulopathy. Return to the operating room for removal of packs should be performed in 24–48 h, at which time the vast majority of patients will have stopped bleeding. Variations on this strategy include the use of a saline bag or tissue expander to achieve the same effect.
Postoperative Pelvic Bleeding
Pelvic hemorrhage can occur after rectal resection but rarely requires reoperation or other intervention. Most cases result in pelvic hematomas, which have a high risk of developing into the pelvic abscesses that may require percutaneous drainage. However, patients with ongoing active bleeding require resuscitation and correction of coagulopathy and may require angiographic embolization or reoperation for control of bleeding.
Pseudoaneurysms of the internal iliac artery or its branches are a rare cause of bleeding after resection for locally advanced rectal cancer [68, 69]. Pelvic radiation therapy is thought to increase the risk of pseudoaneurysm development. Chronic anastomotic leaks or pelvic recurrences may also erode into these vessels and cause pelvic bleeding. Most of these cases can be treated with angiography and embolization, although failure of these methods may require operative intervention for surgical ligation.
Genitourinary Complications
Ureteral Injury
Urologic and gynecologic operations are the most common cause of iatrogenic ureteral injury, followed by colorectal operations with an incidence of about 0.2% [70, 71]. Ureteral injuries were independently associated with higher morbidity and mortality, longer lengths of stay, and greater hospital charges. Risk factors for this complication include diagnosis of rectal cancer or metastatic cancer, adhesions, weight loss or malnutrition, and teaching hospitals [71].
There are several locations where ureteral injuries can occur in the course of an operation for rectal cancer (Fig. 21.6a). An injury to the middle third of the left ureter may occur during ligation of the inferior mesenteric artery; this can be avoided by identifying the ureter prior to vessel ligation and ensuring that the dissection plane is between the retroperitoneum and the colon mesentery . Another potential spot for ureteral injury is during mobilization of the upper mesorectum at the level of the sacral promontory; this risk can be minimized by ensuring that the dissection is proceeding within the proper planes and identifying the course of the ureter. It may be difficult to identify the ureter at this location due to obesity, radiation, or other associated conditions such as diverticulitis. In these instances it is helpful to identify the more proximal ureter behind the descending colon and follow it distally to the pelvis. Injury to the distal ureter as it enters the bladder can occur in the deep pelvis during proctectomy as well as the perineal dissection of an abdominoperineal resection. Patients with locally advanced tumors, prior radiation therapy, and prior operations in the area have a higher risk of injury due to the obliteration of the normal planes, allowing the ureter and other retroperitoneal structures to be pulled up during retraction of the colon and rectum during dissection. Again, identification of the more proximal ureter in healthy tissue and tracing it down to the point of concern is helpful.
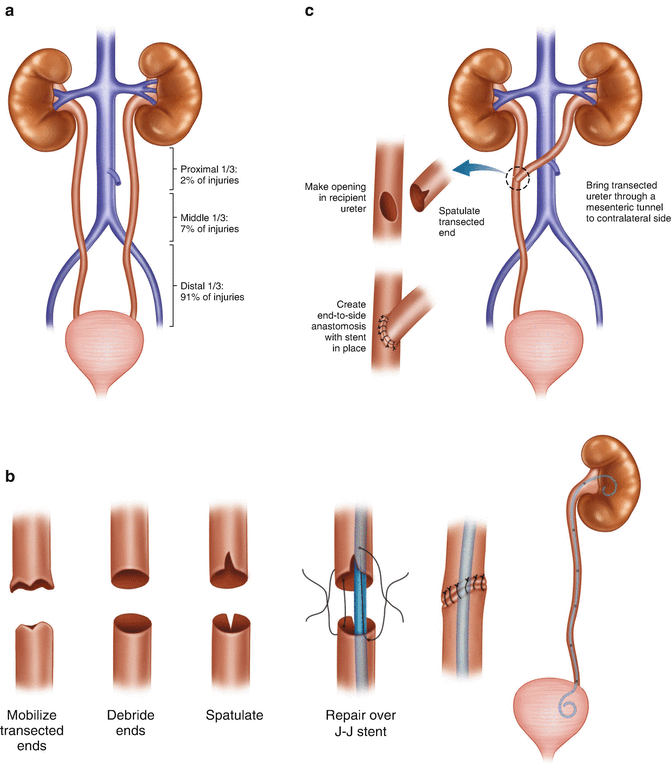
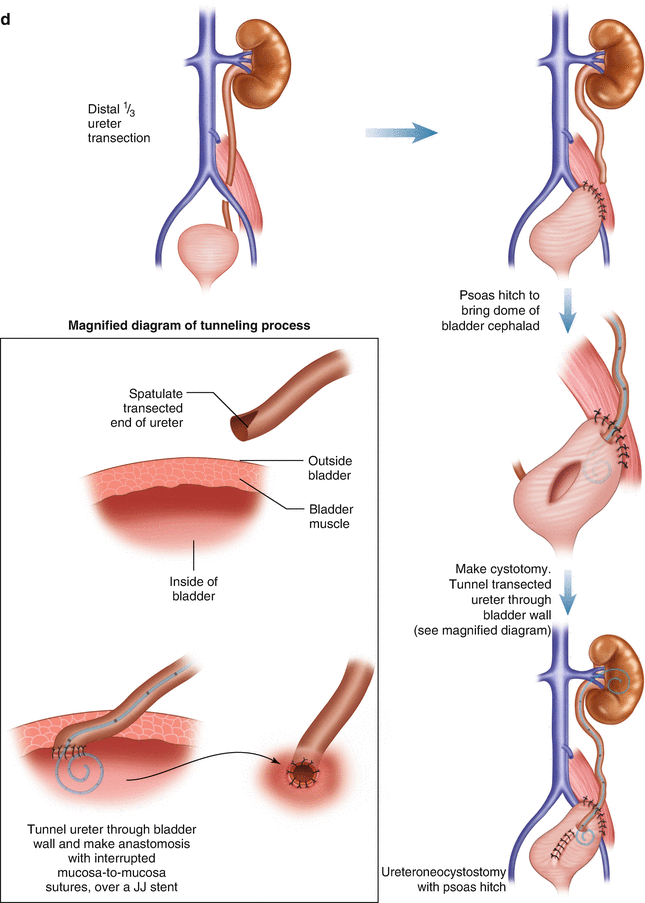


Fig. 21.6
(a) The majority of ureteral injuries occur in the distal third. (b) Injuries in the proximal and middle third of the ureter are generally treated with ureteroureterostomy. (c) Injuries in the middle or distal third of the ureter are occasionally managed with transperitoneal ureteroureterostomy, if the ureter cannot be reconstructed primarily or brought to the bladder. Primary ureteroureterostomy and neoureterocystostomy are preferable to this strategy. (d) Injuries to the middle or distal third of the ureter may best be managed with anastomosis of the ureter to the bladder, often with the help of a Boari flap or psoas hitch to decrease tension on the anastomosis
While the use of ureteral stents has not been shown to reduce the risk of ureteral injury, they may be helpful in immediate identification of the complication and thus prevent missed ureteral injuries [71, 72]. Intraoperative recognition of ureteral injuries allows for immediate reconstruction and avoids the potential morbidities such as renal failure due to ureteral obstruction, urinomas, and the need for additional interventions and operations to manage the injury [73]. For these reasons, preoperative ureteral stent placement should be considered in cases of particularly extensive or complicated pelvic surgery, such as locally advanced or recurrent rectal cancers. However, as ureteral stents can also cause iatrogenic ureteral injury, albeit rarely, it is not advisable to routinely place stents for uncomplicated cases [74].
General principles for ureteral repair include the use of absorbable suture to prevent stone formation, spatulation to prevent anastomotic stricture, repair over a stent, adequate mobilization to ensure a tension-free anastomosis, and placement of a closed suction drain in the area of the repair. The type of repair depends on the anatomic location of the ureteral injury. Only 2% of iatrogenic ureteral injuries occur in the proximal third of the ureter [75]. These are typically repaired with a simple spatulated ureteroureterostomy over a stent with excellent outcomes (Fig. 21.6b). Injuries to the middle third of the ureter are also infrequent, accounting for only 7% of ureteral injuries. Short segment injuries can be repaired with a ureteroureterostomy, while injuries involving longer segments may require ureteroneocystostomy often with the aid of a psoas hitch or Boari flap to decrease tension on the anastomosis (Fig. 21.6d). Injuries to the distal third of the ureter are the most common, accounting for 91% of the injuries, and may be repaired by primary ureteroureterostomy for short segment injuries or ureteroneocystostomy with or without a psoas hitch or Boari flap depending on the mobility of the ureter and the bladder [76] (Fig. 21.6d). Injuries to the middle or distal ureter are occasionally managed with transperitoneal ureteroureterostomy if the ureter cannot be primarily reconstructed or if an anastomosis to the bladder is not possible (Fig. 21.6c). This technique is less preferable than the other methods of reconstruction because it places the contralateral kidney and ureter at risk for stricture.
Postoperatively, an indwelling catheter is left in place to decompress the bladder for at least 2 weeks. Increased output from the surgical drain may be an indicator of ongoing urine leak; this can be confirmed by checking a creatinine level on the drain fluid. Anastomotic leaks from ureteral repairs usually heal with continued drainage and stenting; very rarely do they require reoperation. A contrast study may be obtained at a week after the repair to rule out a leak, prior to removal of the urinary catheter. The double-J ureteral stent typically remains in place for several more weeks. There is no indication for prophylactic antibiotics while the stent is in place.
Patients who have unrecognized ureteral injuries may present with increased pelvic drain output, elevated creatinine due to either obstruction if the ureter was ligated or due to systemic absorption of a urinoma, or hydronephrosis noted on imaging studies. Delayed diagnosis of ureteral injuries typically requires percutaneous nephrostomy placement and possible percutaneous drainage of a urinoma. Retrograde stenting of an injured ureter is occasionally successful; antegrade stenting can also be attempted through the nephrostomy. Reoperation for definitive repair at a later date is usually necessary. Delayed ureteral strictures can occur due to thermal injury and may be managed with stenting and/or nephrostomy depending on the degree of stricture.
Overall long-term outcomes are much better if the ureteral injury is identified and repaired at the time of the initial operation [77]. Complications specific to the type of repair performed include ureteral strictures in 10% of ureteroureterostomy repairs, which can be managed with endoscopic balloon dilation. Reflux or stricture occurs in 5% of ureteroneocystostomy repairs and can be managed with drainage and dilation, respectively. Finally, fistulas form in 1% of patients and may require drainage and operative repair.
Urethral Injuries
Injuries to the urethra can occur during the perineal phase of abdominoperineal resections and most commonly involve the membranous or prostatic portions. If the injury is recognized at the time of the operation, the defect can be closed primarily in layers and a urethral catheter left in place for at least 2 weeks. Most iatrogenic urethral injuries are identified immediately due to the appearance of the indwelling urethral catheter, but delayed injuries may present with urinary leakage into the pelvis or through the perineal wound. Replacement of a urethral catheter, preferably under direct vision with cystoscopy, should be the first step in management. The injury may heal over several weeks with the catheter, but if it does not, then delayed repair with the use of muscle flaps may be necessary [76, 78].
Bladder Injuries
Factors that increase the risk for iatrogenic bladder injuries during rectal cancer surgery include a history of pelvic radiation, locally advanced tumors, and prior pelvic surgery. Rectal cancers that invade directly into the bladder should be managed with en bloc resection of the involved portion of the bladder. If there is a concern for bladder injury during the operation, the bladder can be distended with saline or a dilute methylene blue solution instilled through the indwelling urinary catheter to test for leaks. Bladder injuries that are identified at the time of the operation should be repaired [76]. Small extraperitoneal injuries can be managed with indwelling urinary catheter drainage for 1 to 2 weeks, followed by cystogram to exclude an ongoing urine leak prior to removal. Injuries to the dome of the bladder can be repaired in two layers with absorbable suture. Injuries to the trigone or near the ureteral orifices are more difficult to manage, requiring an anterior cystotomy and closure of injury from the inside of the bladder. If possible, an omental flap should be placed between bladder and the colorectal anastomosis to decrease the risk of fistulization.
Delayed diagnosis of bladder injuries may present with urinoma formation or increased pelvic drain output, which can be sent for a creatinine level. Cystogram will confirm the diagnosis and help quantify the size of the injury. Small injuries require at least urinary drainage for 2 weeks, during which they may heal; larger injuries in patients who have had prior pelvic radiation therapy may require more prolonged bladder decompression or even operative repair.
Urinary Dysfunction
The incidence of urinary dysfunction after rectal cancer surgery ranges from 30% to 70% [79, 80]. At 5 years after rectal cancer resection, 33% of patients had urinary incontinence and 31% had difficulty with bladder emptying [80]. The majority of these patients had normal urinary function prior to surgery. Risk factors for urinary dysfunction include: preoperative urinary dysfunction, T stage, inability to identify the pelvic autonomic nerves during the operation, and possibly neoadjuvant pelvic radiation therapy [81, 82]. Damage to the superior hypogastric plexus and hypogastric nerves causes reduced bladder capacity and may result in urge incontinence. Damage to the sacral splanchnic nerves may lead to difficulty in bladder emptying, urinary retention, and overflow incontinence [83].
Intraoperative identification and preservation of the pelvic autonomic nerves reduces the risk of urinary dysfunction [84, 85]. Anatomic studies have identified a prehypogastric nerve fascial layer between the fascia propria of the rectum and the parietal presacral fascia; sharp dissection anterior to this prehypogastric nerve fascia would spare the pelvic autonomic nerves and improve function [86]. Since the nerves can be challenging to identify, a nerve stimulator may improve identification, prevent injury, and improve rates of urinary dysfunction [87].
Sexual Dysfunction
Sexual dysfunction is common after proctectomy for rectal cancer due to dissection near the pelvic nerves. While postoperative sexual dysfunction in men is well studied with complication rates of 15–60%, the data for women is sparse but appears to be in that same range [83, 88, 89]. Damage to the sympathetic nerves during high ligation of the IMA or posterior dissection at the level of the sacral promontory can lead to retrograde ejaculation, which is the most commonly diagnosed type of sexual dysfunction in men and also the most likely to resolve with time. Damage to the parasympathetic plexus (nervi erigentes and cavernous nerves) during the anterior dissection or the pelvic plexus during the lateral dissection can lead to erectile dysfunction. The greatest risk likely occurs during the process of separating the anterior wall of the rectum from the prostate and seminal vesicles. Thus, preservation of Denonvilliers’ fascia if oncologically feasible may reduce the rate risk of injury to these nerves and preserve erectile function [90].
In addition to surgical dissection, the risk of sexual dysfunction following proctectomy is worsened by advanced patient age, abdominoperineal resection, diminished preoperative libido, and neoadjuvant radiation therapy [89, 91, 92]. Surgical approach may also be a risk factor as several studies have found increased rates of sexual dysfunction after laparoscopic total mesorectal excision as compared to an open approach [93, 94]. Intraoperative nerve stimulation of the parasympathetic nerves during total mesorectal excision has been shown to correlate with long-term sexual function [95, 96].
Infertility
Infertility is defined as 1 year of unprotected intercourse without conception in women of childbearing age. Much of the research regarding infertility after proctectomy comes from women undergoing proctocolectomy for ulcerative colitis or familial adenomatous polyposis since these diseases often result in proctectomy for patients during their reproductive years. Preoperative infertility rates are around 20% and increase to 63% after total proctocolectomy [97]. Women of childbearing age who undergo pelvic radiation prior to surgery or systemic chemotherapy afterward are likely to experience premature ovarian failure. Options for fertility preservation, such as oocyte cryopreservation, should be discussed [98].
Trapped Ovary Syndrome
Trapped ovary syndrome can occur in young women who have undergone any type of pelvic surgery and is due to adhesions that create septations in the pelvis. When the patient ovulates, fluid released into the pelvic cavity collects in these spaces, and as the trapped fluid expands, patients may develop pelvic pain. The diagnosis may be confirmed with an ultrasound or CT scan demonstrating a cystic lesion without air or surrounding inflammation. In severe cases, operative intervention can be undertaken to lyse the adhesions, release the fluid, and suspend the ovaries to the pelvic brim to prevent it from recurring. One may consider suspending the ovaries at the time of proctectomy in young women to prevent this syndrome from occurring. Placement of an adhesion barrier film in the pelvis may also aid in decreased adhesive formation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree