INTRODUCTION
Colorectal cancer is a major cause of cancer-related mortality in the world. It is currently the third-most-common cancer in incidence in the United States and accounts for about 8.5% of all cancer-related mortality (nearly 136,000 new cases and 50,000 deaths each year) (1). This chapter reviews our current understanding of colorectal cancer, describes the known genetic mutations and risk factors, and outlines emerging screening, prevention, and therapeutic strategies, with particular emphasis on the approach taken at MD Anderson Cancer Center (MDACC).
EPIDEMIOLOGY AND ETIOLOGY OF COLORECTAL NEOPLASIA
Colorectal neoplasia results from accumulation of alterations over years, ultimately transforming normal epithelium to intraepithelial neoplasia (dysplasia) and then malignant epithelium. Three different pathways driving carcinogenesis include chromosomal instability, microsatellite instability (MSI), and CpG island methylation. The chromosomal instability pathway identifies early mutations in genes such as the tumor suppressor APC and the K-ras proto-oncogene and later genetic events, including mutations in the deleted in colon cancer (DCC) gene and the tumor suppressor gene p53.
Genetic predisposition and acquired risk factors progress stepwise from normal colonic mucosa to adenomatous polyps to invasive adenocarcinoma in individuals with acquired (somatic) or inherited genetic (germline) mutations, with further environmental, dietary, or other less-well-understood factors. Personal or family histories of colorectal cancer or polyps, older age, and inflammatory bowel disease (IBD) have all been associated with an increased risk of colorectal cancer (Table 24-1).
| Characteristic | Incidence |
|---|---|
| General population | 5% |
| Personal history of colorectal cancer | 15%-20% |
| Inflammatory bowel disease | 15%-40% |
| Adenomatous polyps: personal | Variable |
| Hereditary nonpolyposis colorectal cancer mutation | 70%-80% |
| Familial adenomatous polyposis | >95% |
A “Western” diet rich in saturated fat has been associated with an increased risk of colon cancer. Fiber may decrease the fecal carcinogen concentration and transit time, thus reducing the period of exposure to colonic mucosa. However, the prospective Nurses Health Study of 88,757 women aged 34 to 59 years found no association between fiber intake and the risk of colorectal cancer after a median follow-up of 16 years (2).
Increased body mass index (BMI), and central obesity are emerging as risk factors for colorectal cancer. The Framingham Study found that a BMI >30 increased the risk of colon cancer by 50% among middle-aged (30-54 years) individuals and by 2.4-fold for those aged 55 to 79 years, and waist circumference was a stronger predictor than BMI (3).
Carcinoma is present in 5% of adenomas, where the potential for malignant transformation is 8 to 10 times higher for villous and tubulovillous adenomas than tubular adenomas. Just over 1% of adenomatous polyps less than 1 cm in size are malignant, whereas up to 40% of adenomas larger than 2 cm are malignant (4).
Patients with IBD (ulcerative colitis or Crohn disease) are at increased risk of developing colorectal carcinoma based on the duration and extent of active disease, colitis, and mucosal dysplasia (5). Recognizing the increased risk of colorectal cancer for patients with IBD, appropriate screening should be instituted as detailed in Table 24-2.
| Patient Populations | Screening Tests |
|---|---|
| General population AND | FOBT annually and sigmoidoscopy every 3-5 years or colonoscopy every 10 years, beginning at age 50 |
| Patient with any distant relative with CRC or polyps | |
| Patient with first-degree relative with CRC | FOBT annually and sigmoidoscopy every 3-5 years or colonoscopy every 10 years; begin at age 40 |
| Moderate-risk patients | Polyp removal; repeat colonoscopy at 3 years; if normal, extend interval to 5 years |
| Patient with two first-degree relatives with CRC | Colonoscopy every 3-5 years |
| Begin screening at age 40 or 10 years younger than youngest affected relative | |
| OR | |
| Patient with one first-degree relative with colorectal cancer diagnosed at 50 years of age or younger | |
| Patient with HNPCC risk | Colonoscopy every 2 years, then yearly after age 40; begin screening at age 25 or 10 years younger than the youngest affected relative; consider genetic counseling and testing |
| Patient with FAP risk | Sigmoidoscopy every 1-2 years; begin screening at age 12 years; genetic counseling and testing |
| Patient with personal history of CRC | Total colon examination (TCE: ACBE or colonoscopy) within 1 year after resection; repeat at 3 years; repeat at 5 years if normal |
| Patient with personal history of adenoma | Polyp removal; repeat at 3 years; repeat at 5 years if normal |
About 20% of all colorectal cancer cases are attributed to inherited autosomal dominant syndromes, including familial adenomatous polyposis (FAP), Gardner syndrome, and hereditary nonpolyposis colorectal cancer (HNPCC) (Table 24-1). Most genetic abnormalities involve deletion of fragments of chromosomes, known as allelic loss or loss of heterozygosity (LOH), or errors in DNA mismatch repair.
Familial adenomatous polyposis is caused by a mutation of APC leading to the functional loss of both APC alleles, one inherited as a germline mutation and the other mutated in early childhood. Familial adenomatous polyposis has high penetrance, manifesting as thousands of adenomatous polyps—some invariably progress to cancer, thereby warranting a prophylactic colorectal resection. The onset of malignancy in untreated patients occurs at about 42 years, with invasive cancer developing 20 to 30 years later.
The genetic penetrance of HNPCC (also known as Lynch syndrome) is caused by defects in DNA mismatch repair through germline mutations in the repair genes. Additional mutations involving tumor suppressor genes and oncogenes rapidly accumulate within these DNA repair–deficient cells, leading to malignant transformation in only 3 to 5 years.
MutY Homolog-associated polyposis is caused by biallelic mutation in the base excision repair gene MUTYH. Patients with the syndrome are characterized by oligopolyposis, usually more than 15 but fewer than 100 polyps. The onset of adenomas is older than in classic FAP, but similar to attenuated adenomatous polyposis (45-55 years of age).
SCREENING FOR COLORECTAL NEOPLASIA
Researchers have attempted to identify individuals at greatest risk of developing colorectal cancer who would benefit most from screening (see Table 24-2). Despite the benefits associated with screening, the majority of colon cancers continue to be diagnosed in symptomatic patients.
Meta-analysis of four randomized trials investigating the role of fetal occult blood testing (FOBT) demonstrated an increased percentage of early-stage colorectal cancers discovered through FOBT and a reduction in mortality from colorectal cancer (6). The current recommendation of the US Preventative Services Task Force is to screen using FOBT, sigmoidoscopy, or colonoscopy in adults from age 50 to 75 years (7).
High-quality air contrast barium enema (ACBE) plus flexible sigmoidoscopy was considered in lieu of a full colonoscopy but has fallen out of favor due to its lack of sensitivity for small polyps (<1 cm), its highly operator-dependent nature, and reliance on the patient’s mobility to optimize imaging.
Flexible sigmoidoscopy is a relatively safe and inexpensive procedure that may be suitable for screening large populations at low risk, in combination with FOBT. However, adenomas in the distal colon are not indicative of proximal lesions, and sigmoidoscopy may miss nearly 50% of all colonic lesions (8). Patients with adenomas in the distal colon detected by flexible sigmoidoscopy should have a full colonoscopy.
Virtual colonoscopy (VC) involves reconstruction of three-dimensional images of the colon from the two-dimensional data obtained by a spiral CT scanner. Bowel preparation is required, but the technique is less invasive and does not require sedation. However, VC lacks the advantage of a colonoscopy for direct access to colonic tissue for biopsies.
Colonoscopy not only enables full visualization of the entire colon but also allows for biopsy or removal of any suspicious lesions. In one retrospective study, 1,994 patients were examined to determine whether the size and histologic features of distal lesions are predictive of proximal lesions, as identification of these factors would help determine who should undergo full colonoscopy after sigmoidoscopic screening. The findings in the distal and proximal colon are shown in Table 24-3 (9).
Despite widespread use, the colonoscopy for the purposes of cancer screening has not been studied in a randomized prospective trial until the Nordic-European Initiative on Colorectal Cancer (NordICC) study. This multinational trial randomizes patients aged 55 to 64 years to either once-only screening colonoscopy with removal of all lesions or no screening, which is the standard of care in those trial countries (10). After a 15-year follow-up, the primary end points of cumulative colorectal cancer–specific death and incidence will be evaluated. Results are anticipated beyond 2020, given that the study began accrual in 2009.
Genetic testing for APC mutations, MUTYH mutations, and DNA mismatch repair gene mutations are now available to identify carriers. A patient with classic FAP or with oligopolyposis but negative APC mutation testing should undergo MUTYH mutation testing given the incidence (7%-29%) of biallelic MUTYH in patients with polyposis with negative APC testing (11). At MD Anderson, suspected FAP patients are referred to a genetic counselor to discuss screening recommendations, genetic testing, and intervention for themselves and family members.
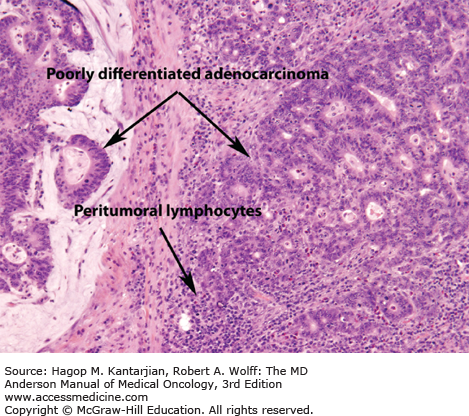
All patients with colorectal cancer at MD Anderson are screened for HNPCC. Table 24-4 summarizes the Amsterdam Criteria (both original and modified) to assess the risk of HNPCC (12). Microsatellite instability, which is a hallmark of HNPCC, also occurs in about 15% of spontaneous colon cancers. Histology that suggests an MSI tumor may include mucinous features, poor differentiation, or the presence of tumor-infiltrating lymphocytes (Fig. 24-1). In female patients less than 50 years of age with proximal tumors, poorly differentiated histology, or mucinous tumors, HNPCC should be considered even when the patient’s family history is not suggestive.
| Original Criteria (Amsterdam Criteria I) | Revised Criteria (Amsterdam Criteria II) |
|---|---|
| There should be at least three relatives with colorectal cancer; all the following criteria should be present: | There should be at least three relatives with an HNPCC-associated cancer (colorectal cancer; cancer of endometrium, small bowel, ureter, or renal pelvis): |
| One should be a first-degree relative of the other two. | One should be a first-degree relative of the other two. |
| At least two successive generations should be affected. | At least two successive generations should be affected. |
| At least one colorectal cancer should be diagnosed before age of 50. | At least one should be diagnosed before age of 50. |
| Familial adenomatous polyposis should be excluded. | Familial adenomatous polyposis should be excluded in the colorectal in cancer case(s) if any. |
| Tumors should be verified by pathological examination. | Tumors should be verified by pathological examination. |
Immunohistochemical (IHC) stains done on sections from the diagnostic biopsy assess tumors for loss of heterozygosity in hMSH2, hMSH6, or hMLH1 gene loci. At MD Anderson, we test all patients with surgically resection for MSI status as a predictive and prognostic marker. Further testing for germline mutations may follow an uninformative IHC stain. In particular, the absence of the MLH1 protein on IHC staining calls for the testing of the BRAF gene, where a BRAF mutation signifies the downregulation of MLH1 gene expression not through a germline mutation, but rather somatic promoter hypermethylation (13). Furthermore, 10% to 15% of all MSIs are not detected through conventional IHC methods, highlighting standardized MSI testing by polymerase chain reaction (PCR) as is done at MD Anderson (14). Thus, genetic counseling and testing are strongly recommended.
PREVENTION STRATEGIES
Prevention of colorectal neoplasia is usually considered primary or secondary. In primary prevention, broad-based interventions may decrease the risk of colorectal cancer for those at average risk. Americans currently have a 1-in-20 lifetime risk of developing colorectal cancer. Consequently, primary preventive strategies may have a significant impact on the overall incidence of colorectal cancer.
For over 20 years, data suggested that nonsteroidal anti-inflammatory agents such as sulindac slow or prevent the formation of adenomatous polyps, particularly in patients with FAP (15). Prostaglandin E2 (PGE2), an important modulator of cell proliferation and malignant transformation, is formed by the catalytic activity of two predominant isoforms of cyclooxygenase. Cyclooxygenase 1 (COX-1) is constitutively active and widely expressed; it appears to regulate tissue repair and homeostasis. Cyclooxygenase 2 is an inducible enzyme that appears to play a role in inflammation and tumor promotion. In a study of patients with FAP, the selective COX-2 inhibitor celecoxib at a higher dose significantly reduced the number of adenomas when compared with placebo or a lower dose of celecoxib (16).
However, the increased incidence of stroke and myocardial infarctions make the use of COX-2 inhibitors as primary chemoprevention unclear (17). Aspirin has primary chemopreventive properties, with the risk of colorectal cancer substantially reduced among women who were regular users of aspirin for at least 20 years (18). An additional prospective cohort study of male physicians followed over 4 years showed that regular users of aspirin (≥2 times per week) had a lower risk of developing colorectal cancer during the study period (RR = 0.68; 95% CI, 0.52-0.92) (19).
Outside the clinical trial setting, patients with FAP are treated with celecoxib 400 mg twice daily. Recognizing the cardiovascular risk associated with COX-2 inhibitors, patients with cardiovascular disease or risk factors need aggressive risk management (eg, hypertension, diabetes, hyperlipidemia) or forgo therapy with celecoxib.
Aspirin also has secondary chemopreventive benefits. In a large randomized, placebo-controlled trial, aspirin demonstrated a benefit for patients with a prior history of colorectal cancer, showing a significantly reduced risk of developing adenomatous polyps compared to the placebo group (RR = 0.65; 95% CI, 0.46-0.91) (20). In a separate study, 1,121 patients with recent adenomatous polyp removal were randomized to receive aspirin 81 mg daily, aspirin 325 mg daily, or a placebo. Both groups receiving aspirin had a reduced risk of subsequent colorectal adenomas, with the 81-mg dose superior to the 325-mg dose (21). In a prospective cohort study of 1,279 patients with stage I to III colorectal cancer, after a median follow-up of 11.8 years, participants who regularly used aspirin had lower colorectal cancer–specific mortality, including those who initiated aspirin after diagnosis.
DIAGNOSTIC EVALUATION AND STAGING
Colonic lesions in any location can cause change in bowel habits and bleeding, which may manifest as melena, hematochezia, a positive hemoccult test, or iron deficiency anemia in addition to weight loss, anorexia, and other constitutional symptoms. Unexplained iron deficiency anemia warrants an evaluation of the gastrointestinal tract.
Preoperative evaluation is not always possible, particularly in the setting of acute bowel obstruction, and the staging evaluation in these cases should be completed within several weeks of surgery. Accurate postoperative staging may be confounded by the preceding surgery and therefore should not be obtained for at least 3 to 6 weeks after the operative procedure. Patients at MD Anderson are advised to wait a minimum of 4 weeks after surgery before undergoing imaging and a colonoscopy to allow wound healing and minimize risk to the surgical anastomosis.
In patients found to have a colonic neoplasm not requiring urgent surgery, a complete history, physical exam, and full colonoscopy with biopsies should be performed. Laboratory evaluation should include a complete blood cell count with differential, electrolytes, liver function studies, carcinoembryonic antigen (CEA) level, serum urea nitrogen (BUN), and creatinine. Imaging studies should include CT of the chest and CT scan or magnetic resonance imaging (MRI) of the abdomen/pelvis.
Patients with newly diagnosed rectal cancer at MD Anderson are staged with an endorectal ultrasound (EUS) or pelvic MRI. The EUS is more accurate than CT for assessing the depth of tumor invasion into the bowel wall and perirectal lymph node involvement. A pelvic MRI to evaluate the mesorectal planes and perirectal lymph nodes allows improved accuracy of preoperative staging. At MD Anderson, all patients with rectal cancer are staged with a CT scan of the chest and abdomen, with a dedicated MRI of the pelvis in the preoperative setting.
Positron emission tomographic (PET) scanning using (18F)-fluorodeoxyglucose (FDG-PET) is obtained in patients with a rising CEA without clinical or radiographic evidence of disease or in the setting of equivocal CT findings. However, inflammation may increase 18FDG uptake, thus confounding accurate assessment. At MD Anderson, FDG-PET is not part of routine staging for newly diagnosed colon or rectal cancer.
More than 95% of all colorectal malignancies are adenocarcinomas that are well differentiated, moderately differentiated, poorly differentiated, and undifferentiated. Other subtypes include mucinous and signet ring cell, which confer a poorer prognosis. These tumors are more likely to be present in younger patients and more commonly spread to the peritoneum. Treatment, however, does not differ from the more typical adenocarcinoma subtypes.
Currently, the widely accepted system is the American Joint Committee on Cancer (AJCC) TNM classification system (Table 24-5) to guide treatment.
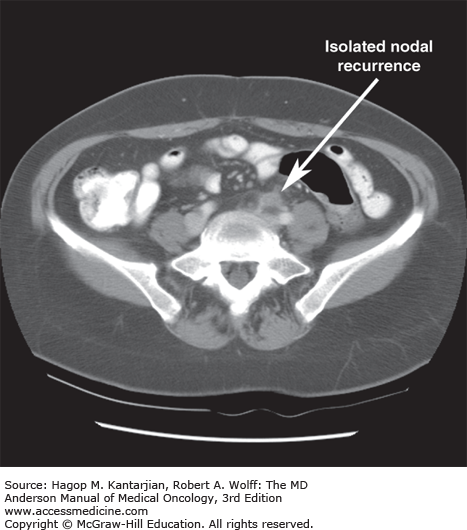
At the time of resection, tumor removal should involve segmental resection of the involved colon or rectum along the appropriate vascular pedicles with careful removal of all regional lymph nodes. Failing to do so may lead to relapse in lymph nodes draining the affected segment of bowel, as shown in Fig. 24-2. Therefore, tumors are considered high-risk stage II (T3N0M0 or T4N0M0) unless at least 12 lymph nodes are negative for metastatic disease (22).
FIGURE 24-2
Computed tomographic image of a 54-year-old woman with a history of T3N0M0 adenocarcinoma of the sigmoid colon found incidentally at the time of hysterectomy for benign disease. Surgical resection of the sigmoid mass was performed by her gynecologist. The patient received adjuvant therapy for 6 months but subsequently developed a rising serum CEA level and a nodal mass at the base of the inferior mesenteric artery (IMA). After a course of chemotherapy for metastatic disease, she underwent repeat laparotomy with the finding of an isolated nodal mass, which was removed and was positive for adenocarcinoma. In retrospect, inadequate resection of the sigmoid mesentery to the level of the IMA was thought to explain the recurrence.
THERAPEUTIC APPROACHES
Almost half of the patients undergoing curative resection will ultimately die of metastatic disease as a result of residual microscopic disease not evident at the time of surgery. Patients with stage II colon cancer at high risk of relapse have been defined by the National Comprehensive Cancer Network (NCCN) as those individuals with T4 tumors (stage IIB/IIC); poorly differentiated history (excluding MSI-H cancers); lymphovascular invasion; perineural invasion; bowel obstruction; localized perforation; margins that are close, indeterminate, or positive; and inadequate sampling of lymph nodes (<12 nodes examined) (23).
Resection for localized colon cancer removes the affected segment of bowel, the adjacent mesentery, and the draining lymph nodes. Asymptomatic patients with stage IV disease with their primary malignancy intact do not require surgical resection of their primary except for impending bowel obstruction. Laparoscopic colectomy was noninferior to an open colectomy in several prospective randomized studies, with the laparoscopic surgery group having a shorter perioperative recovery, hospital stay, duration of parenteral narcotic use and oral analgesics, as well as comparable intraoperative complications and postoperative mortality (24).
Patients with stages II and III colon cancer have a risk of relapse after surgical resection of macroscopic disease. Systemic chemotherapy has been employed to eradicate micrometastases. Currently, patients with stage III colon cancer (node positive without clinically detectable metastases) receive 6 months of adjuvant chemotherapy. The MOSAIC (Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer) trial demonstrated an improved 5-year disease-free survival (DFS) from 67% to 73% for patients receiving FOLFOX (5-fluorouracil [5-FU], leucovorin calcium, and oxaliplatin) versus 5-FU and leucovorin alone (25) with the DFS and overall survival (OS) benefits achieving statistical significance in patients with stage III disease.
The evidence for adjuvant therapy for stage II disease is less robust. To date, the largest study of patients with stage II disease, QUASAR (Quick and Simple and Reliable), showed a modest survival benefit of 3.6% in patients receiving adjuvant 5-FU versus observation following surgical resection (26). The 2012 subset analysis of the 889 patients with stage II disease in the MOSAIC (Multicenter International Study of Oxaliplatin/5FU-LV in the Adjuvant Treatment of Colon Cancer) trial showed no statistically significant benefit in either OS or DFS with the addition of oxaliplatin to 5-FU in the adjuvant setting for stage II (27). Furthermore, the analysis of the patients with low- and high-risk stage II demonstrated that neither subgroup unequivocally derived benefit from the addition of oxaliplatin.
Multigene assays have been in development for prognostic and predictive value in patients with stage II colon cancer. Among the assays furthest along in development is the Oncotype Dx colon cancer assay (Genomic Health, Inc.), which provides a prognostic classification of low, intermediate, or high risk of recurrence based on the expression of seven recurrence risk genes and five reference genes. In two large trials, QUASAR and NSABP C-07, of patients with stage II and III disease, this score was validated as prognostic for recurrence, DFS, and OS but not predictive of benefit from chemotherapy (28).
A second assay, ColoPrint (Agendia), identifies the expression of 18 genes and produces one of two recurrence risk categories, high or low. Although it was studied in stages I-IV, it has emerged to be of the most value in patients with stage II in identifying the risk of recurrence between high- and low-risk groups (hazard ratio [HR] 3.34, P = .017) (29). It is currently being prospectively validated in patients with stage II in the PARSC trial, which will predict the 3-year recurrence-free survival using ColoPrint and clinical factors (30). Both assays share limitations particularly the inability to predict clinical benefit from chemotherapy.
Irinotecan has no established role in the adjuvant setting. Three randomized phase III trials failed to show an improvement in DFS or OS in the adjuvant setting (31,32,33). An exploratory analysis of CALGB 89803 (IFL versus bolus 5-FU/LV) indicates that patients with MSI-H may have an improved DFS from irinotecan-based therapy (HR 0.76; 95% CI, 0.64-0.88; P = .03). However, the PETACC 3 study confirmed that while patients with stage II disease with MSI-H tumors have a survival advantage over MSS patients with a 5-FU-based treatment, the addition of irinotecan produced no additive benefit, affirming the overall consensus against using an irinotecan-based regimen in the adjuvant setting (34).
Furthermore, data have not supported the addition of bevacizumab, cetuximab, or panitumumab in the adjuvant setting. The NSABP trial C-08 showed no improvement in DFS or OS with the addition of bevacizumab to adjuvant FOLFOX6 in stage II and III colon cancer (35). A second phase III randomized controlled study, the bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT) trial of resected stage III or high-risk stage II colon cancer (36) also failed to show any improvement in DFS and, in fact, suggested a poorer OS with the addition of bevacizumab.
The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration is a prospective combined analysis of phase III trials investigating duration of adjuvant chemotherapy (3 vs 6 months) for stage III colon cancer (37). The ongoing US CALGB/SWOG 80702 colon trial, which is among the six trials that are part of the IDEA collaboration, further randomizes patients beyond the duration of adjuvant therapy to 3 years of celecoxib versus placebo (37). Overall, few therapeutic changes appear to be on the horizon for the treatment of adjuvant colon cancer.
When patients present to MDACC with a diagnosis of colon cancer, a detailed history, including family history; routine laboratory tests, including CEA level; and imaging (CT chest, CT or MRI abdomen, pelvis) are obtained. Previous endoscopic findings and pathology are reviewed and are tested for MSI by IHC or by PCR. Patients without metastatic disease or contraindications to surgery should undergo primary resection with curative intent (Fig. 24-3). If there is an obstruction, colonoscopy is usually performed. Surgery may consist of segmental resection or subtotal colectomy, depending on the underlying colonic pathology (multifocal cancer, FAP, HNPCC, etc.); pathologic staging is then determined from the surgical specimens, which is the standard for all such resections at MD Anderson irrespective of age. Patients with stage 0 or I tumors are placed on surveillance only. Patients with stage II colon cancer have a 75% to 80% chance of long-term DFS with surgical resection alone. Patients with stage II colon cancer are referred for discussion of adjuvant chemotherapy with full consideration provided to all patients with stage III disease.
Our current approach favors FOLFOX or XELOX (Table 24-6) for 6 months for all stage III patients unless chemotherapy is contraindicated. Those who are better candidates for a single-agent fluoropyrimidine are offered capecitabine over intravenous 5-FU. Adjuvant therapy should begin within 4 to 8 weeks after surgery, unless postoperative complications warrant a delay.
| Adjuvant Chemotherapy |
|---|
| Capecitabine: 1,000 mg/m2 by mouth twice daily on days 1-14 (3-week cycle, total 8 cycles) |
| 5-Fluorouracil/leucovorin: Leucovorin 400 mg/m2 IV on day 1; 5-fluorouracil 400 mg/m2 IV bolus on day 1, followed by 5-fluorouracil 2,400 mg/m2 IV continuous infusion over 46 hours (2-week cycle, total 12 cycles) |
| Modified FOLFOX 6: Oxaliplatin 85 mg/m2 IV on day 1; leucovorin 400 mg/m2 IV on day 1; 5-fluorouracil 400 mg/m2 IV bolus on day 1, followed by 5-fluorouracil 2,400 mg/m2 IV continuous infusion over 46 hours (2-week cycle, total 12 cycles) |
| XELOX: Oxaliplatin 130 mg/m2 on day 1; capecitabine 850 mg/m2 by mouth twice a day on days 1-14 (3-week cycle, total 8 cycles) |
| Therapy for Metastatic Disease |
| Capecitabine: 1,000 mg/m2 by mouth twice a day on days 1-14 (3-week cycle) |
| • With or without bevacizumab (7.5 mg/kg IV every 3 weeks) |
| 5-Fluorouracil/leucovorin: Leucovorin 400 mg/m2 IV on day 1; 5-fluorouracil 400 mg/m2 IV bolus on day 1, followed by 5-fluorouracil 2,400 mg/m2 IV continuous infusion over 46 hours (2-week cycle) |
| With or without bevacizumab (5 mg/kg IV every 2 weeks) |
| Modified FOLFOX 6: Oxaliplatin 85 mg/m2 IV on day 1; leucovorin 400 mg/m2 IV on day 1; 5-fluorouracil 400 mg/m2 IV bolus on day 1, followed by 5-fluorouracil 2,400 mg/m2 IV continuous infusion over 46 hours (2-week cycle) |
| • With or without bevacizumab (5 mg/kg IV every 2 weeks) |
| • With or without cetuximaba (400 mg/m2 IV first infusion followed by 250 mg/m2 IV weekly or 500 mg/m2 IV every 2 weeks) or panitumumaba (6 mg/kg IV every 2 weeks) |
| XELOX: Oxaliplatin 130 mg/m2 on day 1; capecitabine 850 mg/m2 by mouth twice a day on days 1-14 (3-week cycle) |
| With or without bevacizumab (7.5 mg/kg IV every 3 weeks) |
| • With or without cetuximaba (400 mg/m2 IV first infusion followed by 250 mg/m2 IV weekly or 500 mg/m2 IV every 2 weeks) or panitumumaba (9 mg/kg IV every 3 weeks) |
Modified FOLFIRI:
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|