There are two major groups of hematologic neoplasms: Lymphoma and leukemia. Lymphoma is lymphoid tumors initially confined to lymphoid organs or extranodal tissue, whereas leukemia includes lymphoid and myeloid neoplasms originating from the bone marrow and circulating in the peripheral blood. However, with the advent of new technology, especially immunophenotyping and molecular biology, lymphoma cells can be detected in blood and bone marrow even in the relatively early stage, and the demarcation between lymphoma and leukemia is sometimes blurred. Lymphoma and leukemia have their counterparts, such as lymphoblastic lymphoma versus acute lymphoblastic leukemia (ALL) and small lymphocytic lymphoma (SLL) versus chronic lymphocytic leukemia (CLL). The tumor cells of the counterparts may have the same morphology, immunophenotype, immunogenotype, karyotype, and clinical characteristics, such as the presence of a mediastinal mass in both lymphoblastic lymphoma and ALL of T-cell origin. It has recently been found that the difference in location (tissue vs. blood) between lymphoma and leukemia may be associated with the presence or absence of adhesion molecules or lymphokine receptors on the tumor cells. For instance, the difference in phenotype between SLL and CLL is the presence of the adhesion molecule, CD11a/CD18 (LFA-1), on SLL cells (
1). In contrast, CLL cells express the lymphokine receptors, CXCR4 and CCR7, which are not present on the SLL cells (
2). The high frequency of the leukemic phase in Burkitt lymphoma is due to its lack of LFA-1 (
3).
Leukemia can be further divided into acute and chronic groups. In acute leukemias, the clinical course is rapidly progressive and the leukemic cells are immature blasts. Chronic leukemias are just the opposite: The clinical course is slow and indolent, and the leukemic cells are mature appearing in lymphoid leukemia or intermediate forms (promyelocytes, myelocytes, and metamyelocytes) in myeloid leukemia. Although lymphoma is not divided into acute and chronic forms, lymphoma cell types can also be differentiated on the basis of maturation. For instance, T-cell lymphoma can be divided into thymic and postthymic (peripheral T cell) subtypes. The homogeneity of leukemia and lymphoma cells in terms of their maturation stage prompted the theory of maturation arrest as the mechanism of tumorigenesis (
4). The proliferation of new stage-specific monoclonal antibodies will certainly help to pinpoint the hematologic neoplasms in highly defined stages.
The classification of hematologic neoplasms, therefore, depends on many parameters, including cell morphology, size, stage of maturation, and clinical course. With the recent advances in new technologies, the classification is also based on immunophenotypes, immunogenotypes, and karyotypes. However, the basic requirement for diagnosis is still cytologic recognition of the neoplastic cells. Therefore, it should be useful to be acquainted with the ontogeny of blood cells. This is particularly relevant to biphenotypic lymphoma and/or leukemia and to those tumors derived from a pluripotent stem cell or progenitor cells. Having this knowledge can also help us to understand why, for instance, chronic myelogenous leukemia may have a blast crisis of myeloid, lymphoid, erythroid, or megakaryocytoid origin and why erythroid leukemia has coexistent myeloblasts and trilineage dysplasia.
DEVELOPMENTAL STAGES OF HEMATOPOIETIC CELLS
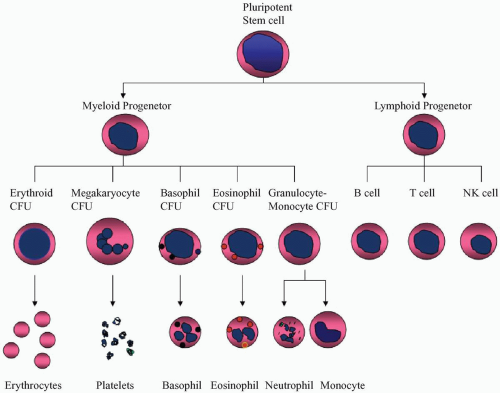
During fetal life, hematopoiesis initially takes place in the yolk sac and occurs later in the liver and spleen. After birth, the hematopoietic function is taken over by the bone marrow. All the blood cells are derived from a pluripotent stem cell that can differentiate into various lineages, including erythrocytes, megakaryocytes, basophils, eosinophils, neutrophils, monocytes, and lymphocytes (
Fig. 5.1). The precursors of these lineages are called colony-forming units. Precursors of T lymphocytes must go to the thymus, where they develop through several stages of thymocytes and finally become mature T lymphocytes. The mature T lymphocytes exit the thymus, enter into the blood and lymph, and reach the peripheral lymphoid tissues. B lymphocytes do not go through the thymus but mature in the bone marrow and enter the lymphoid tissue via blood and lymph. The third lineage of lymphoid cells was initially called null
cell, because it lacks surface immunoglobulin and does not form sheep erythrocyte rosettes. It is now recognized that most null cells are large granular lymphocytes that are able to lyse a variety of tumor cells and virus-infected cells, and are thus called natural killer (NK) cells (
5). The ontogeny of NK cells is still not clear, but NK cells probably share the same stem cells with T lymphocytes.
The proliferation and maturation of various precursors are stimulated by cytokines called colony-stimulating factors (CSFs) (
5). T lymphocytes produce interleukin-3 (IL-3), which simulates all precursors, and granulocyte-monocyte CSF (GM-CSF). Macrophages and marrow stromal cells also produce granulocyte-monocyte CSF and additional CSFs specific for granulocytes (G-CSF) or monocytes (M-CSF), as well as IL-1 and IL-6. The interleukins can enhance colony formation by hematopoietic precursors in the presence of CSFs. The bone marrow stromal cells can also produce IL-7, which preferentially stimulates the maturation of B lymphocytes.
The development of T lymphocytes starts in the thymus. The earliest stage of thymocyte is called
stage I thymocyte or
prothymocyte, which represents 13% of the entire thymocyte population (
Table 5.1) (
6,
7). It expresses terminal deoxynucleotidyl transferase (TdT) in the nucleus; CD3 in the cytoplasm; and CD38, CD71, and the earliest T-cell surface marker, CD7, on the surface. Some studies also indicated the presence of surface HLA-DR, CD34, and possibly CD2 at this stage.
Stage II thymocytes, also called
common thymocytes, include subcapsular and cortical cells and represent approximately 75% of the total thymocyte population. In this stage, CD1, CD2, CD5, and CD7 are expressed. CD4 and CD8 are usually coexpressed, or only one of these two is expressed.
Stage III thymocytes, also called
medullary or
mature thymocytes, consist of about 15% of the thymocyte population. The mature thymocytes express surface CD3 and T-cell receptor protein, and start to divide into CD4+, CD8−, and CD4−, CD8+ are two subgroups. When the mature thymocytes enter into the peripheral circulation, they become
postthymic or
peripheral T cells, which lose the CD38 and TdT markers.
The development of B cells is confined to the bone marrow. In the
B-cell progenitor stage, only TdT, HLA-DR, CD34, and cytoplasmic CD79 are expressed (
Table 5.2) (
6,
7). Additional antigens, CD10, CD19, and cytoplasmic
CD22, appear in the next stage, the
pre-pre-B-cell stage. The
pre-B-cell stage is characterized by the presence of cytoplasmic µ chain without accompanying light chain and the loss of CD34. CD20 first appears at this stage. In the
immature B-cell stage, surface immunoglobulin light chains and CD21 start to appear. CD10 usually disappears at this stage, but it is frequently present on Burkitt lymphoma cells, which are considered immature B cells (
8). When B cells become mature, immunoglobulin D (IgD) appears on the surface side-by-side with IgM. The
mature B cells are considered resting or “virgin” until becoming activated by their contact with antigens. As heavy-chain switching occurs on B-cell activation, the activated B cells express surface IgM, IgA, or IgG instead of IgM/IgD. CD21 disappears at this stage, but activation antigens, such as CD38 and CD71, and proliferation-associated antigen, Ki-67, are frequently expressed. Activated B cells finally develop into the terminal stage,
plasma cell. Plasma cells synthesize cytoplasmic immunoglobulin and express surface CD38, CD138, PCA-1, and PC-1. Some activated B cells may become
memory B cells, which have an immunophenotype similar to that of either a resting B cell (
7) or an activated B cell (
9).
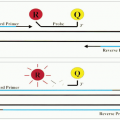
The circulating lymphocytes may migrate to the lymph nodes, the mucosal follicles, and other extranodal sites depending on their surface-homing receptors for highendothelial venules in various tissues (
10). In the lymph node, lymphocytes travel from one compartment to another, undergoing further morphologic changes.
Intranodal B-Cell Differentiation
The lymph node contains four compartments: (i) the cortex, (ii) paracortex, (iii) medullary cords, and (iv) sinuses. The cortex is composed of lymphoid follicles. A
primary follicle contains aggregates of resting or virgin B cells (
9,
11). After antigenic stimulation, resting B lymphocytes become activated, leading to proliferation and blastic transformation. A
germinal center is formed and is surrounded by a
mantle zone, which is made up of the same resting B cells as those in the primary follicles. The follicle with a germinal center is called a
secondary follicle. In the germinal center, B cells are activated by antigens and start to proliferate and undergo somatic mutation. Generation of memory cells and plasma cells and heavy-chain class switch also take place in the germinal center (
12).
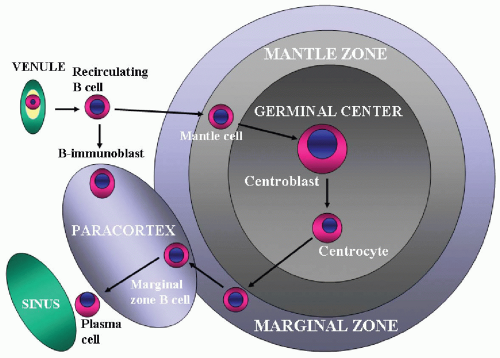
On the basis of current studies, the sequence of normal B-cell differentiation within the lymph node is suggested as follows (
Fig. 5.2) (
9,
13). In the mantle zone, small lymphocytes develop into intermediate lymphocytes (
mantle cells) and finally into blastoid lymphoid cells. The blasts then move into the germinal center and evolve through the stages of small noncleaved cells, large noncleaved cells, and small cleaved cells (
follicular center cells).
Some activated B cells transform into memory B cells and migrate to the marginal zone to become
marginal zone cells (
14). Under certain conditions, the marginal zone cells move to the parafollicular perisinusoidal area and become parafollicular B cells. These cells have ovoid nuclei and relatively abundant clear cytoplasm resembling monocytes and are thus called
monocytoid B cells. Some B cells transform into effector cells, which are plasma cells. The plasma cell is the terminal stage of the B cell, which moves to the medullary cord and finally migrates back to the bone marrow.
Pregerminal Center, Germinal Center, and Postgerminal Center Lymphomas
Lymphoma can develop at each stage of intranodal differentiation. Accordingly, there are mantle cell lymphoma, follicular lymphoma, nodal marginal zone lymphoma, lymphoplasmacytic lymphoma, and plasma cell myeloma. The origin of a lymphoma can be determined by the status of VH gene mutation. Lymphomas that show no VH gene mutation represent a tumor from the pregerminal center. Lymphomas that express VH gene mutation and intraclonal diversity are derived from the germinal center; whereas those that have V gene mutation but not intraclonal diversity are originated from postgerminal center B cells.
Pregerminal center lymphoma is represented by BCLL/SLL and mantle cell lymphoma. Germinal center lymphoma includes follicular lymphoma, Burkitt lymphoma, and a subset of diffuse large B-cell lymphoma. Postgerminal center lymphoma includes nodal marginal zone B-cell lymphoma, extranodal marginal zone B-cell lymphoma, splenic marginal zone lymphoma, plasma cell myeloma, and lymphoplasmacytic lymphoma.