General Introduction
With the progress in subclassification of hematologic neoplasms and the refinement in treatment of these tumors, diagnosis of lymphoma and leukemia has become increasingly complicated. The World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues relies not only on morphology of the tumors but also requires immunophenotyping and genotyping for an accurate diagnosis. Although genotyping with molecular genetic techniques are frequently the tools to make a definitive diagnosis, immunophenotyping is the most important means to provide a prompt diagnosis. Immunophenotyping can be achieved either by flow cytometry (FC) or immunohistochemistry (IH) techniques. Although each of these two techniques has its advantages and disadvantages, each is complementary to the other, and they should be used together. This book will discuss the pros and cons in the application of these two techniques as well as their respective roles in diagnosing various hematologic neoplasms.
The flow cytometer has been hailed as a new product of technical revolution, but the concept of FC has existed for >50 years, and cell counters have been used extensively for >20 years. Nevertheless, the emergence of the fluorescence detector in the new generation of cytometers greatly enhanced their versatility. The availability of a great variety of monoclonal antibodies finally pushed FC to the forefront. The outbreak of acquired immunodeficiency syndrome (AIDS) incidentally accelerated the acceptance of flow cytometers as routine laboratory instruments because of the tremendous demands for testing the helper: suppressor T-cell ratio as a screening technique in the early epidemic of AIDS. However, the flow cytometer has been promptly adopted by hematology and oncology laboratories as a routine tool because most monoclonal antibodies are cell lineage-or developmental stage-specific for blood cells.
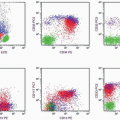
FC has the advantage of being more efficient, sensitive, accurate, and reproducible than manual techniques. With FC, multiple specimens can be simultaneously processed with a panel of 10 or more monoclonal antibodies, and tests can be completed within several hours. When there is a sufficient specimen, FC counts 3,000 to 5,000 cells for the study of each antigen, compared with 100 to 200 cells counted in manual techniques. The examination of large numbers of cells enhances the sensitivity and accuracy of FC and makes it possible to detect small numbers of neoplastic cells. The percentage of various cell groups obtained by FC is highly reproducible and is thus comparable between different laboratories. However, the major merit of FC is its capability of simultaneously measuring multiple parameters (forward light scatter, side scatter, and fluorescence), and the data thus obtained can be stored for further analysis. In addition, the electronic gating gadget enables the study of separate cell groups without requiring tedious isolation techniques.
The major limitations of FC, at this stage, are the high cost of the instrument, the special skill required to operate it, and the lack of a morphologic correlation with the markers. The failure of the machine to distinguish a normal cell from a tumor cell leads to the indiscriminate counting of both populations; thus a wrong conclusion may be drawn if not enough tumor cells or parameters are analyzed. However, with the availability of increasing numbers of specific monoclonal antibodies and fluorochromes, and the emergence of four- to five-color analyzers, the function of FC in terms of diagnosis and therapeutic monitoring has been markedly improved in recent years.
The current simplification of this automated instrument makes it feasible to use as a routine laboratory procedure, not only in large medical centers but also in medium-sized hospitals. Although it is now technically possible for clinical laboratories to use flow cytometers, the lack of expertise in interpreting the results still hampers the broad use of this instrument. It is apparent that a wellillustrated guidebook with detailed clinical cases is needed to help flow cytometer workers to understand how to apply FC to identify malignant cells and to diagnose hematologic neoplasms.
However, the more popular technique for immunophenotyping is IH for obvious reasons. IH can be conveniently performed in a histology laboratory with a minimal investment in equipment and reagents. It can be done on frozen as well as permanent sections. The feasibility of performing IH on archived material retrospectively is a great advantage of
IH. Nevertheless, the major merit of IH is the direct correlation of morphology with markers, which makes surgical pathologists more confident in interpreting the results leading to a diagnosis.
IH. Nevertheless, the major merit of IH is the direct correlation of morphology with markers, which makes surgical pathologists more confident in interpreting the results leading to a diagnosis.
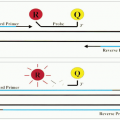
At this stage, the application of IH is limited by the availability of monoclonal antibodies that can be used for histologic staining. After fixation and embedding, many antigenic epitopes are altered so that they can no longer react to most antibodies that are used for FC. The major drawback of IH is its inability to demonstrate surface immunoglobulins and thus the inability to demonstrate a clonal B-cell population in most cases of lymphomas. The antigen-retrieving technique using a microwave oven may help to detect immunoglobulins in some tumors, especially in those with abundant cytoplasm, such as immunoblastic lymphoma and plasma cell neoplasms. The inability to distinguish surface from cytoplasmic antigens is another drawback. For instance, cytoplasmic CD3 is present in early T-cell stage (thymocytes) and surface CD3 is detected in mature T-cell stage (peripheral T cells). When a tumor is stained positive for CD3 by IH, the developmental stage of the tumor cells cannot be pinpointed. The same is true for the distinction between natural killer (NK) cells and NK-like T cells.
There are other limitations of IH. Multiple staining cannot be performed on the same cells. Therefore, the characterization of tumor cells by IH is not as comprehensive as by FC, which can detect 2 to 4 antigens on the same cells. IH using sequential horseradish peroxidase and alkaline phosphatase can label two different antibodies, but it is difficult to demonstrate two antigens on the same cell.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree