Principles of Immunohistochemistry
For many years, pathologists had depended on morphology alone to make a histologic diagnosis until the availability of “special stains.” Special stains were developed because pathologists always realized the fallacy of the morphologic approach and felt the need to have some accessory tests to substantiate the diagnosis. The first kind of special stains are cytochemical stains, which mainly identify cell lineage and cellular chemistry. When cytochemical techniques are applied to histologic sections, it is called histochemistry, which further improves the accuracy of a morphologic diagnosis. However, it is the development of immunohistochemistry that finally makes histologic diagnosis highly objective. Immunohistochemistry is particularly indispensable for the practice of hematopathology, but the molecular cytogenetic techniques have also played an increasingly important role in the diagnosis of hematologic neoplasms. The advent of nonradioactive in situ hybridization (NISH) techniques represents the current effort to combine histologic staining with the studies of nucleic acids (DNA and RNA). These various entities will be discussed briefly in this chapter.
CYTOCHEMISTRY
Cytochemistry is an integral part in the diagnosis of acute myeloid leukemia (AML) required by the French-American-British Cooperative Group (1,2). Although its role has been gradually replaced by immunophenotyping with flow cytometry, cytochemistry is still useful in identifying cell lineages, especially the immature monocytes in the bone marrow, which are frequently difficult to recognize morphologically.
Myeloperoxidase (MPO) is the first screening test to distinguish AML from acute lymphoblastic leukemia (ALL). This enzyme is present in neutrophilic, eosinophilic, and monocytic lineage but not in lymphocytes (3). However, the minimally differentiated myeloblasts may not stain for MPO as seen in the AML-M0 cases, which can be distinguished from ALL only by immunophenotyping. The MPO in eosinophils is resistant to cyanide, so that eosinophil and its immature forms can be identified by this specific reaction. The peroxidase of megakaryocytes and platelets cannot be visualized by light microscopy, but it can be demonstrated by electron microscopy.
Sudan black B reaction is slightly more sensitive than, but similar to, MPO reactions. Sudan black B is a fat-soluble substance, but it probably stains for substances related to MPO, because the Sudan black B reaction becomes negative in patients with MPO deficiency (4). In addition to the MPO-positive cells, Sudan black B also stains fat cells, macrophages, and cytoplasmic vacuoles in Burkitt lymphoma cells.
Specific esterases are a group of enzymes capable of hydrolyzing halogenated naphthol esters (3). The most commonly used substrate is naphthol AS-D chloroacetate. Chloroacetate esterase is most frequently used for the identification of neutrophilic series and occasionally used for mast cells. Because chloroacetate esterase is stable even in paraffin-embedded tissue, it can be used for the diagnosis of myeloid sarcoma and extramedullary hematopoiesis. Chloroacetate esterase is negative for monocytes, megakaryocytes, erythroblasts, and lymphocytes. Only abnormal eosinophils, such as those seen in AML with bone marrow eosinophilia, are positive for chloroacetate esterase.
Nonspecific esterases are a group of enzymes capable of hydrolyzing various aliphatic and aromatic short-chain esters (3). They are called nonspecific esterases because these enzymes exhibit a wide range of substrate specificity. The substrates used to detect nonspecific esterase activity include α-naphthyl butyrate, α-naphthyl acetate, naphthol AS-D acetate, and naphthol AS acetate. The first two substrates are most frequently used because they do not stain for granulocytes. Thus sodium fluoride inhibition is not needed to distinguish monocytes from granulocytes. Besides monocytes and histiocytes, α-naphthyl acetate esterase is also positive in megakaryocytes and platelets, and the reaction is sodium fluoride sensitive (4). T lymphocytes usually show a focal paranuclear staining for nonspecific esterases. Because nonspecific esterases are sensitive to heat, storage, and fixative, they cannot be demonstrated in paraffin-embedded tissue sections.
Acid phosphatases (APs) are a group of enzymes capable of hydrolyzing monophosphate esters in an acid environment (3). By electrophoresis, APs can be separated into seven nonerythrocytic isoenzymes. Isoenzymes 2 and 4 are present in neutrophils and monocytes; 3, in lymphocytes and platelets; 3b, in primitive blood cells and blasts; and 5, in hairy cells of hairy cell leukemia. The most important function of AP is to identify hairy cells, which show a strong, diffuse, tartrate-resistant AP. This reaction may be demonstrated occasionally in other lymphomas or leukemias, but the reaction is seldom as intense and diffuse as in hairy cell leukemia. The focal paranuclear staining pattern of AP is helpful in identifying T lymphocytes.
Periodic acid-Schiff (PAS) is capable of reacting with R-CHO groups in tissues to form an insoluble bright-red complex (aldehyde-fuchsin-sulfurous acid compound) (5). Therefore, tissue and cells containing glycoproteins, mucoproteins, and high-molecular-weight carbohydrates are positive for PAS. The PAS reaction is positive in most blood cells; it is detected in 80% to 90% of cases of ALL and in 10% to 15% of cases of AML (3). It is particularly useful when a block pattern is demonstrated in pronormoblasts and lymphoblasts. A positive PAS stain in erythroblasts is a common finding in erythroleukemia; it is a coarsely granular pattern in cells of early stage and a finely granular pattern in cells of later stage. Normoblasts in healthy persons are PAS negative, but they can be PAS positive in erythrodysplasia. PAS staining of the periphery of cytoplasm, especially in cytoplasmic protrusions, is characteristic for megakaryocytes and megakaryoblasts. This pattern, if present, is helpful for the diagnosis of acute megakaryoblastic leukemia.
IMMUNOHISTOCHEMISTRY
Immunohistochemistry is the application of a labeled or enzyme-bound antibody to identify a specific antigen, which is visualized under light microscopy by means of a color signal. There are several important milestones in the history of immunohistochemistry development (5). In 1940, Coon first used immunofluorescence techniques to detect antigens in frozen sections (6). Avrameas et al. (7) developed enzymatic labeling to demonstrate the antigenantibody immune complex in tissue sections. Taylor and Burns (8) first applied immunohistochemical techniques to formalin-fixed, paraffin-embedded tissue sections. The subsequent progression from the one-step direct conjugate method to the multiple-step indirect method as well as to the discovery of the hybridoma technique by Kohler and Milstein (9) greatly facilitated the versatility of immunohistochemistry. The staining technique is further enhanced by enzyme digestion (10) and finally by antigen retrieval (AR) techniques (11,12) that make immunohistochemistry the indispensable tool in the practice of surgical pathologists. The current goal is to make immunohistochemistry quantitative so that it is not only a tool for diagnosis, but one for therapeutic monitoring and prediction of prognosis in various tumors (13,14).
Immunohistochemical staining involves multiple and somewhat complicated steps; therefore, there are many technical problems to watch for, and there are many technical decisions to make depending on the target antigens. Due to space limitation, only a few important technical matters are discussed in this chapter. For a comprehensive review, the reader is referred to the textbooks by Dabbs (15) and Elias (16).
Fixatives
There are two groups of fixative: The cross-linking fixatives (e.g., formaldehyde) and coagulant or precipitation fixatives. The latter group includes acid fixatives (e.g., Bouin’s solution) and heavy metal fixatives (B5 and Zenker’s fluid). Williams et al. (17) compared the effects of various fixatives in immunohistochemical stain results on tonsil tissue and found that 10% neutral buffered formalin (NBF), 10% zinc formalin, and 10% formal saline gave the most consistent results overall and showed excellent antigen preservation. In contrast, 10% formal acetic acid, B5, and Bouin’s fixative showed poor antigen preservation.
Others feel that there is no particular optimal fixative because the staining results depend on a complex interaction among the fixative, pH, osmolarity, temperature, length of treatment, and tissue types (16). For instance, there is no difference between formalin and Bouin’s fixative for the staining of insulin, pancreatic polypeptide, and gastrin, but better results are obtained by Bouin’s fixative than by formalin for the staining of glucagons and somatostatin (18). Elias (16) suggested dividing specimens into multiple fixatives, including NBF, 10% formal saline, 95% ethanol, Omni, modified methacarn, and B5 for subsequent processing. B5 is considered most suitable for fixation of lymphoid tissues but, because it contains mercury, many laboratories avoid using it.
Immunohistochemical Staining Procedures
Direct Conjugate-Labeled Antibody Method
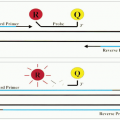
The direct method is the application of labeled monospecific antibody directly to the tissue section (Fig. 3.1). The label can be an enzyme, biotin, fluorochrome, or colloidal gold. There are several procedures that are used to enhance the direct method. The most common one is the use of biotin to conjugate the antibody, and then the biotin will bind to the receptor of either the labeled avidin or streptavidin. With the second layer of labeling, the staining becomes amplified.
Indirect or Sandwich Method
The indirect method includes two major procedures: The indirect labeled procedure and the indirect unlabeled antibody procedure. The first procedure uses two layers of antibodies; the unlabeled primary antibody directly reacts to the tissue antigen, and the labeled secondary antibody reacts to the primary antibody (Fig. 3.2). The unlabeled antibody procedure uses three layers of antibodies (Fig. 3.3). The tertiary antibody is an anti-enzyme antibody that will conjugate with a specific enzyme, such as peroxidase, alkaline phosphatase, or glucose oxidase. When the enzyme reacts to its specific substrate in the system, a color product
will be demonstrated. The indirect method is generally more sensitive than the direct method, mainly because the polyvalent secondary antibody is able to detect multiple sites on the Fc and Fab portions of the primary antibody, and the primary antibody is more accessible to the secondary antibody than to the tissue antigen, which is being modified by fixation and embedding (16).
will be demonstrated. The indirect method is generally more sensitive than the direct method, mainly because the polyvalent secondary antibody is able to detect multiple sites on the Fc and Fab portions of the primary antibody, and the primary antibody is more accessible to the secondary antibody than to the tissue antigen, which is being modified by fixation and embedding (16).
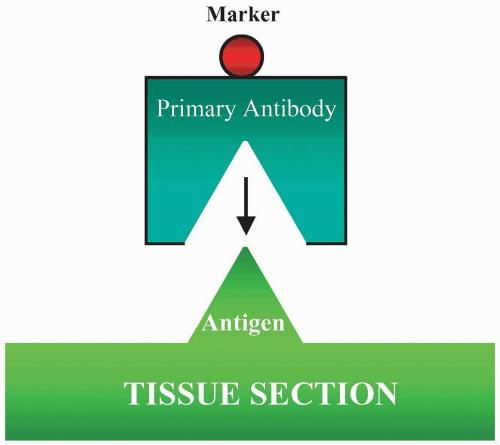 FIGURE 3.1 Direct method is to apply a labeled antibody directly to tissue sections. The marker then demonstrates the cellular location of the antigen in the section. |
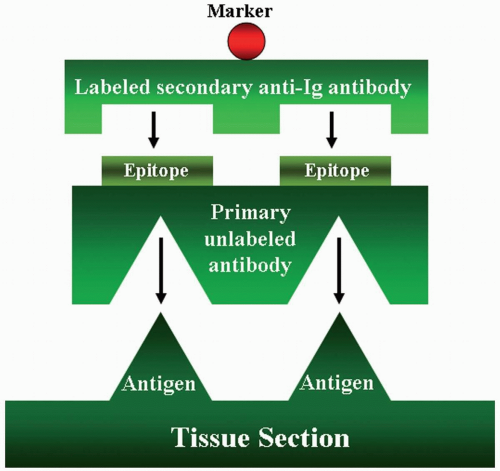 FIGURE 3.2 Indirect labeled method has two layers of antibodies. The primary unlabeled antibody is specific for a particular tissue antigen. The secondary labeled antibody is an anti-immunoglobulin antibody. The marker then identifies the sites of the tissue antigen.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|