Key points
- •
Although relatively rare, cholangiocarcinoma is the second most common primary hepatobiliary malignancy in the United States, and its incidence is increasing.
- •
Surgical treatment remains the cornerstone of curative therapy. Resection frequently requires a major procedure but can be done with relatively low morbidity and near zero mortality.
- •
Locoregional therapies have an important role in the management of cholangiocarcinoma, with radiation therapy more commonly used for hilar cholangiocarcinoma and intraarterial therapy possibly having a role for intrahepatic cholangiocarcinoma.
- •
The prognosis of cholangiocarcinoma remains poor. Five-year survival following resection ranges from 25% to 40%. More effective systemic and locoregional therapies are needed.
Introduction
Cholangiocarcinoma is an epithelial carcinoma of the bile ducts, which can be divided anatomically into intra- and extrahepatic. Intrahepatic and extrahepatic cholangiocarcinoma are distinct entities that have different considerations with regard to diagnosis, staging, and treatment. Intrahepatic cholangiocarcinoma (ICC) arises directly from the hepatic ducts within the liver, whereas extrahepatic cholangiocarcinoma (ECC) can include both hilar and distal bile duct cancers. We herein review the management of ICC and extrahepatic hilar cholangiocarcinomas, also known as Klatskin tumors.
Epidemiology and risk factors
Cholangiocarcinomas are relatively rare in the United States. Cholangiocarcinoma is, however, the second most common primary hepatobiliary malignancy after hepatocellular carcinoma. , Around 25% of cases involve the common/hepatic bile duct, and the remaining are intrahepatic, nonperihilar tumors. , Cholangiocarcinoma is the ninth most common gastrointestinal cancer and it accounts for approximately 13% of cancer-related deaths worldwide (3% in the United States).
The incidence of cholangiocarcinoma varies widely with geography. The reported incidence is highest in Southeast Asia (96 per 100,000) and lowest in Australia (0.1 per 100,000). In the United States, the incidence is approximately 1 per 100,000 for both ICC and ECC. , The incidence of ICC has been increasing over the past 30 years worldwide, , , and this increase does not seem to be solely attributable to improvements in diagnostic modalities or better pathologic characterization of the disease. , In the United States, age-adjusted incidence of ICC increased from 0.32 in 1975 to 0.85 per 100,000 in 2000, and has not yet reached a plateau. At the same time, incidence of ECC has declined.
The prevalence of cholangiocarcinoma is approximately equal between the two genders, with a slightly higher prevalence in males. , Almost all patients are older than 50 years at the time of diagnosis, with most patients in the United States diagnosed in their sixth decade of life. Asian Americans and Hispanics have a 2.5 times and 1.8 times, respectively, increased incidence of cholangiocarcinoma compared with non-Latino whites in the United States.
Cholangiocarcinoma is associated with conditions that result in chronic biliary inflammation and cholestasis. For example, the risk of cholangiocarcinoma has been associated with primary sclerosing cholangitis and inflammatory bowel disease, biliary fluke infestations, hepatitis (due to hepatitis B and C virus or alcoholic steatohepatitis), and cirrhosis. In addition, biliary malformations, hepatolithiasis, carcinogens (i.e., thorotrast), as well as chronic conditions like diabetes mellitus or HIV infection have also been reported to be associated with a higher risk of cholangiocarcinoma. , , ,
In the United States, one of the most common risk factors for cholangiocarcinoma is primary sclerosing cholangitis (PSC). PSC can cause fibrosis and stricture formation. Patients with PSC often present with cholangiocarcinoma at a younger age—often up to two decades earlier than non-PSC patients. On average, the diagnosis of cholangiocarcinoma usually occurs 2.5 years after the diagnosis of PSC. , In contrast, the increased incidence of cholangiocarcinoma in Southeast Asia is associated with increased infection rates with Clonorchis sinensis or Opisthorchis viverrini . The World Health Organization has labeled both of these organisms as grade 1 carcinogens. Humans can become infected by both types of flukes through ingestion of raw or undercooked seafood. ,
Patients with malformations such as choledochal cysts or Caroli disease are also at increased risk of cholangiocarcinoma. Choledochal cysts are cystic dilations of the biliary tract, and Caroli disease is associated with multiple intrahepatic cysts. Both are associated with an approximately 10%–15% lifetime risk of cholangiocarcinoma. , Hepatolithiasis is another well-recognized risk factor for cholangiocarcinoma. In these cases, the stones are usually found in proximity to the tumor site. Some authors have reported a 10% lifetime risk of cholangiocarcinoma with hepatolithiasis. , Thorotrast, a past radiocontrast agent, and dioxins also increase the risk of cholangiocarcinoma. Both hepatitis B and hepatitis C are considered potential risk factors for ICC but not ECC.
Tumor markers and molecular pathogenesis
The biomarkers carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), and carbohydrate antigen 125 (CA 125) are often used during the diagnostic workup for cholangiocarcinoma. These markers are, however, not specific for cholangiocarcinoma and may be elevated in other conditions, including cholangitis, hepatolithiasis, or other malignancies such as colorectal cancer. The sensitivities of CA 19-9, CEA, and CA 125 have been reported to be less than 70%. The sensitivity and specificity of CA 19-9 is higher among patients with PSC at 78.6% and 98.5%, respectively. However, even in PSC, CA 19-9 does not prove to be an effective screening tool. In areas with high incidences of liver fluke infestations, serum or stool tests, as well as urinary 8- oxo -7,8-dihydro-2′-deoxyguanosine levels may be potential screening tests.
Biliary fluke infestation and hepatolithiasis have aided in the study of the molecular pathogenesis of cholangiocarcinoma. Specifically, biliary flukes can cause mechanical and toxic damage to bile ducts, causing chronic inflammation of the biliary epithelia leading to carcinogenesis. Similarly, hepatolithiasis can also cause biliary inflammation and bile stasis, leading to increased exposure of carcinogenic bile acids. Biliary injury and inflammation leads to the release of cytokines, which increases the production of nitric oxide (NO) and reactive nitrogen oxide species (RNOS) through the expression of inducible nitric oxide synthase in epithelial cells. NO and RNOS can cause DNA mutations and inactivate DNA repair proteins. Through a multistep process, normal biliary epithelium can be transformed into hyperplastic, dysplastic, and eventually malignant epithelium. Notably, two distinct precursors have been identified for cholangiocarcinoma: biliary intraepithelial neoplasia and intraductal papillary mucinous neoplasia of the liver. , Several growth factors or cytokines have been found to increase biliary proliferation. These factors include hepatocyte growth factor, transforming growth factor α (TGF-α), endothelial growth factor, leukocyte inhibitory factor, and interleukin 6 (IL-6).
Pathologic and morphologic classification
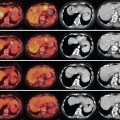
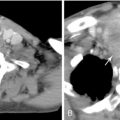
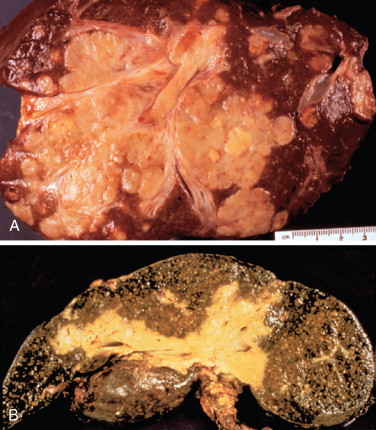
There have been many classification systems proposed to describe the pathologic and gross appearance of ICC and ECC. For ICC, the Liver Cancer Study Group of Japan’s (LCSGJ’s) classification scheme proposed dividing cholangiocarcinoma into three morphologies: mass forming, periductal infiltrating, and intraductal growth types ( Figure 10-1 ). , In the mass-forming type, a nodular, gray-white lesion or mass is typically found in the hepatic parenchyma ( Figure 10-2 , A ). The mass is generally solid and well defined, with significant variations in size. The center of the mass can often be necrotic or fibrotic, with rare calcifications. The periductal infiltrating type grows along the bile duct wall and appears as a concentric thickening that is ill defined and frequently not recognized ( Figure 10-2 , B ). The bile ducts may appear to be strictured, narrowed, or obstructed, resulting in stasis of bile. The liver often appears green and enlarged. Both the intra- and extrahepatic bile ducts are often involved in the periductal-infiltrating type. Intraductal-growing cholangiocarcinoma is characterized by intraluminal papillary tumors of the bile ducts that partially obstruct and dilate the ducts. The projections form sessile or polypoid masses covered with columnar epithelial cells. The papillary tumors produce mucin that is often retained within the tumor or may leak out into the bile duct, leading to obstructive jaundice in excessive cases. Traditionally, ECC has been described as nodular, sclerosing, and papillary, corresponding to mass-forming, periductal infiltrating, and intraductal-growing types of intrahepatic cholangiocarcinoma, respectively.
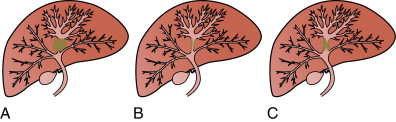
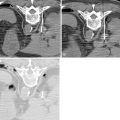
In contrast, hilar cholangiocarcinomas are typically classified anatomically based on the location within the biliary tree using the Bismuth-Corlette classification, types I-IV (see Figure 11-2 ). , Type I cholangiocarcinoma begin distal to the confluence of the right and left hepatic ducts, involving the common hepatic duct. Type II cholangiocarcinoma affect the confluence of the right and left hepatic ducts. Type IIIa cholangiocarcinoma involve the junction of the right and left hepatic ducts along with part of the right hepatic duct, and type IIIb also involves the junction and part of the left hepatic duct. Type IV cholangiocarcinoma include tumors that are multifocal or affect the union of the right and left hepatic ducts along with both the right and left hepatic ducts.
Overview: Intrahepatic cholangiocarcinoma
Presentation and diagnosis
The presentation of ICC is often nonspecific and delayed. Symptoms may include abdominal pain, fever, night sweats, cachexia, and general malaise. Symptoms of biliary obstruction, such as jaundice and pruritus, are uncommon in ICC and usually signify very advanced disease. Often asymptomatic patients are diagnosed during routine evaluation of liver function tests. ,
The diagnosis of cholangiocarcinoma relies on a combination of laboratory, cytology, and imaging studies. Laboratory studies include liver function tests, which can indicate biliary tract obstruction with elevated bilirubin, as well as possible elevation in alkaline phosphatase or γ-glutamyltransferase; however, it is not uncommon for patients to have a normal battery of laboratory exams. CA 19-9 may be useful, especially among patients with PSC. As noted, one study of 218 PSC patients reported sensitivity and specificity of 78.6% and 98.5%, respectively, using a CA 19-9 cut-off value 129 U/mL. However, a different study using the same cut-off reported a 37% false-positive rate. Other cut-off points have yielded similar results in two smaller studies. A CA 19-9 >180 U/mL had a sensitivity of 66.7% and specificity of 97.7% in a study of 55 PSC patients, whereas the respective values in a study of 37 PSC patients were 89% and 86% for a CA 19-9 >100 U/mL. , For patients without PSC, the sensitivity and specificity were 53% and 76% for CA 19-9 >100 U/mL. CEA can also be elevated in patients with intrahepatic cholangiocarcinoma.
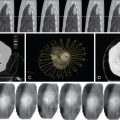
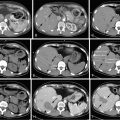
Both computed tomography (CT) and magnetic resonance imaging (MRI) are used routinely in the evaluation of cholangiocarcinoma. However, their specificity is low. Dynamic CT or MRI may be useful in distinguishing between ICC and hepatocellular carcinoma (HCC). Approximately 80% of ICC will take up contrast progressively throughout both the arterial and the venous phase, whereas HCC enhances rapidly during the arterial phase and washes out quickly in the delayed venous phase. The utility of positron emission tomography (PET) in the workup of ICC remains unclear. One study showed that in 4 of 11 (36%) patients with ICC, PET was able to identify distant metastases that were not found on CT or MRI, even though it was not superior in detecting regional lymph node metastases. Similar results were seen in a study from the Memorial Sloan-Kettering Cancer Center (MSKCC), which reported that PET scan identified metastatic disease in 24% of patients that eventually altered management. PET-CT does not appear to be superior in assessing the primary liver lesion. Evaluation of extrahepatic disease is extremely important in the workup process and may drastically affect the management plan, and therefore PET should be strongly considered ( Figure 10-3 ).
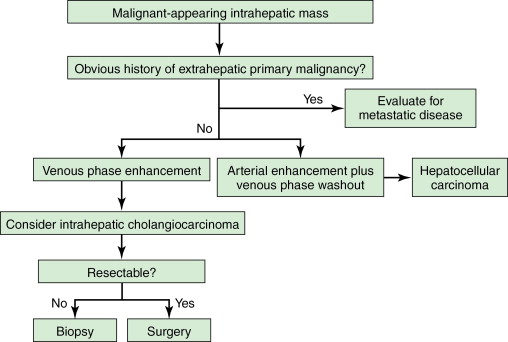
Staging
There is no consensus on the best staging system for ICC. Although factors such as tumor number, tumor differentiation, lymph node metastases, vascular invasion, and preoperative CA 19-9 have all been shown to be associated with survival, the effect of other factors such as tumor size and serosal invasion on survival is controversial. Currently, there are three main staging systems utilized for ICC: the American Joint Cancer Committee/Union Internationale Contre le Cancer (AJCC/UICC) TNM (tumor, node, metastasis) staging system seventh version ( Table 10-1 ), the LCSGJ, and the National Cancer Center of Japan (NCCJ) staging system. The biggest difference between the three staging systems resides in the disparate criteria for the T stages ( Table 10-2 ).
| TNM classification | |
|---|---|
| Tx | Primary tumor cannot be assessed |
| T1 | Solitary tumor without vascular invasion a |
| T2a | Solitary tumor with vascular invasion a |
| T2b | Multiple tumors, with or without vascular invasion a |
| T3 | Tumor perforating the visceral peritoneum or involving local extrahepatic structures by direct invasion |
| T4 | Tumors with periductal invasion b |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis c |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Stage groupings | |
| Stage I | T1 N0 M0 |
| Stage II | T2 N0 M0 |
| Stage III | T3 N0 M0 |
| Stage IVA | T4 N0 M0, any T N1 M0 |
| Stage IVB | Any T Any N M1 |
a Includes major vascular and microvascular invasion.
b Includes tumors with periductal-infiltrating or mixed mass-forming and periductal-infiltrating growth pattern.
c Nodal involvement of the celiac, periaortic, or caval lymph nodes is considered to be distant metastasis (M1).
| NCCJ Okabayashi et al. | LCSGJ Nathan et al. | AJCC/UICC 6th Edition | AJCC/UICC 7th Edition | |
|---|---|---|---|---|
| Primary Tumor (T) | ||||
| T1 | Solitary tumor without VI | Solitary tumor without VI, any size | Solitary tumor without VI, any size | Solitary tumor without VI |
| T2 | Solitary tumor with VI | Multiple tumors and/or VI, any size | Solitary tumor with VI, or multiple tumors <5 cm | T2a: Solitary tumor with VI |
| T2b: Multiple tumors with or without VI | ||||
| T3 | Multiple tumors with or without VI | Extrahepatic extension | Multiple tumors >5 cm or major VI | Direct invasion of adjacent organs (except gallbladder) or with perforation of visceral peritoneum |
| T4 | Direct invasion of adjacent organs (except gallbladder) or with perforation of visceral peritoneum | Periductal invasion |
Nathan et al. showed that the T stages of the AJCC/UICC sixth edition staging system failed to correlate well with survival and therefore proposed a new staging system, which was subsequently adopted in the seventh edition of the AJCC/UICC. A recent independent study validated the seventh version of the AJCC/UICC system showing a strong correlation between the new proposed T stages and survival in nonmetastatic ICC. Other groups such as the LCSGJ have proposed alternative staging systems using tumor size >2 cm, multiple tumor nodules, and vascular/serosal membrane invasion as negative prognostic factors. However, the LCSGJ staging system has been shown to have poor prognostic discriminatory power. Similarly, another staging system proposed for mass-forming ICC that was based on regional lymph node metastases, multiple tumors, symptom manifestations, and vascular invasion was shown to be somewhat poor. As such, the seventh edition AJCC/UICC staging system is currently the preferred staging system for ICC prognosis.
Surgical treatment
The use of staging laparoscopy at the time of surgery in order to detect metastatic disease remains controversial. Several groups have shown evidence of potential benefit for patients who undergo staging laparoscopy and have advocated its routine use. In a French study, the authors showed that 36% (4/11) of ICC patients were found to have metastatic disease upon laparoscopy. They noted 67% accuracy for detection of hepatic and peritoneal metastasis in patients with ICC, since 4 of the remaining 7 were found to have unresectable disease owing to peritoneal metastasis, vascular involvement, or distal lymph node spread at the time of laparotomy. Both the yield and the accuracy for ICC exceeded that for hilar cholangiocarcinoma; however, laparoscopy was unable to detect lymph node metastasis or vascular invasion, which contributed to a significant false-negative rate. In a separate study from the MSKCC, the authors reported a 27% yield for laparoscopy. Metastatic disease was found in 6 of 22 (27%) patients, whereas 5 of 11 (45%) with unresectable disease remained unidentified with laparoscopy. Despite these studies, the evidence to support routine use of laparoscopy to evaluate patients with ICC is still lacking.
Complete surgical resection is the only option that offers a potential cure for ICC. The contraindications to surgical resection for ICC are similar to most other cancers involving the liver: diffuse bilobar involvement, peritoneal and/or distant metastases, presence of major vascular invasion, advanced fibrosis/cirrhosis, or a small liver remnant. , In addition, presence of retropancreatic or paraceliac nodal metastases and direct invasion of adjacent organs are contraindications for surgery.
At the time of surgery, resection often requires a major procedure. Specifically, anywhere from 50% to 80% of resectable patients will require an extended hepatectomy in order to achieve an R0 resection. In addition, approximately 20%–40% will also need bile duct resection and reconstruction. , The role of routine lymphadenectomy was traditionally controversial, but now many groups advocate for it. , De Jong et al. reported that up to one-third of patients may have lymph node metastasis and that therefore lymphadenectomy may not only help with local control but also provide important prognostic information. Similar to other solid gastrointestinal-hepatopancreatobiliary malignancies, lymphadenectomy probably does not provide a substantive survival benefit. Shimada et al. noted no difference in survival or overall recurrence between patients who underwent routine lymphadenectomy and those who did not. Another study comparing patients who underwent routine formal lymphadenectomy versus patients who had lymph node sampling found no difference in overall survival. However, because of the staging and prognostic value of lymph node metastasis, as well as possible decrease in locoregional recurrence, routine lymphadenectomy at the time of resection should be strongly considered.
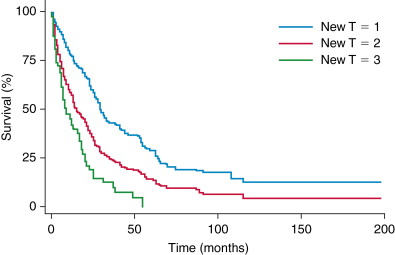
Survival following resection of ICC has been reported to range from 25% to 40%. , , Poor prognostic indicators include lymph node metastasis, positive margins, vascular invasion, multiple tumor sites, periductal-infiltrating types, bilobar disease, and elevated markedly elevated CA 19-9. , Survival based on AJCC seventh edition T stage is shown in Figure 10-4 .
Locoregional therapy
The role of adjuvant radiation therapy in the treatment of ICC is undetermined. Although a few retrospective studies have suggested a benefit for patients with completely resected cholangiocarcinoma, others have not found a benefit. Interpretation of these studies is difficult given their retrospective nature and because patients with ICC and ECC are often included. As such, routine adjuvant radiation therapy is not commonly utilized, but should be strongly considered in patients with positive surgical margins. Other types of locoregional therapy have not been well studied.
Several small studies have examined the role of intraarterial therapy for unresectable ICC and have shown some modest improvement in survival. Because most patients with ICC present with large tumors that may be central in location, thermal ablation is often not a therapeutic option. External beam radiotherapy may be beneficial for some patients with unresectable disease. One study found that the use of radiation therapy for inoperable disease was associated with some survival benefit, whereas a different study reported that radiotherapy also can lead to improved quality of life and relief of jaundice in some patients with ICC.
Systemic therapy
Because of the high number of patients who present with locally advanced or metastatic disease, there is a significant need for effective systemic therapy for cholangiocarcinoma. The traditional single agents used include 5-fluorouracil (5-FU), gemcitabine, oxaliplatin, and docetaxel. Recently, a phase III randomized controlled trial evaluating chemotherapeutic agents in biliary tract cancers known as the ABC-02 trial was reported. This study noted that the combination of gemcitabine plus cisplatin significantly increased progression-free survival compared with gemcitabine-only group (11.7 months vs. 8.1 months, respectively). Adverse effects were similar between the two groups except for an increase in neutropenia in the combination therapy group, which did not lead to increased neutropenia-associated infection. The combination of gemcitabine plus a platin-based therapy is now considered the standard of care for treatment of metastatic and locally advanced cholangiocarcinoma for most patients. Oxaliplatin may be used in the place of cisplatin in patients with poor performance status; other gemcitabine combinations under investigation include gemcitabine–capecitabine , and gemcitabine–irinotecan.
The role of such treatment in the adjuvant therapy for patients with resected disease is less clear. Adjuvant therapy should be considered in patients with lymph node metastasis, given the higher incidence of recurrence and worse overall prognosis for this subset of patients.
Overview: Hilar cholangiocarcinoma
Presentation and diagnosis
In contrast to the often asymptomatic presentation of ICC, patients with hilar cholangiocarcinoma frequently present with jaundice, pruritus, dark urine, pale stools, and sometimes cholangitis. , Symptoms of fatigue, malaise, and weight loss may also be part of the presenting symptomatology. Whereas laboratory exams can be normal in many patients with ICC, most patients with hilar cholangiocarcinoma will present with elevations in direct and total serum bilirubin, alkaline phosphatase, and sometimes γ-glutamyltransferase. Transaminase levels and prothrombin time may initially be normal, although later in the course of the disease these studies can increase due to chronic hepatocellular damage. As discussed in the diagnosis of ICC, tumor markers can be indicative, but not diagnostic of cholangiocarcinoma. CA 19-9 has a sensitivity and specificity of 76% and 92%, respectively, in determining the difference between nonmalignant and malignant strictures. The addition of CEA can also improve detection.
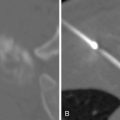
Diagnosis of hilar cholangiocarcinoma relies on multiple methods of assessment ( Figure 10-5 ). Ultrasonography is often the first mode of imaging used to evaluate a patient with jaundice, but is of little help except in identifying biliary dilatation. Cross-sectional studies are essential. Imaging with contrast is necessary for determination of vascular involvement, location of the bile duct obstruction, and tumor extent. It is usually preferable to obtain cross-sectional imaging prior to stenting, as bile duct stents can obscure definition of the extent of disease. It is also important to determine the existence and extent of hepatic atrophy due to biliary obstruction and portal vein encasement, which can be suggestive of a malignancy. CT has traditionally been the initial cross-sectional imaging obtained and was traditionally deemed inferior to MRI. With the introduction of multi-detector CT (MDCT), the resolution and detection accuracy of CT has drastically improved. On CT imaging, hilar cholangiocarcinomas can appear as either a focal or ill-defined mass with vascularity changes ranging from hypovascular to hypervascular on delayed imaging. Most clinicians believe that MRI and magnetic resonance cholangiopancreatography (MRCP) are still superior to CT in the diagnosis and determination of resectability of hilar cholangiocarcinoma. In addition to evaluating the location of the stricture and the extent of vascular involvement, MRI can also determine the presence of choledocholithiasis and hepatolithiasis. MRCP can provide information similar to invasive procedures such as endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC). The accuracy of MRI combined with MRCP is generally high, from 78% to 96%. , As with ICC, the use of PET scan in the management of hilar cholangiocarcinoma still remains to be elucidated. Although PET is not as accurate as CT in the detection of hilar cholangiocarcinoma, PET may be most useful in detecting metastasis not recognized by CT or MRI. Typically, PET scan is not employed as much for ECC as with ICC.