6 Chemoprevention of Colorectal Cancer
Introduction
Colorectal cancer is a leading cause of cancer-related deaths in the United States. Currently, the goal of screening programs is early detection of a preinvasive lesion or early cancer. An alternative or conjunctive approach is chemoprevention of colon cancer. Chemoprevention involves using drugs or natural compounds to inhibit or reverse carcinogenesis. Nonsteroidal anti-inflammatory drugs (NSAIDs), vitamins, hormones, and dietary factors have been implicated in primary prevention of colon cancer (Box 6-1).
Nonsteroidal Anti-inflammatory Drugs
NSAIDs, cyclooxygenase-2 (COX-2) inhibitors, and aspirin all have been studied in the prevention of colon cancer. Strong support for a chemopreventive effect of NSAIDs is provided by in vitro experiments, animal models, epidemiologic studies, and clinical trials.1 The mechanism of action of NSAIDs is inhibition of cyclooxygenase, which is a key enzyme in the conversion of arachidonic acid to prostaglandins, prostacyclin, and thromboxanes. Prostaglandins affect cell proliferation and tumor growth through activation of second messengers in signal transduction pathways, and prostaglandin levels are increased in many cancers including colorectal adenomas and adenocarcinomas.2 COX-1 is present in most tissues, whereas COX-2 is expressed in response to growth factors, cytokines, mitogens, and tumor promoters.3 COX-2 inhibition also has anti-angiogenic4 and apoptotic effects.5 COX-2 independent targets have been suggested as well, including β-catenin, transcription factor NF-κB, and transforming growth factor-β (TGF-β).6–8
Much of the initial data on NSAIDs and chemoprevention of colon cancer in humans came from literature on familial adenomatous polyposis (FAP), which is an autosomal dominant disorder characterized by innumerable colorectal adenomas and eventual carcinoma. Giardiello and colleagues9 conducted the first randomized controlled trial evaluating sulindac in patients with FAP who had not undergone colectomy. Twenty-two patients with FAP received either sulindac 300 mg daily for 9 months or placebo, and the number and size of polyps were evaluated every 3 months for 1 year. The group treated with sulindac had a statistically significant reduction in both the number and size of polyps when compared with the placebo group. Many studies have evaluated sulindac in FAP, and all report either complete or partial regression of adenomas after 3 to 6 months of treatment with sulindac at doses of 300 to 400 mg per day.10
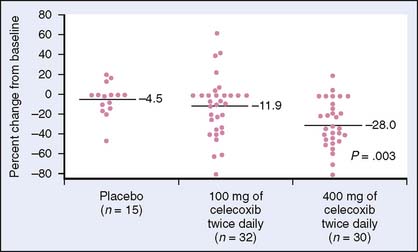
NSAIDs such as sulindac have gastrointestinal toxicity that may limit use for prevention in colorectal cancer. This has led to studies on COX-2 inhibitors. COX-2 is expressed in inflammatory states, premalignant lesions, and colorectal cancer. Thus, COX-2 inhibitors were speculated to have a role in the chemoprevention of colon cancer. Again, using patients with FAP, Steinbach and colleagues11 randomized patients to celecoxib at 100 mg or 400 mg twice daily or placebo for 6 months. Endoscopy done at the beginning and end of the 6 months demonstrated a significant reduction in the mean number of polyps and the polyp burden in the group receiving high-dose celecoxib (Fig. 6-1).
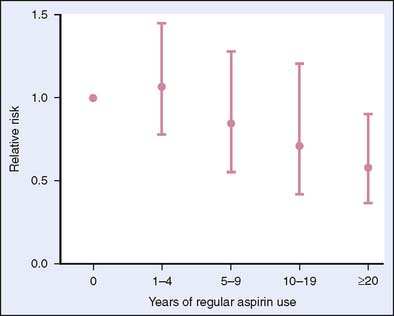
The finding that NSAIDs lead to polyp regression in patients with FAP led to investigation of NSAIDs in sporadic adenomas and colorectal neoplasia (see Box 6-1). Epidemiologic studies have shown a reduction in colorectal cancer in individuals taking NSAIDs.1,12 A series of case-control studies revealed a 40% to 50% reduction in the risk of colonic adenomas or colorectal cancer among patients taking aspirin.1 Thun and colleagues13 reported results of a large observational study on aspirin and colon cancer showing that aspirin reduced the relative risk of cancer mortality by 40% when consumed more than 16 times per month for at least 1 year. Giovannucci and associates14 measured cancer incidence in a cohort of women and found that regular aspirin use at doses similar to those recommended for cardiovascular disease prevention reduced the risk of colorectal cancer; however, this effect may require more than a decade of use to occur (Fig. 6-2). A controlled trial with the primary endpoint of cardiovascular events involved 22,071 male physicians randomized to aspirin 325 mg or placebo every other day for 5 years found no difference between the two groups in the incidence of colorectal cancer. These findings may be accounted for by the short treatment period.15
Two large randomized trials have evaluated the role of aspirin in colorectal adenoma recurrence. Baron and colleagues16 randomized 1121 patients (with removed polyps) to aspirin 81 mg or 325 mg daily or placebo. Ninety-seven percent of patients had a follow-up colonoscopy at least 1 year after randomization, and the incidence of adenomas was 38% in the low-dose aspirin group compared with 47% in the placebo group and 45% in the high-dose aspirin group. These investigations concluded that low-dose aspirin prevents recurrence of adenomas in the colon. Of note, a higher but not statistically significant incidence of stroke was found in the aspirin group. Also, the reason why higher-dose aspirin did not have the same effect as low-dose aspirin is unclear.
A second study randomized 635 patients with previous colorectal cancer to either aspirin 325 mg daily or placebo and found that the aspirin group had fewer adenomas and a longer time to detection of a first adenoma (Fig. 6-3).17 This study concluded that daily aspirin can significantly reduce the incidence of colorectal adenomas in patients with previous colorectal cancer. In a smaller randomized control trial, Benamouzig and colleagues18 showed that daily soluble aspirin was associated with a reduction in the risk of recurrent adenomas found at colonoscopy 1 year after beginning treatment (Table 6-1).
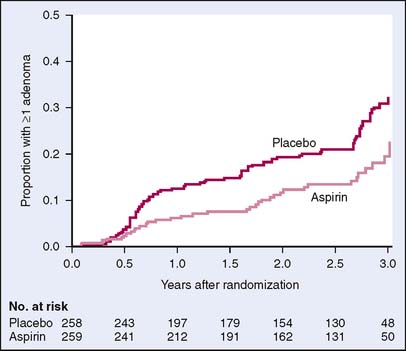
Figure 6-3 Kaplan-Meier estimates of the time to a first adenoma.
(From Sandler RS, Halabi S, Baron JA, et al: A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348:883–890, 2003, Fig. 1.)
Ursodeoxycholic Acid
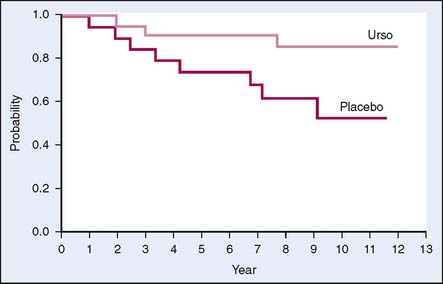
Another drug that may show benefits in chemoprevention is ursodeoxycholic acid (UDCA), also called ursodiol, a synthetic bile acid. Preclinical studies demonstrate that UDCA has chemopreventive effects. In a cross-sectional study on patients with ulcerative colitis and primary sclerosing cholangitis, the use of UDCA was associated with lower incidence of colonic dysplasia. The hypothesized mechanism of action is that UDCA reduces levels of the secondary bile acid deoxycholic acid, which is cytotoxic to colonic epithelial cells and induces hyperproliferation.19 Pardi and colleagues20 studied UDCA in a similar population and found that those taking the drug had a relative risk of 0.26 (95% confidence interval [CI], 0.06–0.92) for developing colorectal dysplasia or cancer (Fig. 6-4). A phase III, double-blind, placebo-controlled trial of UDCA was conducted to evaluate its ability to prevent colorectal adenoma recurrence. This trial found a nonstatistically significant reduction in total adenoma recurrence but did have a 39% reduction in recurrence of adenomas with high-grade dysplasia.21 Further investigation is needed to evaluate the role of UDCA in chemoprevention of high-grade dysplasia.
Hormones
Over the last 20 years, mortality from colon cancer has decreased slightly in both sexes but more prominently in women.22 From 1982 to 1992, there was a 2.3-fold increase in the use of oral menopausal estrogens and a 4.9-fold increase in oral medroxyprogesterone use.23 This increase in hormonal therapy led to the postulation that hormones may reduce the risk of colon cancer. Biologic evidence includes findings that estrogens decrease the production of secondary bile acids, which can promote malignant change in colonic epithelium. Also, estrogens decrease the production of insulin-like growth factor I, an important mitogen, which may exert a direct effect on colorectal epithelium.22 Epidemiologic studies of postmenopausal hormone replacement therapy (HRT) showed a 20% reduction (relative risk [RR] 0.80, 95% CI, 0.74–0.86) in risk of colon cancer in postmenopausal women who had ever taken HRT compared with women who had never used hormones.24
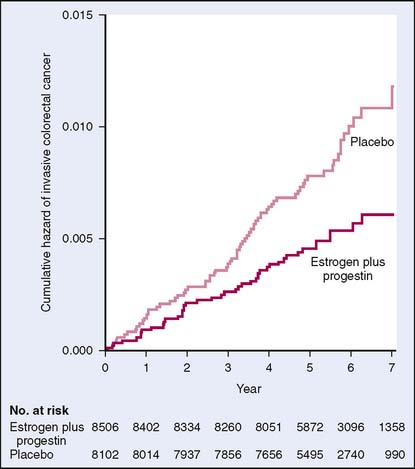
The Women’s Health Initiative (WHI) evaluated 16,608 postmenopausal women in a randomized controlled trial. Participants were given either estrogen .625 mg daily plus medroxyprogesterone acetate 2.5 mg daily or placebo. The primary outcomes of this study were coronary heart disease and adverse outcome of invasive breast cancer. A global index summarized the risks and benefits of HRT, including colorectal cancer. The study did show a hazard ratio for colorectal cancer of 0.63 (0.43–0.93), indicating a reduction in colorectal cancer in the HRT group. However, the study was stopped after 5.2 years of follow-up because of an increased risk of breast cancer, stroke, coronary disease, and venous thromboembolism.25 Using the data from the WHI, Chlebowski and colleagues26 showed a statistically significant decrease in colorectal cancer among users of HRT. However, patients who were diagnosed with colorectal cancer were more likely to have a more advanced stage of cancer than women taking placebo (Fig. 6-5). These findings were not easily explainable. Again using the WHI data, a randomized controlled trial evaluated use of estrogen alone in postmenopausal women with hysterectomy and found no significant differences in rates of colorectal cancer when compared with those with placebo.27 This study and others28 may suggest that progesterone, rather than estrogen, is the chemopreventive agent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree