CHEMICAL NEUROANATOMY
HORMONES AS CHEMICAL NEUROTRANSMITTERS
The hypothalamus is one of the most complicated areas of the brain with respect to chemical neurotransmitters because of the small amount of tissue occupied by the hypothalamus and the great number of transmitter substances located within hypothalamic nerve cells and associated fiber systems.12 The classic studies of Bargmann13 and of Scharrer and Scharrer,14 who are credited with describing the principles of neurosecretion, brought to light the unique chemical characteristics of neurons of the supraoptic and paraventricular nuclei. The introduction in the early 1960s of the Falck-Hillarp histofluorescence method15 allowed identification of the catecholamines dopamine and norepinephrine within the hypothalamus. Most notable were the dopaminergic neurons of the tuberoinfundibular system, which are involved in regulating the release of anterior pituitary substances.16 A decade later, immunohistochemical methods17 increased the list of known hypothalamic chemical neurotransmitters and modulators to include substances such as luteinizing hormone–releasing hormone, corticotropin-releasing hormone, vasoactive intestinal peptide, neurotensin, somatostatin, enkephalin, endorphin, cholecystokinin, galanin, and several others.18,19 Although the discovery of luteinizing hormone–releasing hormone20 and somatostatin within hypothalamic, preoptic, and adjacent regions of the endocrine hypothalamus was not surprising, the finding of “gut peptides” and the discovery of a complex system of opioid neurons21 have redefined the hypot halamus, based on the chemical cytoarchitecture of transmitters and hormones.18,21a
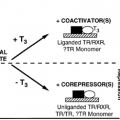
The application of in situ hybridization techniques to localize messenger RNA for these peptides indicates that this wide array of peptides is synthesized in the hypothalamus and that neurons have the capacity to synthesize multiple regulatory peptides simultaneously.22 The specific peptides produced by a given neuron are not static but depend on the stimuli received by that cell.22,23 For example, hypophysectomy dramatically increases the expression of galanin in the vasopressin neurons of the supraoptic nucleus and of cholecystokinin in the oxytocin neurons, but salt loading induces the expression of tyrosine hydroxylase in the vasopressin neurons and of corticotropin-releasing hormone in the oxytocin neurons.24 The role of these simultaneously released peptides is not completely defined, but in at least some instances, they interact to regulate hormone release from the anterior pituitary or to modulate hormone release from the posterior pituitary.
VASOPRESSIN RELEASE AS A MODEL OF INTERACTIVE CHEMICAL CIRCUITRY
NOREPINEPHRINE REGULATION OF VASOPRESSIN AND OXYTOCIN
The role of afferents to the paraventricular and supraoptic nuclei is considered relative to the regulation of vasopressin release as exemplary of the kind of functionally interactive chemical circuitry that is being revealed with respect to hypothalamic neuroanatomy. The supraoptic and paraventricular nuclei receive dense, diverse afferent inputs, many arising from the brainstem reticular formation. One of these is a well-defined system of noradrenergic afferents to vasopressin neurons of the supraoptic and paraventricular nuclei (Fig. 8-8). The paraventricular nuclei in turn send reciprocal peptidergic fibers to the reticular core of the brainstem. Norepinephrine-containing perikaryal groups in the brainstem have been designated A1 to A7.25 These noradrenergic neurons originally were seen primarily within the reticular formation of the pons and medulla. Later, attention focused on groups A1, A2, A5, and A7 as projecting to the hypothalamus through a ventral pathway that ascends within the dorsal portion of the reticular formation of the brainstem, entering the medial forebrain bundle at hypothalamic levels in association with serotonergic and dopaminergic systems from the brainstem.26 On reaching the hypothalamus, these fibers exit the medial forebrain bundle to supply the supraoptic and paraventricular nuclei. The densest patterns of noradrenergic fibers—perhaps the densest patterns in the entire brain—are seen in the mammalian hypothalamus27,28 (Fig. 8-9). These fibers appear in contact with the cell bodies of the magnocellular neurons, lending further support to the concept that norepinephrine plays a role in the regulation of neurohypophysial peptides.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree