Fig. 14.1
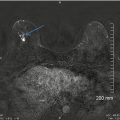
Intraductal papilloma in a 44-year-old female with a spontaneous unilateral serous nipple discharge. (a) Mediolateral oblique view of the left breast demonstrates a branching subareolar tubular density with calcifications. (b) Craniocaudal view showing a similar finding. (c) Ultrasound image of the subareolar region showing a distended duct with an intraductal mass in the radial plane. (d) Ultrasound image of the subareolar region showing a distended duct with an intraductal mass in the antiradial plane
Follow-up After Concordant Percutaneous Biopsy
Calcifications
For benign concordant pathologic results, a single 6-month follow-up is adequate with magnification views to ensure that calcifications are stable [16]. In case of a specific diagnosis such as a fibroadenoma, a 12-month follow-up may be sufficient. At follow-up if there is no increase in the number of calcifications or a change to a suspicious morphology, no further follow-up is warranted.
Masses
There is no consensus on follow-up after a benign concordant biopsy. Some advocate that no follow-up is needed, while others recommend a 6-, 12-, and 24-month follow-up;, the latter seems excessive. At our institution a single 6-month follow-up is performed for benign concordant histology. If there is a significant increase in the size of the mass or if there are new morphologic features that are suspicious such as marginal irregularity, excisional biopsy is appropriate [16].
Aspiration of Cysts
A simple cyst is a benign finding and requires intervention only for symptomatic relief. Complicated cysts are those which do not fulfill all of the criteria of a simple cyst such as when internal echoes or septations are seen within a cyst or when there is no increased through transmission. These are mostly benign and may not require aspiration. In one series only 1 of 243 lesions (0.4 %) proved malignant; this lesion was 1 of 33 complicated cysts that did not yield fluid [17]. Even when cytology yields atypical cells, the final histology is mostly benign [17]. In a large series of 6,782 cyst aspirations over a 7-year period, the incidence of intracystic papillomas was 5 [0.1 %] [18]. All cases of papilloma showed blood-stained fluid. Overall only 2 % of cyst fluids were blood stained. Cytology of six cases of papilloma was positive in two, negative in two, and falsely positive in two cases [18]. These investigators recommended cytology only when aspirate is blood stained. A cyst that demonstrates thick indistinct walls, thick internal septations, or mixed solid and cystic components requires core biopsy sampling. Clustered microcysts and septate cysts are generally benign [19]. A recently published large series of 5,375 aspirations performed over a 16-year period of noncomplex cysts reported a malignancy rate of 0.3 % [20]. Atypical cytology revealed malignancy in 21 % of cases. All atypical results should undergo further workup. Malignant cytology revealed malignancy in 91 % of cases and hence all patient with malignant histology need to undergo biopsy [20].
Dense Breast Law
Sensitivity of mammography in women with dense breasts has been reported to be as low as 48 % [21]. About 41 % of women may have dense breasts on a mammogram [22]. Increased breast density is an independent risk factor for breast cancer and increased the risk by a factor of 5 [23]. Supplemental ultrasound has been shown to detect additional cancers in women with dense breasts in those with an elevated risk as well in those with an average risk [24–27].
Recently based on these facts and a public campaign undertaken by a breast cancer survivor, several states have passed a law called the “Henda’s law” or the “dense breast law” requiring women with dense breasts to be informed by their clinician about their breast density and discussing the option of undergoing supplemental screening depending on their risk factors.
The ACRIN 6,666 trial showed that 4.2 additional cancers were identified by ultrasound in women with an elevated risk for breast cancer [24]. The dense breast law passed in Connecticut requires notification of women with a greater than 50 % density and recommendation for supplemental screening ultrasound; the law also required insurance to pay for supplemental ultrasound screening. A study from Connecticut looking at such women showed that ultrasound lead to an additional yield of 3.25 cancers per 1,000 in women with dense breasts, normal mammograms, and no additional risk factors [25]. Although the NPV [99.9 %] and sensitivity was very high [96.6 %], the positive predictive value was low at 6.7 % [25]. Yet in another study of 5,519 women with dense breasts who underwent sonographic screening, the supplemental yield was only 1.8 per 1,000 and positive predictive value was low at 5.5 %, and mean tumor size was 9.7 mm [26]. Post enactment of the Connecticut law, a study that looked at women with low risk [614/935], intermediate risk [149/935, 15.9 %], and high risk [87/935, 9.3 %] found one cancer in each of the three groups. All of these three cancers were small solid masses in postmenopausal women for a cancer detection rate of 3.2 per 1,000 women screened again with an expected low positive predictive value of only 6.5 % [27].
Double Reads
About half of the countries that use screening mammography have implemented double reading, although direct evidence of its effectiveness in the context of a national screening program is lacking [28]. Analysis of ten cohort studies showed that overall double reading increases the cancer detection rate by 3–11 per 10,000 women screened and most of the cancers thus found are small cancers. The effect on recall rate depended on the methodology. Double reading with unilateral recall increased the number of women recalled from 38 to 149 per 10,000 women screened. In programs where a consensus or arbitration policy was in place, the recall rate decreased between 61 and 269 per 10,000 women screened [28]. In a large majority of cases, double reads do not lead to disagreement; when there is one mutual consultation, this further diminishes the number of recall. In some facilities cases with disagreement are referred to an arbitration panel. The effectiveness of this methodology of referral to an arbitration panel has been studied. In a series from Netherlands involving screening of 65,779 women, there was concordance in the reads of double readers in 98.7 % of women, and there was agreement on the need for referral in 0.8 % of cases and disagreement on the need for referral in 0.5 % of cases which decreased to 0.3 % after mutual consultation. These 183 studies were referred to the arbitration panel which referred 89 of these for further workup that resulted in a cancer diagnosis in 20/89 [22 %]. Among the 94 cases that were not referred, there were 3 cancers [3 %] at the site of the discrepant mammographic findings [29]. Screening mammograms with discrepant findings form a small but significant subset that may lead to a diagnosis of breast cancer.
Double Reads vs Single Reads With CAD
The effectiveness of double reading has been compared to single reader using a CAD [computerized aided detection] by several investigators [30–32]. In a study of 10,267 mammograms, single reading with CAD led to a cancer detection rate that was higher albeit with a higher recall rate of 8.6 % vs 6.5 % achieved with a double read [30]. The cancer detection rate though increased in the CAD group by 15 %. Others have reported similar results. A meta-analysis of ten studies that looked at efficacy of single readers using CAD vs single readers’ found that CAD did not significantly increase the cancer detection rate and increased the recall rate. The same report in a meta-analysis of 17 studies that assessed the value of double reads over single reads found that double reads increased both cancer detection rate and the recall rates; however, double read with arbitration increased cancer detection rate with decreased recall [31]. A literature review of six studies that compared single reads with CAD vs double reads showed that three of these studies did not show any differences in sensitivity or specificity: one showed increased sensitivity with same specificity, one showed higher specificity with the same sensitivity, and one had higher sensitivity with lowered specificity [32].
Cost-Effectiveness of Double Reading
The cost-effectiveness resulting from double reading has also been studied [33, 34]. Double reading followed by consensus involving 33,734 consecutive screening mammograms detected an additional 9 cancers per 10,000 women screened. A nonconsensus double reading policy detected an additional 10 cancers per 10,000 women screened. However, nonconsensus double reading resulted in a recall rate was significantly higher than single read; recall rate in consensus double read was significantly lower than with single reads. From a cost-effectiveness perspective consensus double read costs less than single reading (4,853 £ saved per 10,000 women screened) and nonconsensus double reading costs more than single reading (difference of 19,259 £ per 10,000 women screened) [33]. The cost-effectiveness in terms of the cost per cancer detected has also been studied. Data from 255,000 women from Scotland showed that costs per cancer detected by double reading compared to single reading range from $ 1,859 to $ 3,553 [34].
Clinical Breast Exam with Screening Mammography [35–37]
Clinical breast examination [CBE] in conjunction with screening mammography can be implemented concurrently when administered in a breast center by a registered nurse or when a screening mammogram is done following a well woman exam as is more often the case. The former has been studied to determine the added benefit of increasing cancer detection. There were 232,515 women in the group receiving CBE and 57,715 in the group undergoing screening mammogram without a clinical breast exam. Sensitivity in the CBE group was 94.9 % vs 88.6 % for the screened group without CBE. However, the false positive was also higher for those women who had CBE with screening mammography compared to those who did not receive CBE [12.5 % vs 7.4 %] [35]. Another large study with dual screening in 300,303 women, CBE increased the rate of detection of small invasive cancer by 2–6 %. Without the concurrent use of CBE, three cancers would be missed for every 10,000 screens [36]. The cost-effectiveness of offering CBE in a comprehensive breast center was reported in a cohort of 60,000 women who received CBE by a nurse practitioner. Four hundred and seventy four had a positive exam leading to a diagnostic evaluation. Forty-six cancers were identified, 32 of which would have been identified by mammography alone, and only 14 were not seen on a mammogram. The cost of CBE was 122,598 per cancer detected based solely on CBE findings [37].
Imaging of the Male Breast
A male breast is composed of subcutaneous tissue, atrophic ducts, and stromal elements with preponderance of fat [38]. Conditions that affect the male breast are therefore related to ductal and stromal proliferation and include the most commonly encountered gynecomastia, invasive ductal carcinoma, and papillary neoplasm. Most commonly men are referred for a breast lump, breast enlargement, or tenderness. Mammography is the initial imaging and may be the only modality needed. If abnormality cannot be imaged on mammography or if findings are questionable, sonography is indicated. The most common cause of breast symptoms are due to gynecomastia.
Gynecomastia
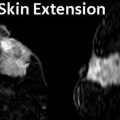
Gynecomastia is the most common breast problem in a male patient and has been reported to be between 87 and 90 % of cases [39, 40]. There are three patterns of gynecomastia, nodular, dendritic, and diffuse glandular. The nodular pattern represents the early florid phase of ductal and stromal proliferation and is seen in the first year of onset and accounts for 34–36 % of gynecomastia [39, 40]. At mammography it produces a fan-shaped subareolar density that blends into surrounding parenchyma (Fig. 14.2a–d). Ultrasound is not needed when mammographic appearance is characteristic. Ultrasound may show the area of gynecomastia as an irregular mass prompting a recommendation for a biopsy of a benign abnormality and should be avoided for this reason (Fig. 14.2d). The presentation is in the form of a painful mass and the process is reversible if the inciting factor is withdrawn [38]. The dendritic phase is the fibrotic quiescent phase characterized by stromal fibrosis and ductal proliferation. This accounts for 31–35 % of cases of gynecomastia [39, 40]. This represents irreversible phase of gynecomastia. Diffuse glandular type accounts for 31–33 % of cases and is seen in patients receiving estrogen therapy and represents a combination of dendritic and diffuse nodular types. Gynecomastia tends to be bilateral in 55–65 % of cases [39, 40].
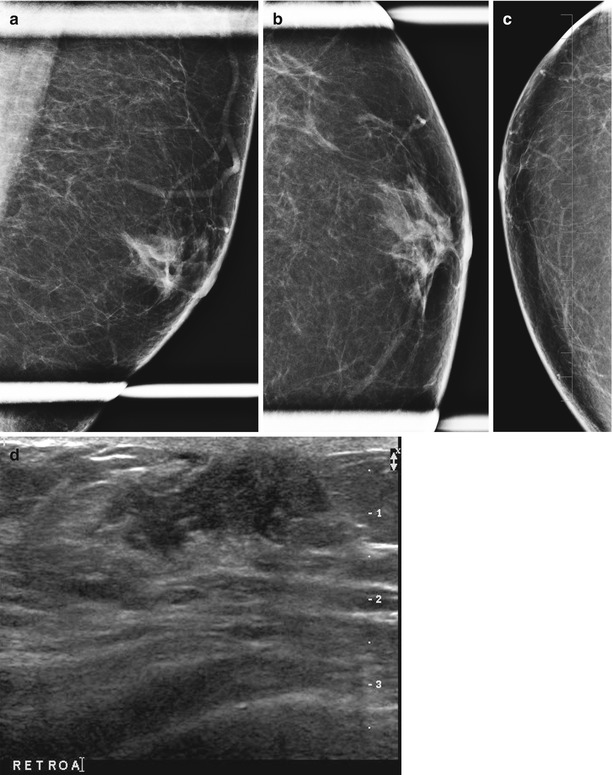

Fig. 14.2
A 65-year-old male with history of liver disease presenting with left breast swelling. (a) Mediolateral oblique view of the left breast demonstrates a fan-shaped subareolar density consistent with gynecomastia. (b, c) Craniocaudal views of the left breast demonstrate a fan-shaped subareolar density consistent with gynecomastia. (d) Ultrasound demonstrates an irregular hypoechoic mass-like abnormality representing gynecomastia
Male Breast Cancer
Breast cancer accounts for 1–8 % of symptomatic breast disease in males [39–41]. About 0.7 % of all breast cancers are diagnosed in men [38]. In 2010 based on cancer statistics, 1,970 new cases of male breast cancers were diagnosed [42]. Mass without calcifications is seen in 86 % of breast cancer cases in men and as calcifications in 7 % [39]. Mean size of the mass is 2.4 cm; prognosis is generally poor due to late stage of presentation. Risk factors for breast cancer in males include Klinefelter syndrome, BRCA1 or BRCA2 mutation, family history of breast cancer in a first-degree male or female relative, hyperestrogenism, exogenous estrogen for feminization purposes, advanced age, and history of chest radiation. Breast cancer typically presents at an age on an average 10 years later than in women, the mean age at diagnosis being 67 years [38]. The disease is often at an advanced stage at diagnosis with axillary node metastasis seen at initial evaluation in 50 % of cases [38]. Secondary signs of breast cancer occur earlier in the male breast because of the smaller size of the breast. These include nipple retraction, skin ulceration and thickening, and axillary adenopathy [41]. Cystic lesions in a male breast have to be worked up as potentially malignant since cystic lesions commonly demonstrate malignant findings. Breast cancer most often presents as a discrete mass with malignant features on a mammogram or ultrasound. The relationship of the mass to the nipple is helpful; an eccentric mass is highly suspicious for cancer [41].
The differential diagnosis of male breast includes gynecomastia, lipoma, epidermal inclusion cyst, pseudoangiomatous hyperplasia, and intraductal papilloma. The most common histological type of breast cancer is the infiltrating ductal cancer accounting for 80 % of all cancers, ductal carcinoma in situ accounts for 5 % of cancers, and rarer types include papillary cancer [38].
Overdiagnosis of Breast Cancer with Screening Mammography
The term overdiagnosis of breast cancer by screening mammography in population-based studies refers to the difference between cancer detection and subsequent treatment of abnormal findings and the corresponding effect on mortality. The percentage of overdiagnosis represents the estimated percentage of cases that were detected and treated but that would not have affected mortality if had been left alone. In other words mammography identifies cancers that are nonlethal and do not lead to mortality [43, 44]. Bleyer and Belch used a model for expectation values and estimated that 31 % of cancers that are diagnosed breast cancer represent overdiagnosis [43]. They then concluded that the reduction in mortality can be attributed to improvements in therapy and not to early diagnosis [43]. In an opinion article in response to this theory, Gur and Sumkin correctly point out that once a decision to screen is made, the role of a breast imager should be to detect disease early and towards this end due diligence is needed to detect and correctly diagnose all abnormalities at the earliest stage possible. It is then the responsibility of other specialties to make the best use of the information provided by the radiologist to decide how best to use the information in the appropriate management of the patient. They state that there can only be a “correct, partially correct or an incorrect diagnosis and there can only be optimally managed, suboptimally managed and mismanaged and over treated disease” [44]. They go on to appropriately state “There should not be any doubt that the overall objective of a screening program is to first and foremost detect, correctly diagnose, and appropriately treat early preclinical cancers that, if left alone, would become life threatening cancers” [44]. This seems to be a reasonable and appropriate response to the criticism of overdiagnosis of breast cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree