- Stem cells are self-renewing cells that possess the ability to generate daughter cells that produce large numbers of differentiated progeny. They can be divided into two broad categories: embryonic stem cells and adult stem cells.
- Embryonic stem cells are derived from the inner cell mass of mammalian blastocysts and can give rise to all of the differentiated tissues of the embryo proper, including the β-cells of the pancreas.
- Induced pluripotent stem cells are embryonic stem cell-like cells derived from adult cells such as skin fibroblasts in a process called reprogramming.
- Human embryonic stem cells and induced pluripotent stem cells have been shown to produce insulin-producing cells by stepwise approach in culture.
- Adult stem cells have been identified in many organs, where they participate in tissue repair and homeostasis. No definitively identified stem cell population has been described in pancreas.
- Lineage-tracing experiments suggest that β-cell mass is maintained primarily by replication of pre-existing β-cells during normal adult life in mice. After injury, stem/progenitor cells may be recruited to produce additional islet cells, but the identity of these pancreatic stem/progenitor cells remains unclear.
- Pancreatic exocrine cells can be directly converted to become β-cells in vivo in a process called lineage reprogramming.
Introduction
The aim of stem cell therapy in the treatment of type 1 diabetes mellitus (T1DM) is to provide a source of cells that are identical or nearly identical to β-cells. Stem cells by definition are self-renewing, and in some cases they can be propagated clonally from a single cell. These properties allow a degree of reproducibility that is unusual for a cell-based therapeutic vehicle. It may be possible to engineer stem cells to evade immune recognition. It is also now possible to derive patient-specific induced pluripotent cells (iPS) that fully match individual patients immunologically. Encouraging progress has been made in recent years to derive insulin-producing cells from either embryonic stem cells or exocrine cells. Future efforts will be focused on continued improvement of the derivation efficiency and fidelity of β-cells, with the ultimate aim of producing sufficient numbers of transplantable mature β-cells that can rescue hyperglycemic conditions.
General definitions: adult and embryonic stem cells
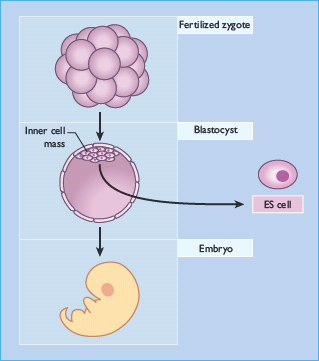
Stem cells are defined functionally. They are capable of self-renewal and possess the ability to generate daughter cells that produce large numbers of differentiated progeny [1]. They can be divided into two broad categories: embryonic stem cells and adult stem cells. Embryonic stem cells are derived from the inner cell mass of the mammalian blastocyst (Figure 61.1) and are said to be pluripotent because they give rise to all of the fully differentiated tissues of the embryo proper, including the products of all three embryonic germ layers and thus the β-cells of the pancreas (Figure 61.2). Fortunately, conditions have been described that permit the long-term culture of these cells in vitro without chromosomal aberration or loss of potency to form diverse tissues (Figure 61.3) [2–6]. Indeed, cell lines can be created by the propagation of a single cell, ensuring homogeneous cell populations that can subsequently be used as starting material for β-cell differentiation experiments.
Figure 61.1 Derivation of embryonic stem cells. After fertilization, the single-cell human or mouse zygote undergoes cleavage to produce a multicellular blastocyst. The blastocyst stage of mammalian development occurs well before cytodifferentiation and organogenesis. Cells from the inner cell mass, which normally give rise to the tissues of the embryo proper, can be cultured in vitro to produce embryonic stem cells. ES, embryonic stem.
Adult stem cells are thought to be rare cells and are known to participate in the repair or regeneration of certain tissues, most notably in the bone marrow [7,8]. Furthermore, stem cell populations are involved in the tissue homeostasis of the liver, the brain, the skeletal muscle and the skin [9–13]. Adult stem cells, like their embryonic counterparts, are capable of self-renewal and retain the ability to generate large numbers of differentiated progeny, but they are traditionally thought to produce a more limited number of cell types. Although there is some evidence to suggest the existence of a pancreatic stem cell function, no cell type or marker of such a putative cell has been identified. In fact, it is unclear whether the concept of an adult stem cell represents a single well-defined cell type or a common property of a heterogeneous population of different cells [1].
Figure 61.2 The pluripotent nature of embryonic stem (ES) cells. Embryonic stem cells can be cultured in vitro indefinitely. When allowed to aggregate in suspension culture, forming embryoid bodies, they differentiate into derivatives of all three embryonic germ layers. This includes ectodermal tissues such as neurons, mesodermal tissues such as muscle cells and blood, and endodermal tissues such as β-cells. Although there are many cell types in embryoid bodies, they are often disorganized and do not adopt the same patterned structure found in mature embryos and adults (e.g. actual muscle, ganglia, gut or islets).
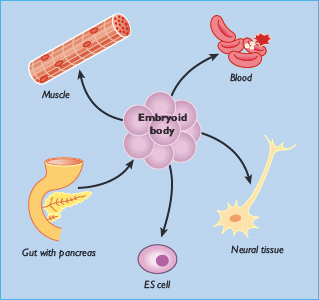
Figure 61.3 A human embryonic stem cell colony is composed of hundreds to thousands of homogeneous cells grown in a Petri dish along with hundreds of other similar stem cell colonies. One colony is shown here. These colonies are grown on mouse embryonic fibroblasts, which provide signals preventing embryonic stem cell differentiation. This allows repeated, near-indefinite subculture of embryonic stem cells. When removed from the murine feeder layer, the stem cells spontaneously differentiate into a wide variety of cell types (not shown).
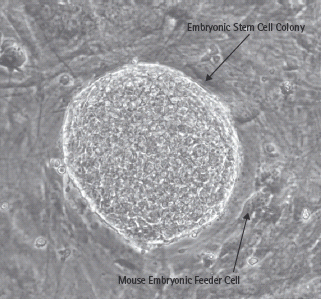
Figure 61.4 Potential stem-cell sources of β-cells. In theory, one can derive pancreatic β-cells from a number of different stem cell sources. Embryonic stem cells from the mammalian blastocyst have the broadest capacity for differentiation. Similarly, induced pluripotent cells (iPS) derived from skin fibroblasts may also give rise to all cell types in the body, including β-cells. Pancreatic stem cells may be the most direct path to β-cells, but their identity is still unclear. ES, embryonic stem.
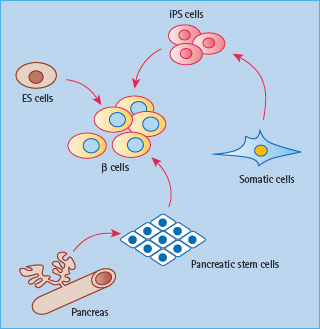
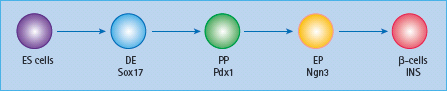
Figure 61.5 Stepwise differentiation from embryonic stem (ES) cells to β-cells. DE, definitive endoderm; PP, pancreatic progenitor; EP, endocrine progenitor; Ngn3, neurogenin 3; INS, insulin.
Differentiating β-cells from human embryonic stem cells
Embryonic stem (ES) cells, with a virtually endless replicative capacity and the potential to differentiate into most cell types, provide nearly unlimited starting material to generate differentiated cells for study and clinical therapy [14]. By mimicking signals used during embryonic pancreatic development, to the extent that they are known, a stepwise protocol is being explored to differentiate human ES cells into functional β-cells (Figure 61.4). This involves directing ES cells first to form definitive endoderm, then Pdx1+ pancreatic progenitors, followed by the formation of endocrine progenitors and, finally, insulin+ β-cells (Figure 61.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







