Introduction
Recent advances in the field of immunotherapy have revolutionized the treatment of cancer and have given hope to patients with cancers that were associated with a poor prognosis. Immune therapies have now been US Food and Drug Administration (FDA)–approved in the frontline setting for metastatic melanoma, non–small-cell lung cancer (NSCLC), renal cell carcinoma, and as second-line therapy for renal cell carcinomas, bladder cancer, Merkel cell carcinoma, gastric cancer, hepatocellular carcinoma, head and neck cancer, Hodgkin’s lymphoma, and microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) cancer. FDA–approved available immune therapies consist of immune checkpoint inhibitors (ICIs), adoptive cell transfer therapies, interferon-α (IFN-α), and interleukin-2 (IL-2). “Immune-like” therapies such as trastuzumab with cardiac side-effects have not been included in this chapter as they are discussed elsewhere. Immune therapies result in durable responses in a significant number of patients. However, varied immune-related adverse events (irAEs) may emerge as a result of nonspecific targeting of normal tissue besides the tumor tissue. The various irAEs associated with the use of ICIs include colitis, pneumonitis, hepatitis, nephritis, and uveitis, and are generally managed with high-dose glucocorticoids, at least initially. Cardiotoxic effects associated with the use of ICIs were not initially recognized but are now a well-recognized rare complication. Clinically severe and even fatal cardiac events have occurred in rare instances with ICIs and, hence, it is important to detect cardiac adverse events early to initiate successful intervention. Other immune therapies are also associated with cardiac toxicities presumed to be by off-target tissue mechanisms or by cytokine release syndrome (CRS). With increasing use of ICI therapies, the incidence of immune-mediated cardiotoxicities may rise, and hence, it is necessary that emergency medicine physicians, internists, oncologists, and cardiologists be vigilant and identify early signs to improve management.
Description
IMMUNE CHECKPOINT INHIBITORS
Currently, there are several ICIs that are approved by the US FDA. Ipilimumab, an anticytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4) antibody, was the first ICI approved to be used in metastatic melanoma. Nivolumab and pembrolizumab, which target programmed cell death protein-1 (PD-1), have been approved for use in melanoma, metastatic NSCLC, head and neck squamous cell cancer, urothelial carcinoma, gastric adenocarcinoma, and dMMR solid tumors, as well as for classic Hodgkin’s lymphoma. Nivolumab is also approved for use in hepatocellular carcinoma and in patients with renal cell carcinoma. The combination of nivolumab and ipilimumab has been approved by the FDA for treatment of metastatic melanoma and renal cell carcinoma. Recently, antibodies targeting the ligand of PD-1, programmed cell death protein-ligand 1 (PD-L1), have been approved, namely, atezolizumab (urothelial cancer and NSCLC), durvalumab (urothelial cancer), and avelumab (Merkel cell carcinoma and urothelial cancer), which also block the PD-1 pathway. This field is rapidly evolving as new agents and combinations continue to be made and tested.
BLINATUMOMAB
Blinatumomab is a newly developed monoclonal antibody. It is a bispecific T cell engager, or BiTE, that is directed against CD19 on B lymphocytes and CD3 on T cells. This novel agent for the treatment of B-cell precursor acute lymphoblastic leukemia (ALL) has demonstrated encouraging response rates in the setting of minimal residual disease (MRD)–positive (80% complete remission) and relapsed/refractory (R/R) patients. Thus, it is approved by the FDA for the treatment of R/R, Philadelphia chromosome (Ph)-negative and positive B-cell precursor ALL in adults and children. Due to this success, the incorporation of blinatumomab in ALL patients in combination with chemotherapy, targeted therapies, and other immunotherapeutic approaches is currently being actively investigated.
ADOPTIVE CELL TRANSFER
In this emerging treatment modality, T cells are isolated from a patient, genetically engineered to express either a receptor that has high affinity for specific tumor antigens (e.g., NY-ESO-1 or MAGE-A3), or a chimeric antigen receptor (CAR), and reintroduced into that patient. These affinity enhanced T cells have been used to treat multiple myeloma, melanoma, and synovial cell sarcoma, whereas CAR T cells have demonstrated efficacy primarily in the treatment of R/R ALL, chronic lymphocytic leukemia, and non-Hodgkin’s lymphoma. In August 2017, the FDA approved the first anti-CD19 CAR T cell product, tisagenlecleucel, for the treatment of pediatric and young adult patients with relapsed and/or refractory B-cell precursor ALL. In October 2017, the FDA approved axicabtagene ciloleucel for treatment of R/R diffuse large B-cell lymphoma (DLBCL).
INTERLEUKIN-2
IL-2 became the first FDA–approved immune therapy for renal cell carcinoma in 1992 and then metastatic melanoma in 1998.
INTERFERON-α
The current indication for IFN-α is in the adjuvant setting for high-risk resected melanoma, and in combination with bevacizumab, for advanced renal cell carcinoma.
Agents and Mechanism of Action
IMMUNE CHECKPOINT INHIBITORS
- ■
Currently approved ICIs include anti–CTLA-4 antibodies and anti–PD-1/anti–PD-L1 antibodies. CTLA-4 and PD-1, through intracellular signaling pathways, help to downregulate T-cell function and, hence, induce apoptosis. Ipilimumab (an anti–CTLA-4 monoclonal antibody), pembrolizumab and nivolumab (anti–PD-1 monoclonal antibodies), and durvalumab and avelumab (anti–PD-L1 monoclonal antibodies), block immune checkpoints, and thereby enhance the cytotoxic immune response to cancer cells.
- ■
The irAEs secondary to ICIs, namely, colitis, hepatitis, endocrinopathies, and dermatitis, lead to significant morbidity but only cause mortality ~1% of the time. Cardiovascular complications associated with ICI treatment are potentially life threatening, with devastating clinical consequences. With increasing clinical use of ICIs and with several evolving combination treatments with ICIs, early recognition and timely intervention is required. Due to the rarity of cardiotoxicities, data are very sparse and generally include case reports or small case series.
Cardiac Manifestations With Anti–CTLA-4 Treatment
Cases of fatal myocarditis, myocardial fibrosis, reversible left ventricular dysfunction, late onset pericardial effusion, cardiac tamponade, constrictive pericarditis, and Takotsubo cardiomyopathy with apical ballooning on echocardiography have all been observed with anti–CTLA-4 therapy.
Cardiac Manifestations With Anti–PD-1 Treatment
Clinically significant cardiotoxic events associated with anti–PD-1 therapy include pericarditis, hypertension, atrial and ventricular arrhythmia, and myocardial infarction. A case series of melanoma patients treated with anti–PD-1 therapy showed a 1% incidence of cardiac disorders including a case of fatal ventricular arrhythmia due to myocarditis, various other arrhythmias (atrial flutter, ventricular arrhythmia), asystole due to cardiomyopathy, hypertension, myocarditis, and left ventricular dysfunction. The onset of these toxicities ranged from 2 to 17 weeks after treatment. There does not appear to be any correlation between the type of anti–PD-1 therapy, tumor response, tumor type, or any particular clinical features that predispose these patients to adverse cardiac events.
Cardiac Manifestations With Combination Immune Checkpoint Inhibitors
- ■
Toxicities associated with ICIs are enhanced with combination therapy compared with monotherapy alone. Combination ICI (ipilimumab-nivolumab) resulted in grades 3 and 4 adverse events in 55% of patients compared with 16% of patients only on nivolumab and 27% of patients only on ipilimumab.
- ■
Combination ipilimumab and nivolumab in two melanoma patients led to fulminant myositis with rhabdomyolysis, early progressive and refractory cardiac electrical instability, and myocarditis. Despite aggressive interventions with high-dose glucocorticoids and in one case, infliximab, both of the patients died. Another case of myocarditis which was salvaged presented with symptoms of heart failure and left ventricular (LV) dysfunction with reduction in left ventricular ejection fraction (LVEF) from 50% to 15% after 3 combination infusions of ipilimumab and nivolumab. However, the LVEF improved to 40% after 2 months of high-dose glucocorticoids and treatment for heart failure.
- ■
Johnson et al. reported the frequency of cardiovascular complications, namely, myocarditis and myositis, in a large population extracted from the of Bristol-Myers Squibb corporate safety database. Among a total of 20,594 patients studied, 0.09% drug-related severe adverse events of myocarditis were reported. Combination therapy was associated with a higher incidence of frequent and severe myocarditis than with nivolumab alone (0.27% or 5 fatal events vs. 0.06% or 1 fatal event, P < .001). Mortality was high, with death occurring secondary to refractory arrhythmias or cardiogenic shock. Median time to development of myocarditis was 17 days (range, 13–64 days). Severe myositis (grade 3 to 4) also appeared more frequently when the combination of drugs was used than when nivolumab was the only agent used (0.24% vs. 0.15%). In clinical trials involving nivolumab, ipilimumab, or both, there was no routine testing for myocarditis by means of either biochemical analysis or cardiac imaging. It is important to recognize, however, that the actual incidence of cardiac events post ICI may be more substantial than what is already known because cardiac monitoring was not a routine part of clinical trials. Also, it is important to remember that the data were collected retrospectively from a single manufacturer in the absence of prospective standardized screening of cardiac issues, and hence, it is very likely that this underrepresents the true incidence.
- ■
A paper published in Circulation analyzed a total of 30 patients with ICI-related cardiotoxicity which included 12 newly diagnosed patients and 24 patients with previous data that had been reported in case series. Cardiotoxicity was diagnosed at a median of 65 days (range, 2–454 days) after the initiation of ICIs, and occurred after a median of 3 infusions (range, 1–33). It was observed in the study that cardiotoxicity was higher after the first and third infusions. The most frequent clinical manifestations observed in the patients were dyspnea, palpitations, and signs of congestive heart failure. The development of LV dysfunction was observed in 79% of patients, and 14% patients developed a Takotsubo-syndrome–like appearance. Atrial fibrillation, ventricular arrhythmia, and conduction disorders were observed in 30%, 27%, and 17% of patients, respectively, who were treated with ICIs; however, after excluding the finding of LV dysfunction, they were observed to occur in 3%, 7%, and 13% of patients, respectively. Myositis was noted to develop in 23% of patients. It was also observed that cardiovascular mortality was significantly associated with conduction abnormalities (80% vs. 16%, P = .003) and ipilimumab-nivolumab combination therapy (57% vs. 17%, P = .04).
- ■
A review of complied case reports and case series by Jain et al. revealed that the onset of cardiovascular irAEs can be seen as early as 2 weeks and as late as 32 weeks after initiation of ICI, with a median onset at 10 weeks after initiation.
Mechanism of Immune Checkpoint Inhibitor–Mediated Cardiotoxicity
A plausible mechanism behind ICI-mediated myocarditis is that shared targeted antigens (epitopes)/high frequency T-cell receptors may exist among tumor cells and the cardiac myocytes that could become a target for activated T cells and thus lead to myocardial lymphocytic infiltration resulting in heart failure and several other conduction abnormalities. Among patients who died from myocarditis, autopsy showed abundant CD4+ and CD8+ T-cell infiltration of the tumor, cardiac muscle (cardiac sinus and the AV node), and skeletal muscle. These were indicative of lymphocytic myocarditis and myositis. Pathology review also showed myocardial fibrosis, and cardiomyopathy predisposing to heart failure, and conduction abnormalities, including heart block and cardiac arrest. Pericarditis and pericardial effusion have also been described. Although rare, there has also been a case report of irAE-associated acute coronary syndrome. PD-L1 expression has been noted on the membranous surface of the injured myocytes and on the infiltrating CD8+ T cells and histiocytes from the inflamed myocardium. Along with this, the over-expression of IFN-γ, granzyme B, and tumor necrosis factor-α (TNF-α), produced by the activated T cells, could also contribute to cardiac damage. On the other hand, the skeletal muscles and the tumor had negative/lower expression for PD-L1. PD-L1 upregulation in the myocardium could be a cytokine-induced cardioprotective mechanism that is abrogated by immune-checkpoint blockade. It remains undetermined as to what are the causative epitopes that are recognized by these T-cell receptors within the multitude of antigens. Mice studies have shown that genetic deletion of PD-1 in mice models results in cardiomyopathy caused by antibodies against cardiac troponin I; however, no such mechanism has been identified in humans. , Hence, Nishimura and colleagues concluded that PD-1 may be an important receptor contributing to autoimmune cardiac diseases. Several mouse models of T-cell–dependent myocarditis exist where genetic deletion of PD-L1/L2, as well as treatment with anti–PD-L1, transformed transient myocarditis into a lethal disease. Preclinical studies have also shown that CTLA-4−/− mice develop severe autoimmune myocarditis mediated by CD8+ T cells, which is rapidly fatal at birth.
Clinical Presentation of Immune Checkpoint Inhibitor-Mediated Cardiotoxicity
Patients may present with varied clinical manifestations and thus careful consideration must be given for this entity. Symptoms can vary from nonspecific symptoms like weakness and fatigue, to typical cardiac symptoms of chest pain, heart failure (shortness of breath, pulmonary or lower extremity edema), palpitations, irregular heartbeat, new arrhythmias (including conduction blocks), and syncope and myalgias, especially in the first few months of treatment. Patients may develop myocarditis/pericarditis along with symptoms of myositis (myalgias, rhabdomyolysis) and present with muscle pain, fevers, pleuritic chest pain, and diffuse ST elevation on electrocardiogram (ECG), and hence, these overlapping manifestations pose a challenge to the accurate diagnosis of the condition. Patients may also present with nonspecific signs of fatigue, malaise, myalgias, and/or weakness alone or along with other irAEs and symptoms may be masked by pneumonitis, hypothyroidism, or other pulmonary symptoms. Severe cases can present with cardiogenic shock or sudden death. Immune-mediated myocarditis can present as heart failure or arrhythmias. The myocarditis may be fulminant, progressive, and life threatening. The dysrhythmias may present as benign supraventricular tachycardia to more fatal advanced heart blocks or ventricular tachycardia. Per expert consensus, it is imperative to have high vigilance for development of cardiac symptoms in all patients, but especially in those with evidence of myocarditis, vasculitis, or myositis.
Patients with known cardiac morbidities should not be denied treatment with ICI but should be carefully monitored with a low threshold of suspicion for any nonspecific presentation of cardiac irAE with the potential to cause rapid deterioration.
Referral and Consultation.
Patients who present with multiple cardiovascular risk factors or established cardiovascular disease prior to starting ICIs should have a cardiology consultation prior to initiation of therapy. Any abnormal cardiac test result, in any patient, during the course of ICI treatment warrants an immediate referral to cardiology, as myocarditis can be fatal, and patients suspected with documented myocarditis should be admitted to the hospital for cardiac monitoring.
BLINATUMOMAB
Tumor-specific T cells play a key role in the immune surveillance of cancer cells. This has been demonstrated by the positive correlation of CD8+ cytotoxic T cells within tumors, antitumor responses, and long-term survival. BiTEs are capable of eliciting polyclonal T cell responses that are unrestricted by T cell receptor specificity, presence of major histocompatibility class (MHC), or additional T-cell co-stimuli. Blinatumomab is a BiTE which has been FDA-approved for the treatment of adult R/R Ph− B-cell precursor ALL and also for MRD-positive ALL. Once CD19+ B-cells and CD3+ T cells have been linked together via blinatumomab, there is formation of a cytolytic synapse between the T cell and the cancer target cell. The cytotoxic T cell releases granzymes and perforin via exocytosis and the perforins, in the presence of calcium, bind to the target B-cell membrane, thus creating a pore for the entry of granzymes. They also release inflammatory cytokines. The granzymes activate programmed cell death. The activated T cells enter the cell cycle, expanding the T-cell compartment and, thus, increasing the number of T cells present in the target tissue. T-cell activation and release of various proinflammatory cytokines results in the development of CRS. It is also important to recognize that malignant cell lysis induced by activated T cells results in development of hypocalcemia, hyperkalemia, hyperphosphatemia, and hyperuricemia and release of several proinflammatory cytokines contributing to the development of tumor lysis syndrome (TLS), a potentially life-threatening condition.
Cardiac Manifestations With Blinatumomab
A phase I/II study of blinatumomab in pediatric patients with R/R ALL in 70 patients showed that in the phase I portion, 3 patients experienced grade 4 CRS (one of which had grade 5 cardiac failure). , Severe (grade 3) CRS occurred in 2% of 189 patients who received blinatumomab for approved indication in a large phase II study; 17% of patients had any grade arrhythmias; 2% of patients developed grade 3 or higher arrhythmias; 12% of patients developed any grade hypotension; grade 3 or greater hypotension occurred in 3% of patients; any grade chest pain in 11% and 1% of patients had grade 3 or greater chest pain. A study by Kantarjian et al., comparing blinatumomab with chemotherapy for advanced ALL, showed an incidence of any grade hypotension in 12% of patients, tachycardia in 6.7%, and hypertension in 6.4% of patients.
Mechanism of Blinatumomab-Mediated Cardiotoxicity
Cytokine release syndrome (CRS) is mediated by the release of IL-2, TNF-α, IFN-γ, IL-6, and IL-10 from blinatumomab-engaged effector T cells. These cytokines, which reach peak on day 1 of therapy, and then decline rapidly thereafter, are responsible for the adverse effects seen in CRS. The above cytokines result in capillary leak syndrome, which results in hypotension and arrhythmias. Several cytokines have specific cardiac effects which mediate cardiac toxicity. IL-1-β and TNF-α result in a decrease in cardiac contractility, induction of fibrosis, and cardiac hypertrophy. IL-2 also results in a decrease in cardiac muscle contractility. IL-6 causes a decrease in cardiac contractility and cardiac hypertrophy induction. These proinflammatory cytokines and the subsequent elevation in nitric oxide (NO) result in both inotropic and chronotropic alterations in the myocardial excitation-contraction coupling, myocardial contractility suppression, and desensitization of the β-adrenergic receptors stimulation, thereby resulting in development of cardiac failure. Of note, the TLS induced by blinatumomab can also lead to life-threatening conditions, including cardiac failure due to hypocalcemia, hyperkalemia, and hyperuricemia, and hence, monitoring electrolytes during treatment is critical.
ADOPTIVE CELL TRANSFER
- ■
Adoptive cell transfer (ACT) is another form of immunotherapy which has shown promise for a wide variety of solid tumors. In ACT, patients’ T cells are genetically engineered to specifically target tumor cells. This is based on previous studies wherein preexisting tumor-infiltrating lymphocytes (TIL) were collected, expanded, and then reintroduced into the patient’s microenvironment in the presence of IL-2 in order to stimulate their survival and expansion.
- ■
With the additional component of modification in the form of genetic engineering of the T-cell receptors (TCRs), the T cells are able to have higher affinity towards tumor antigens that are not normally well engaged by wild-type TCRs. The targeted antigens are expressed in immunologically protected germline cells but become abnormally expressed in various cancers, including NY-ESO-1 and MAGE-3. ACT is currently being utilized for multiple different malignancies.
- ■
CAR T-cell therapy is a form of ACT that has been approved for B-ALL and DLBCL. In CAR T cells, the naive T cells are genetically engineered to express a CAR on the cell membrane and coupled with an external binding domain to bind to the tumor antigens (targeting CD19 on malignant cells and differentiated B-cells). In refractory B-ALL patients, CAR T-cell therapy is effective in 70% to 90% of cases. IL-6 has been increasingly recognized as a key mediator of systemic toxicity associated with CAR T-cell therapy.
Cardiac Manifestations of Adoptive Cell Transfer
One of the most common cardiovascular toxicities following CAR T-cell administration is tachycardia, which is frequently associated with fever. As the grade of CRS increases, hypotension, arrhythmias, and decreased ejection fraction may be seen. Grades 3 to 4 hypotension has been reported in 22% to 38% of patients. Most of these cardiovascular toxicities are reversible and can be managed with supportive care. Cardiac arrest was noted in one patient 7 days after infusion with CAR T-cell therapy with subsequent reduction in LVEF to less than 25% from baseline. Reversible reduction in ejection fraction has been reported in multiple other patients. Reversible increases in serum troponin can concomitantly occur. Asymptomatic prolongation of the QTc interval on ECG as well as atrial fibrillation have also been reported. Two patients with T cells expressing receptor against MAGE-3 presented with diffuse ST elevations on an ECG and elevation of cardiac biomarkers preceding cardiopulmonary arrest. One of the patients developed a large pericardial effusion and cardiogenic shock, and ultimately died of multisystem organ failure. In both patients, robust cytokine production and T cell infiltrates were observed in the heart, with histopathological analysis demonstrating myocyte necrosis in a pattern similar to allograft rejection.
Mechanism of Adoptive Cell Transfer–Mediated Cardiotoxicity
- ■
Most of the observed cardiac side effects appear to be secondary to the preparative regimen or IL-2, but there have been reports of significant toxicity from cross-reactivity to other normal cells with fatal consequences. A case series showed that the use of genetically modified TCR against MAGE-3, a cancer germline antigen, as mentioned above, led to the development of fatal cardiogenic shock in two patients. Though cross-reactivity was thought to be the mechanism of action in these cases, there was no evidence of MAGE-3 expression on cardiac tissue. On the other hand, significant myocardial damage with T-cell infiltration targeted against titin, an unrelated myocardial protein, was observed. Another case report with MART-1 TCR in melanoma resulted in neurological dysfunction and cardiac arrest 6 days after the T-cell infusion. Infused T cells were noted in the cardiomyocytes, but no cross-reactivity was identified, thus suggesting that an alternate mechanism exists.
- ■
Cardiotoxic side effects with CAR T-cell therapy are generally reversible and are part of the CRS, which is a systemic inflammatory response that correlates with the in vivo activation and proliferation of CAR T cells. It is believed that the pathophysiology for the cardiac dysfunction is similar to that of stress (stress-induced Takotsubo cardiomyopathy) and sepsis.
- ■
For both CRS-mediated and off-target/cross-reactivity–mediated cardiotoxicity, the literature describing cardiovascular adverse events remains limited to case reports, and the overall incidence is yet to be defined.
INTERLEUKIN-2
Clinical data have shown a significant number of cardiac toxicities associated with the use of high-dose IL-2 (HDIL-2). IL-2 is a cytokine, or biological response modifier, produced by activated natural killer (NK) cells and promotes clonal T-cell expansion during an immune response. It also helps develop and mature T regulatory cells, promotes NK cell activity, and mediates immune tolerance by activation-induced cell death. IL-2 binds to its receptors of either high-affinity containing α(CD25)-, β(CD122)-, and γ(CD132)-chains, or low-affinity receptors that contain only α- and β-chains. This induces proliferation and differentiation of both CD4+ and CD8+ cells to effector cells or memory cells. IL-2 elicits a combination of immune responses, both activation of innate immune effectors including NK cells and macrophages, and specific immune responses mediated by T effector and memory cells for long-term control of tumor recurrence. Subsequent release of various cytokines, including other interleukins, interferons, and colony stimulating factors, is also believed to be important in induction of tumor regression.
Cardiac Manifestations With Interleukin-2
The cardiac manifestations with IL-2 can range from severe hypotension (up to 65%), arrhythmias (up to 57%), and ischemia (up to 20%). HDIL-2 can induce vascular leak syndrome that can result in hypotension and tachycardia. Up to 10% of patients can develop arrhythmias including atrial fibrillation, which can lead to hypotension. In a series of 199 patients treated with HDIL-2 over 310 courses of therapy, 19 cases of dysrhythmias were reported that included atrial fibrillation in 16 patients (5.2%), prolonged atrial arrhythmia with hypotension in 2 patients (0.6%), and nonsustained ventricular tachycardia in 1 patient (0.3%). Sinus tachycardia should be expected during therapy and is the most frequent dysrhythmia. There have been rare cases of myocardial infarction and myocarditis with IL-2 therapy. Myocarditis may present with elevated cardiac enzymes in the presence of a normal ECG, patients may have fever, mild chest pain, or discomfort, and may present even days after stopping IL-2 therapy. Peripheral edema may also result from capillary leak syndrome and patients may gain 10% to 15% of their body weight due to fluid overload. Due to these toxicities, IL-2 is reserved for patients with excellent performance status and good organ function.
Mechanism of Interleukin-2–Mediated Cardiotoxicity
The previously mentioned clinical manifestations such as hypotension, tachycardia, and atrial arrhythmias are secondary to capillary leak syndrome induced by IL-2. Although these changes are secondary to cardiac stress and certain hemodynamic changes, preclinical studies have suggested that IL-2–activated lymphocytes could also directly damage endothelial cells and myocytes. Myocarditis has been confirmed and myocardial infiltrating lymphocytes are seen on biopsy.
INTERFERON-α
IFN-α induces the transcription of several genes via the JAK-STAT (Janus tyrosine kinase—signal transducer and activator of transcription) and other signaling pathways. The antitumor mechanism of IFN-α is the cumulative result of cytostatic, antiangiogenic, and immune-modulatory activities. The immune-modulatory effect of IFN-α includes induction of cytokines, upregulation of major histocompatibility antigen expression, enhancement of phagocytic activity of NK cells, macrophages, and augmentation of T-cell cytotoxicity against the tumor cells.
Cardiac Manifestations With Interferon-α
No relationship between cardiotoxic effects and dose of IFN-α has been found. Eight phase I trials with IFN-α therapy have not reported any significant cardiotoxic adverse events. One small case series of 44 patients treated with IFN-α therapy showed that the cardiotoxic effects ranged from arrhythmia (in 25 patients), dilated cardiomyopathy (in 5 patients), ischemic heart disease (in 9 patients), and sudden death (in 22 patients). Tachycardia and hypotension have been reported in 5% to 15% of IFN-α2b–treated patients during the first day of treatment.
Mechanism of Interferon-α–Mediated Cardiotoxicity
In two cases of reversible cardiomyopathy in patients who received IFN-α2b, endomyocardial biopsy revealed myocardial inflammation. Hypothyroidism may also be a contributing factor for cardiac dysfunction, and can have a variety of effects on the heart, such as ventricular dilation, pericardial effusion, and poor contractility.
Pharmacological Approaches for Management and Treatment
IMMUNE CHECKPOINT INHIBITORS
- ■
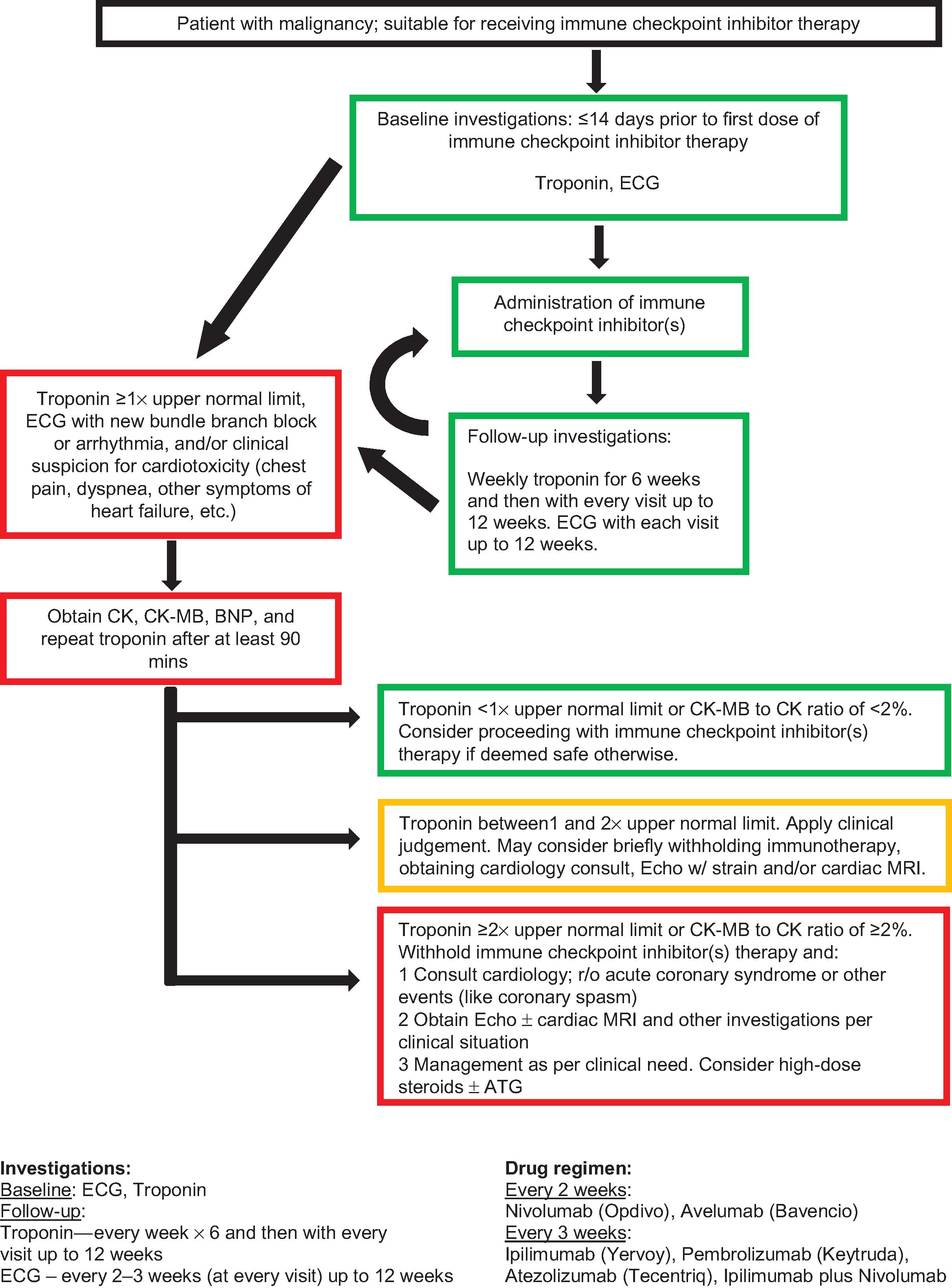
Due to the potential for myocarditis-related mortality, some institutions recommend monitoring troponin every week for the first 6 weeks, and then prior to each cycle for the first 12 weeks of treatment. ECG should be done at baseline and be repeated prior to every cycle for the first 12 weeks. Fig. 23.1 shows the Roswell Park Cancer Institute (RPCI) schema for monitoring patients on immune checkpoint inhibitors.