Introduction
- ■
A multitude of cancer treatments are known to cause thrombocytopenia (TCP), termed treatment-related thrombocytopenia. Chemotherapy-induced thrombocytopenia (CIT) is a term that refers specifically to thrombocytopenia caused by chemotherapy drugs. Notably, many nonchemotherapy cancer treatments may also cause thrombocytopenia.
- ■
The normal platelet range is 150,000 to 450,000/µL. The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) defines TCP according to grade. Grade 1 is 75,000 to 150,000/µL, Grade 2 is 50,000 to 75,000/µL, Grade 3 is 25,000 to 50,000/µL, and Grade 4 is less than 25,000/µL. Although the risk of bleeding with TCP is difficult to ascertain fully, general practice is to avoid surgical procedures for platelets less than 50,000/µL (100,000–75,000/µL for central nervous system [CNS] procedures). Furthermore, spontaneous bleeding is possible at platelet counts less than 20,000/µL, but more consistently at levels of less than 5000 to 10,000/µL. ,
Mechanisms of Thrombocytopenia
- ■
Chemotherapy-induced thrombocytopenia: CIT comprises the bulk of treatment-related TCP. Chemotherapy causes TCP by altering the megakaryocyte to platelet production pathway, either at the stem cell level or at later stages in platelet maturation. Some of the more commonly implicated chemotherapeutic agents are gemcitabine and carboplatin. Regimens such as ICE (ifosfamide, carboplatin, and etoposide) for non-Hodgkin’s lymphoma and MAID ( m esna, d oxorubicin, i fosfamide, and d acarbazine) for sarcoma are associated with particularly high rates of TCP. Aside from cytotoxic effects on the platelet production line, some chemotherapeutic agents are associated with immune-mediated TCP, such as fludarabine. ,
- ■
Drug-induced thrombotic microangiopathy (DITMA): DITMA often presents with hemolytic anemia and thrombocytopenia and has been associated with several cancer-directed therapeutic agents such as gemcitabine, bortezomib, carfilzomib, bevacizumab, sunitinib, and others. DITMA is a potentially serious condition and is first managed by discontinuation of the offending drug followed by supportive care.
- ■
Targeted therapy-related thrombocytopenia: Targeted therapies, such as tyrosine kinase inhibitors (TKIs), can have off-target effects that can be problematic for patients taking them. These TKIs can have platelet decreasing effects and cause a decrease in platelet function, thus require special consideration.
- ■
Immunotherapy-related thrombocytopenia: Although rare, checkpoint inhibitors are known to cause immune-related thrombocytopenia (irTCP) in some patients. Aside from a history of autoimmune conditions, risk factors for irTCP are not well known. Patients, whose TCP has a temporal relationship with treatment with immunotherapy, have no alternate explanations for TCP, are found to have positive antiplatelet antibodies, and do not respond to platelet transfusions, should be considered to have irTCP. These patients can be treated with immunosuppressive drugs (steroids will be first line) if the TCP is severe.
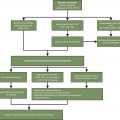
Diagnostic Workup of Thrombocytopenia
- ■
The diagnostic workup of cancer treatment–related thrombocytopenia is similar to that of thrombocytopenia from other causes. All patients receiving cancer-directed therapy should undergo evaluation with a complete blood count and examination of the peripheral smear (to evaluate for the presence of abnormal cells such as schistocytes), and metabolic testing including determination of liver enzyme levels of lactate dehydrogenase (LDH), and haptoglobin. This testing is important to rule out other confounding etiologies which also need to be considered in these patients.
Treatment of Thrombocytopenia
In general, treatment-related thrombocytopenia is managed identically to other causes of thrombocytopenia:
- ■
Remove the offending agent: In this case, it is often as simple as delaying future chemotherapy or holding oral-targeted therapy. Patients who experience DITMA should be treated with supportive therapy and discontinuation of the offending drug. Plasma exchange or plasmapheresis (PLEX) and anticomplement therapy are not recommended for DITMA because the pathophysiology differs from that of thrombotic thrombocytopenic purpura (TTP).
- ■
Manage risk of complications: The risk of life-threatening bleeding increases as platelet concentration decreases. The American Society of Clinical Oncologists (ASCO) guidelines from 2018 suggest offering platelet transfusions when platelet counts drop below 10,000/µL. Higher platelet goals can be maintained for certain situations, such as for procedures and surgeries. Patients who experience active bleeding should have a minimum platelet goal of 50,000/µL.
- ■
Consider risk of repeated thrombocytopenia: Future treatment may expose the patient to repeated TCP; consideration must be given to either dose-reduction of the likely offending agent(s) or choosing an alternative agent.
Thrombopoietin receptor agonists (TPO-RAs) have garnered much press for the prevention and treatment of CIT. However, studies have shown mixed results and, their utility has not been proven in large clinical trials, thus their use has not become standard of care.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree