Cardiotoxicity of Cancer Therapy
Justin D. Floyd
Michael C. Perry
It is well recognized that antineoplastic agents may have adverse effects on multiple organs and normal tissues. The most commonly associated toxicities occur in tissues composed of rapidly dividing cells and may spontaneously reverse with minimal long-term toxicity. The myocardium, however, consists of cells that have limited regenerative capability, which may render the heart susceptible to permanent side effects from chemotherapeutic agents. In addition, several transient cardiac toxicities occur that may remit upon withdrawal of the offending agent. The effects of antineoplastic agents on the myocardium can be predictable or unpredictable, fixed or cumulative, and potentiated or abrogated by the addition of other chemotherapeutic agents. Furthermore, increasing age and preexisting heart disease increase the potential of developing cardiac toxicity. As patients continue to live longer and be exposed to rapidly expanding treatment algorithms, treatment-related cardiac toxicity is becoming a more common problem for patients and practitioners. In fact, this phenomenon has resulted in an increase in both formal and informal collaboration between cardiology and oncology practitioners; this field is increasingly being known as “cardio-oncology.” We review herein the cardiac complications associated with chemotherapy, biologic therapy, hormonal therapy, and targeted therapy by discussing the individual therapeutic agents.
ANTITUMOR ANTIBIOTICS
Anthracyclines
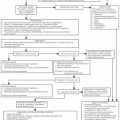
The anthracyclines are a class of red-pigmented antibiotics (rhodomycins) isolated from a soil bacillus, actinomycete Streptomyces.1 They include daunorubicin, doxorubicin, epirubicin, and idarubicin. Anthracycline-associated cardiac toxicity may have two clinical presentations: acute/subacute and chronic. Acute/subacute toxicities typically arise any time from the initiation of therapy to several weeks after the completion of therapy. These toxicities include supraventricular tachycardia (SVT), ventricular ectopy, myopericarditis, significant ECG changes, abnormal cardiac enzymes, cardiomyopathy, and death.2 Fortunately, these are rarely clinically significant and ECG monitoring is not routinely used. However, detection of early toxicity, may prove to be more relevant than previously thought. For example, elevated levels of troponin may prove to predict chronic anthracycline-induced cardiomyopathy. Chronic cardiomyopathy presents months or even years after therapy with anthracyclines. Anthracycline-related cardiomyopathy is characterized by a dose-dependent, symptomatic or asymptomatic, progressive decrease in left ventricular function (LVEF) often resulting in congestive heart failure (CHF). Anthracycline-induced cardiomyopathy is clinically indistinguishable from other forms of CHF. Serious arrhythmias, including ventricular tachycardia, ventricular fibrillation, and sudden cardiac death, have been identified in both symptomatic and asymptomatic patients with late cardiomyopathy. The proposed pathogenesis of anthracycline-induced cardiomyopathy consists of the following mechanisms:
Mitochondrial dysfunction resulting in adenosine triphosphate (ATP) depletion
Free radical lipid peroxidation mediated through an iron-doxorubicin complex
Decrease in glutathione peroxidase; endomyocardial biopsies demonstrate sarcoplasmic reticulum dilation, vacuole formation, myofibrillar dropout, and necrosis3
Risk factors for anthracycline-induced cardiac toxicity include cumulative dose (especially >550 mg per m2), hypertension, preexisting cardiac disease, advancing age, and prior mediastinal irradiation.4,5 In addition, female sex has been demonstrated to be an independent risk factor.6 Finally, multiple antineoplastic agents including trastuzumab, cyclophosphamide, actinomycin D, mitomycin C, etoposide, melphalan, vincristine, bleomycin, paclitaxel, docetaxel, and dacarbazine all may have an additive effect on anthracycline-induced cardiomyopathy.
Based on risk factors, multiple strategies (including serial endomyocardial biopsy, radionucleotide studies, and echocardiography) have been proposed for the prevention and early detection of anthracycline-induced cardiomyopathy.1 In addition, cardiac troponins and natriuretic peptides have been investigated as markers of cardiac injury and as a potential method of monitoring cardiac injury. Specifically, troponin T (TnT) levels have been demonstrated to correlate with the histopathologic changes associated with anthracycline-induced cardiac toxicity.7 In humans, TnT levels have been demonstrated to be associated with the severity of myocardial damage in children previously treated with doxorubicin.8 In addition, elevated TnT levels have been demonstrated to be predictive of subsequent LV dilatation and wall thinning identified by echocardiography.9 Furthermore, troponin I (TnI) levels are elevated soon after administration of high-dose chemotherapy and may be beneficial in predicting patients at risk of subsequent cardiac toxicity.10,11 TnT levels have also been used to monitor cardiac
protection by dexrazoxane.12 Doxorubicin-associated elevations in TnT levels have been identified immediately following the first dose of doxorubicin. The incidence of TnT elevation has been shown to increase substantially throughout the duration of doxorubicin therapy.12 Both atrial natriuretic peptide and brain natriuretic peptide appear to be elevated in cases of doxorubicin cardiomyopathy.13,14 However, the decrease in LVEF may precede the elevation of natriuretic peptides.15
protection by dexrazoxane.12 Doxorubicin-associated elevations in TnT levels have been identified immediately following the first dose of doxorubicin. The incidence of TnT elevation has been shown to increase substantially throughout the duration of doxorubicin therapy.12 Both atrial natriuretic peptide and brain natriuretic peptide appear to be elevated in cases of doxorubicin cardiomyopathy.13,14 However, the decrease in LVEF may precede the elevation of natriuretic peptides.15
Currently, none of the strategies or laboratory tests described earlier have become standard in monitoring for anthracycline-induced cardiomyopathy. However, it is generally accepted that adult patients scheduled to receive an anthracycline should have baseline evaluation of cardiac function by echocardiography or radionuclear ventriculography. Some clinicians advocate serial assessment of LVEF after every one to two cycles in selected high-risk patient populations. Finally, LVEF should be reassessed in patients after receiving a cumulative dose of 300 mg per m2, if further anthracycline-based therapy is planned. A decrease in LVEF of ≥ 10% to a level ≤ 50% should result in consideration of stopping anthracycline administration. Exercise stress testing in patients with borderline ejection fractions has not been useful in predicting future toxicity.
Anthracycline-induced cardiomyopathy should be aggressively managed with combinations of the following medications: diuretics, angiotensin converting enzyme inhibitors, ß blockers, spironolactone, and digitalis. If oncologic cure or stability is present, heart transplant can be considered, if necessary.16 The incidence of heart failure resolution with or without medical therapy, or the incidence of patients requiring heart transplant has not been well studied or documented. However, data published recently suggests a 55% response rate to aggressive medical therapy. Early initiation of therapy and a low NYHA functional class were found to be independent predictors of LVEF recovery.17 This observation, as well as others, suggests that well-designed survivorship studies evaluating the incidence of and therapy for anthracycline-induced cardiomyopathy may identify strategies to decrease treatment-related morbidity and mortality.
Although all of the anthracyclines may be associated with cardiac toxicity, doxorubicin has been extensively studied and is the model for the discussion of anthracycline-induced cardiomyopathy. The generally accepted safe cumulative dose of doxorubicin is 450 to 500 mg per m2. However, some authors have advocated limiting the doxorubicin cumulative18 dose to 400 mg per m2. Conversely, some patients have tolerated doses of >5,000 mg per m2 without cardiac dysfunction, whereas others have received only 40 mg per m2 and developed fatal CHF.19 One patient developed life-threatening CHF after receiving a single dose of 50 mg of doxorubicin through hepatic arterial infusion.20 Hequet et al.21 reported a 20.5% incidence of late subclinical cardiomyopathy in 141 adult lymphoma patients receiving a mean doxorubicin dose of 300 mg per m2. A weekly infusion schedule has been shown to allow up to 200 mg per m2 of additional doxorubicin without increasing cardiotoxicity. Continuous infusion has also been noted to decrease cardiac toxicity, as demonstrated by 96-hour continuous infusion, which permitted 800 to 1,000 mg per m2 with less cardiotoxicity, compared with 450 mg per m2 administered through rapid infusion.1 ACE inhibitors and ß blockers have been evaluated in small clinical trials and may be protective against the development of anthracycline-induced cardiomyopathy.
Though not uniformly accepted by the oncology community, liposomal formulations have demonstrated decreased cardiotoxicity in two published phase III randomized trials.22,23 Of the three liposomal anthracycline formulations, pegylated liposomal doxorubicin demonstrates the most significant difference in pharmacokinetics and toxicity profile. Specifically, low serum-free doxorubicin concentrations and limited myocardial distribution are associated with pegylated liposomal doxorubicin. Although this is an improvement, the safe level of previous anthracycline exposure and the maximum lifetime doxorubicin dose remain unknown. This is a question of particular relevance in women with metastatic breast cancer who previously received prior adjuvant anthracycline treatment as well as in patients affected by diseases, such as epithelial ovarian cancer and Kaposi sarcoma, in which individuals may have prolonged disease control with continued liposomal doxorubicin. Patients receiving prior doxorubicin have been safely treated to a cumulative24,25 dose of >500 mg per m2. For example, patients with Kaposi sarcoma have reportedly been safely treated with cumulative doxorubicin doses up to 2,360 mg per m2 over a 5-year period. In the absence of definitive randomized data, clinicians must combine available data, cardiac and noncardiac risk factors, and clinical judgment when using liposomal anthracyclines. If doses of liposomal doxorubicin given exceed a total lifetime dose of 450 mg per m2, we suggest monitoring the LVEF after each additional 50 to 100 mg per m2.
Finally, dexrazoxane, an iron chelator, has been reported in multicenter randomized trials to significantly reduce anthracycline-related cardiotoxicity in adult patients.26,27,28,29 These studies did not evaluate patients treated with liposomal anthracyclines. Cardioprotection was still conferred if the administration of dexrazoxane was delayed until a cumulative dose of 300 mg per m2 of doxorubicin had been administered.28 However, one multicenter randomized trial suggested decreased antitumor efficacy associated with dexrazoxane.29 Lipshultz et al. reported the effects of dexrazoxane on myocardial injury in 206 doxorubicin-treated children with acute lymphoblastic leukemia (ALL). This study reported a significant reduction in the incidence of myocardial injury, as indicated by TnT elevations, in patients receiving dexrazoxane in addition to doxorubicin.30 Furthermore, when treated with dexrazoxane prior to anthracyclines, pediatric high-risk ALL patients have been shown to have a reduction in long-term subclinical cardiac toxicity, as demonstrated by echocardiography, without compromising efficacy of treatment or increasing the rate of secondary malignancies.31 Concern about potential reduction in chemotherapy efficacy as well as concern about enhanced myelopsuppression and secondary malignancies have limited acceptance of dexrazoxane. ASCO has published detailed recommendations for the use of dexrazoxane.32
Anthraquinones
Mitoxantrone is an anthraquinone designed to yield broadspectrum antitumor activity similar to the anthracyclines. It was
originally postulated that this drug would have minimal cardiac toxicity; however, initial tissue culture studies demonstrated toxicity to myocardial cells.3 Subsequently, phase I and phase II studies from the National Cancer Institute (NCI) reported cases of dose-related cardiac failure and arrhythmias.33,34 Cumulative doses <110 mg per m2 were associated with a decreased incidence of heart failure and doses >160 mg per m2 with an increase in the incidence of cardiac failure.33,35 Immunex laboratories has reported the incidence of a subclinical moderate to severe decline in LVEF to be 13%, and the incidence of cardiac failure, up to a cumulative dose of 140 mg per m2, to be 2.6%.3 Currently, most authorities consider the maximum dose to be 140 mg per m2. Mitoxantrone-induced cardiac failure often responds to standard medical therapy for CHF.36
originally postulated that this drug would have minimal cardiac toxicity; however, initial tissue culture studies demonstrated toxicity to myocardial cells.3 Subsequently, phase I and phase II studies from the National Cancer Institute (NCI) reported cases of dose-related cardiac failure and arrhythmias.33,34 Cumulative doses <110 mg per m2 were associated with a decreased incidence of heart failure and doses >160 mg per m2 with an increase in the incidence of cardiac failure.33,35 Immunex laboratories has reported the incidence of a subclinical moderate to severe decline in LVEF to be 13%, and the incidence of cardiac failure, up to a cumulative dose of 140 mg per m2, to be 2.6%.3 Currently, most authorities consider the maximum dose to be 140 mg per m2. Mitoxantrone-induced cardiac failure often responds to standard medical therapy for CHF.36
Mitomycin C
The mitomycins are a family of antibiotics from Streptomyces caespitosus, which act through alkylation and DNA cross-linking.1,37 Cardiotoxicity in the form of cardiac failure has been observed in patients receiving mitomycin C with an incidence rising with cumulative38,39 doses >30 mg per m2. There is also evidence of an additive cardiotoxicity when used in combination with anthracyclines.40 Finally, data suggests cardiac toxicity associated with mitomycin C histologically resembles radiation-induced cardiac injury.41,42
Bleomycin
The bleomycins are a family of glycoproteins, initially isolated from the fungus Streptomyces verticillus. Pericarditis is an uncommon, but potentially serious, cardiotoxicity associated with bleomycin.43 An acute chest pain syndrome has also been reported with bleomycin therapy.44 The incidence is <3% and it is associated with sudden substernal chest pain. No long-term cardiac sequelae noted. Treatment is supportive and discontinuation of the drug is not needed, as further infusions do not usually cause recurrence of the symptoms. In addition, coronary artery disease (CAD), myocardial ischemia, and myocardial infarction have been observed in young patients during and after treatment with bleomycin-based chemotherapeutic regimens.45,46,47
TOPOISOMERASE INHIBITORS
Etoposide
Etoposide is a semisynthetic podophyllotoxin possessing a doselimiting toxicity of myelosuppression; however, some evidence exist supporting cardiac toxicity in the form of myocardial infarction and vasospastic angina associated with etoposide.48,49,50 In addition, etoposide is often a part of bleomycin- and cisplatin-based regimens that have been associated with cardiac toxicity.
ALKYLATING AGENTS
Cyclophosphamide
Cyclophosphamide is an alkylating agent commonly used in the treatment of both nonmalignant and malignant conditions. At low doses, cyclophosphamide has not been reported to be associated with cardiotoxicity. However, acute cardiac toxicity has been described when doses of 120 to 170 mg per kg over 1 to 7 days were administered in high-dose-conditioning regimens for bone marrow transplantation.51,52,53 Common manifestations of cyclophosphamide-associated cardiotoxicity are decreased amplitude of the QRS complex, nonspecific T wave or ST segment abnormalities, tachyarrhythmias, and complete heart block.3 Furthermore, an asymptomatic transient decrease in ejection fraction has been observed that generally resolves over 3 to 4 weeks. Acute-onset fulminant CHF has been reported in as many as 28% of patients receiving high-dose cyclophosphamide.54 Specifically, in 32 patients, at a dose of 180 mg per kg over 4 days, 28% of patients developed CHF, and 19% died from cardiac failure. Symptomatic CHF is generally treated with supportive measures such as diuretics, angiotensin converting enzyme inhibitors, ß blockers, and inotropic medications.3 Other reported cardiac complications reported include hemorrhagic myopericarditis leading to pericardial effusions, tamponade, and even death.54,55 The pathophysiology of these events may be related to endothelial capillary damage. Pericardial effusions may be treated by pericardiocentesis or may require a pericardial window. Fatal episodes of hemorrhagic myopericarditis have been described.
Cyclophosphamide and the anthracyclines are commonly used together in a variety of chemotherapeutic regimens. Cyclophosphamide has been utilized concurrently, sequentially, and after anthracycline failure, especially in breast cancer therapy.56,57,58 Evidence exists both for and against an additive effect of cyclophosphamide- and anthracycline-induced cardiomyopathy. Therefore, one should be aware of the possible development of CHF in patients older than 50 years receiving cyclophosphamide and anthracyclines. Monitoring patients with multiple risk factors while undergoing treatment may be worthwhile to detect toxicity as quickly as possible.59,60,61 Furthermore, the risk of cardiotoxicity may be minimized by administering cyclophosphamide by infusion or twice-a-day dosing, substituting liposomal anthracyclines, and the addition of cardioprotective agents, such as dexrazoxane.3,22,23,26,29 In addition, an increased incidence of fatal cardiac toxicity may be associated with the addition of pentostatin to bone marrow transplant preparatory regimens containing cyclophosphamide.62
Ifosfamide
Ifosfamide is an alkylating oxazaphosphorine related to cyclophosphamide, which has been associated with arrhythmias, ST-T wave changes, and CHF associated with LV dysfunction in a dose-related manner.63 These events, when symptomatic, are generally reversible with medical management. It is unclear whether there is increased cardiotoxicity when ifosfamide is used in combination with anthracyclines.64,65,66,67
Cisplatin
The platinum compounds produce interstrand DNA crosslinks. Cisplatin is known best for its nephrotoxicity; however, it has also been implicated as a cause of SVT, bradycardia,
ST-T wave changes, left bundle branch block, acute ischemic events, myocardial infarction, and ischemic cardiomyopathy.51,68,69 Such changes may be related to hypomagnesemia secondary to cisplatin-induced tubular defects. In addition, cisplatin has been associated with vascular toxicities including Raynaud phenomenon, hypertension, and cerebral ischemic events. It has been suggested that elevated levels of von Willebrand factor may be predictive of arterial occlusive events. Combination cisplatin-based chemotherapeutic regimens have been reported to be associated with coronary artery fibrosis, acute myocardial ischemia, myocardial infarction, and CHF. Of particular concern is the potential for an increased risk of late cardiovascular toxicity in long-term survivors treated with cisplatin-containing regimens, specifically young men treated with cisplatin-based chemotherapy for germ cell tumors. However, most of these patients have received regimens containing multiple drugs exhibiting potential cardiac toxicity.70,71,72,73,74,75,76,77,78 Conversely, one retrospective study of 270 testicular cancer patients receiving cisplatin-based chemotherapy failed to demonstrate acute cardiovascular toxicity.79 Despite definitive proof, most experts agree that young men who previously received cisplatin-based chemotherapy should at least be screened for hypertension every 2 years and dyslipidemia every 5 years.
ST-T wave changes, left bundle branch block, acute ischemic events, myocardial infarction, and ischemic cardiomyopathy.51,68,69 Such changes may be related to hypomagnesemia secondary to cisplatin-induced tubular defects. In addition, cisplatin has been associated with vascular toxicities including Raynaud phenomenon, hypertension, and cerebral ischemic events. It has been suggested that elevated levels of von Willebrand factor may be predictive of arterial occlusive events. Combination cisplatin-based chemotherapeutic regimens have been reported to be associated with coronary artery fibrosis, acute myocardial ischemia, myocardial infarction, and CHF. Of particular concern is the potential for an increased risk of late cardiovascular toxicity in long-term survivors treated with cisplatin-containing regimens, specifically young men treated with cisplatin-based chemotherapy for germ cell tumors. However, most of these patients have received regimens containing multiple drugs exhibiting potential cardiac toxicity.70,71,72,73,74,75,76,77,78 Conversely, one retrospective study of 270 testicular cancer patients receiving cisplatin-based chemotherapy failed to demonstrate acute cardiovascular toxicity.79 Despite definitive proof, most experts agree that young men who previously received cisplatin-based chemotherapy should at least be screened for hypertension every 2 years and dyslipidemia every 5 years.
Busulfan
Busulfan is an alkylating agent frequently used at high doses as myeloablative therapy for bone marrow transplantation. One case of presumed busulfan-induced endocardial fibrosis has been reported.51
MICROTUBULE-TARGETING DRUGS
Vinca Alkaloids
The vinca alkaloids were the first group of drugs used as chemotherapeutic agents in this class. Initially isolated from the pink periwinkle plant, vincristine was first prescribed for control of hemorrhage and dental pain. Vincristine and vinblastine were approved as antineoplastic agents in the 1970s. In 1994, a semisynthetic derivative, vinorelbine was approved for the treatment of non-small cell lung cancer. Hypertension, myocardial ischemia, myocardial infarction, and other vasoocclusive complications have been implicated with the use of these drugs. Cardiotoxic complications have been described most commonly with vinblastine but have also been reported with vincristine and vinorelbine.80,81,82,83,84,85,86,87 This is not surprising given the fact that the original medical uses of the vinca alkaloids were related to their vasoconstrictive properties.
Paclitaxel
Similar to the vinca alkaloids, the taxanes affect microtubules. Paclitaxel, the prototypical taxane, was discovered and subsequently isolated from the bark of the Pacific yew tree, Taxus brevifolia. Paclitaxel has been demonstrated to cause cardiac arrhythmias, including an asymptomatic bradycardia that is reversible. In one phase II study of 45 patients, 13 of the patients treated with paclitaxel developed bradycardia and 2 patients progressed to a higher grade heart block.88 The occurrence of more clinically significant bradyarrhythmias, such as Mobitz type I and II and complete heart block, have been noted in other series of patients. In one large study of continuously monitored patients, the incidence was 0.1%.89 Most of these patients were asymptomatic and the arrhythmias spontaneously remitted. In rare cases, atrial and ventricular tachycardias, myocardial ischemia, and myocardial infarction have been described.90 These severe conditions often occurred in patients with underlying cardiac disease or an electrolyte abnormality.
The taxanes are frequently used in combination with anthracyclines. Although there is evidence of an additive effect with respect to cardiotoxicity in combination therapy, this topic is controversial.91,92,93 One trial reported that the incidence of cardiac toxicity was increased with combination therapy compared with an anthracycline alone. Of note, CHF may occur at a lower cumulative dose when this combination is used.94,95 It has been suggested that the maximum cumulative doxorubicin dose should be decreased to <380 per mg per m2 when it is used in combination with paclitaxel. However, the premature cardiotoxic effects may be related to decreased renal excretion of the anthracycline caused by paclitaxel, rather than to additive cardiotoxicity. Conversely, paclitaxel has been safely administered to patients with preexisting cardiomyopathy.96
Albumin-bound paclitaxel appears to have the same cardiac toxicity as nonalbumin-bound paclitaxel. Most common are asymptomatic ECG changes consisting of nonspecific ECG changes, sinus bradycardia, and sinus tachycardia.97 Rare cases of chest pain, SVT, and cardiac arrest have been reported.
Docetaxel
Conduction abnormalities, cardiovascular collapse, and angina have been reported with docetaxel.98,99,100,101 However, there is no convincing evidence that directly links docetaxel to these events. Evidence does exist for a potentiating effect of anthracycline cardiomyopathy associated with docetaxel administration. Recently, this combination was studied in the setting of newly diagnosed stage III breast cancer. Patients were treated neoadjuvantly with four cycles of doxorubicin and docetaxel and four cycles of doxorubicin and docetaxel adjuvantly. CHF was reported in 10.5% of the patients receiving this regimen with a mean decrease in ejection fraction of 25%. The total doxorubicin dose was <400 mg per m2 in all of the patients.102 Such data raise concern about the potentiation of anthracycline-related cardiomyopathy by docetaxel.
Eribulin mesylate
Eribulin is the newest microtubule inhibitor. Studies leading to the approval of eribulin identified QT prolongation by day 8 of therapy. Because of this, it is recommended that potassium and magnesium levels be corrected and monitored during therapy. Finally, this drug should be avoided in patients with a prolonged QT interval.103
ANTIMETABOLITES
5-Fluorouracil
The cardiotoxic effects of the antimetabolite 5-fluorouracil (5-FU) were first recognized by Dent and McColl104 in 1975. Since then, 5-FU has become the most widely investigated antineoplastic agent known to cause myocardial ischemia. Ischemic events are more common when this agent is administered in combination with cisplatin.1 By 1990, there were >67 cases reporting cardiotoxicity from 5-FU.105 A large cooperative series of 1,145 patients included 31 patients with cardiotoxicity, which represented 3% of the study population.106 A 1992 prospective study of high-dose continuous infusion reported a 7.6% incidence of cardiotoxic events.107 Silent ischemic changes noted on ECG were as high as 68% in patients undergoing continuous infusion monitored for 24 hours.108 Some authors have suggested as many as 50% of all patients treated with 5-FU have nonspecific changes on ECG, and up to 16% of patients demonstrate ST-segment depression suggestive of ischemia or ST-segment elevation suggestive of infarction. Underlying CAD is present in many of these patients and probably exacerbates the ischemic potential of 5-FU.18 Overall, the incidence of ischemic events is probably higher than that reported in the past.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree