Carcinoma of the stomach
Carl Schmidt, MD  Mariela Blum Murphy, MD
Mariela Blum Murphy, MD  James C. Yao, MD
James C. Yao, MD  Christopher H. Crane, MD
Christopher H. Crane, MD
Overview
Gastric cancer is the second most common cause of cancer-related death worldwide and risk factors include autoimmune gastritis, chronic Helicobacter pylori infection, obesity, and other causes. Some countries with higher incidence have established screening programs but methodology and eligibility remain controversial. Despite knowledge of risk factors and screening for high-risk populations, identification of earlier stage potentially curable disease remains a challenge.
Multiple genetic mutations are involved in gastric cancer pathogenesis, and mutations in the cellular adhesion protein CDH1 result in familial diffuse gastric cancer. Certain patterns of metastatic disease have been associated with particular molecular alterations. Increasing understanding of molecular pathways involved in gastric cancer has led to efficacious use of targeted biologic therapies with cytotoxic chemotherapy in the advanced setting, such as use of trastuzumab in patients with gastric cancer and HER2/neu overexpression.
Surgical resection is the primary potentially curative therapy in those without metastatic disease. Type of resection and extent of lymphadenectomy are not consistent, and standards for best practice are needed. Chemotherapy and radiation play an important role in the adjuvant setting and, therefore, decisions regarding choice and timing of these therapies require accurate staging by thorough evaluation with endoscopy, cross-sectional imaging and, in some cases, endoscopic ultrasound or positron emission tomography.
Palliative interventions and therapy are often important, both for patients with symptoms in the metastatic setting and after recurrence as cure is not achieved in either situation. Results of recent clinical trials are encouraging, and there have been multiple positive phase III trials in the adjuvant and metastatic setting during the past 10 years. Despite this, most people diagnosed with gastric cancer will die from it eventually, so much more research is needed.
Incidence and epidemiology
Despite a worldwide decline in gastric cancer incidence, gastric cancer remains the second most common cause of cancer-related death. Gastric cancer is particularly common in China (accounting for 42% of cases worldwide), South America, Eastern Europe, Japan, and Korea, where it is the most common malignancy.1 The age-adjusted death rate has been on the decline in the United States over the past 30 years and was 3.8 per 100,000 in 2005.2 However, this is mostly due to declining incidence; survival in patients with diagnosed gastric cancer remains poor with only modest improvements during recent years. According to the Surveillance, Epidemiology, and End Results (SEER) registry, the 5-year survival rate for gastric cancer (all stages) increased from 16.3% between 1975 and 1979 to 23% in 2000.2 It should be noted these data predate the publication of major trials of multimodality therapy with positive results and widespread acceptance.
Risk factors
Environmental insults to the gastric mucosa may eventually lead to atrophic gastritis resulting in metaplasia, a precursor condition for some gastric cancers.3 Other factors associated with increased risk include low serum ferritin levels, pernicious anemia, history of distal gastrectomy for peptic ulcer disease, and endemic H. pylori infection.4–6 While some evidence is suggestive, it remains unknown whether eradicating H. pylori infection reduces the incidence of gastric cancer.7 Behavioral associations with gastric cancer have long been thought to include dietary exposure to nitrates, nitrites, and bacterial or fungal contamination of food given decreasing incidence with the advent of refrigeration.8 Studies have also documented increased incidence of proximal gastric cancers with obesity and a protective effect of physical activity on overall rates of gastric cancer.9, 10 Red meat consumption has been examined by multiple observational studies and found to be associated with increased risk of gastric cancer.11, 12 Some studies suggest a dose–response relationship between amount of red meat consumption and risk lending further support to this possibility. Consumption of fruit conversely may reduce the risk of gastric cancer.13
Familial clustering has been noted to occur in approximately 1% of gastric cancer cases. Germ line mutations account for only a small portion of these cases. Hereditary Diffuse Gastric Cancer (HDGC) syndrome results from germ line mutations of the CDH1 gene; however, CDH1 mutation accounts for only a small percentage of families with a history of diffuse gastric cancer. The CDH1 gene is involved in cellular adhesion, and defects in this gene are also associated with lobular breast cancer.14 Affected individuals with gastric cancer inherit one copy of the defective CDH1 gene. Somatic mutation, deletion, or promoter methylation inactivates the other copy. Gastric cancers follow an autosomal dominant inheritance pattern with high penetrance.
Prophylactic gastrectomy is a consideration for members of families affected by HDGC syndrome. When prophylactic gastrectomy is performed, multifocal early gastric cancers are nearly always found in the resected specimen.15 Genetic counseling is recommended in suspected cases, considering the degree of penetrance and age of onset of known cases of gastric cancer in the family and the paucity of long-term outcomes data for prophylactic gastrectomy. Worster et al.16 studied the impact of prophylactic total gastrectomy on health-related quality of life (HRQOL). In 32 patients who underwent total gastrectomy, HRQOL was compared to 28 patients at risk for HDGC who did not undergo total gastrectomy. While physical and mental function returned to baseline by 12 months after operation, some symptoms persist, specifically, loose stools (70%), fatigue (63%), discomfort when eating (81%), reflux (63%), eating restrictions (45%), and body image (44%).
Gastric polyps occur in 27–70% of individuals with FAP (familial adenomatous polyposis).17, 18 While fundic gastric polyps are usually thought to be hamartomas, foveolar dysplasia and invasive adenocarcinoma have been described. Hereditary non-polyposis colorectal cancer (HNPCC) is a genetic disorder characterized by germ line mutations in a group of mismatch repair genes, including hMSH2, hMLH1, hMSH6, hPMS1, and hPMS2. Defects in these genes result in genomic instability characterized by microsatellite instability (MSI). Although colorectal and endometrial cancers are the most common manifestations of HNPCC, gastric carcinoma has also been observed. An analysis of the Korean Hereditary Tumor Registry showed a 3.2-fold increase in the relative risk of gastric cancer in families carrying the HNPCC mutation.19 However, germ line mutation of one of the mismatch repair genes accounts for only a small percentage of gastric cancers with MSI.
- Gastric cancer is the second leading cause of cancer-related death worldwide
- Risk factors include autoimmune gastritis, H. pylori infection and obesity
- Germ line mutations in the CDH1 gene are associated with hereditary gastric cancer
Pathology
Adenocarcinoma is the dominant histology in gastric cancer. The Lauren and World Health Organization (WHO) classifications are the two major systems used. The Lauren’s system classifies cancer as intestinal, diffuse, or mixed.20 The simplicity of this system has resulted in widespread use. Intestinal-type gastric cancer is also called epidemic-type gastric cancer. It features a retained glandular structure and cellular polarity. Grossly, it usually has a sharp margin. It arises from the gastric mucosa and is associated with chronic gastritis, gastric atrophy, and intestinal metaplasia. The diffuse-type histology is associated with an invasive growth pattern. Scattered clusters of uniform-sized malignant cells frequently infiltrate the submucosa with little glandular formation and mucin production is common (Figure 1).
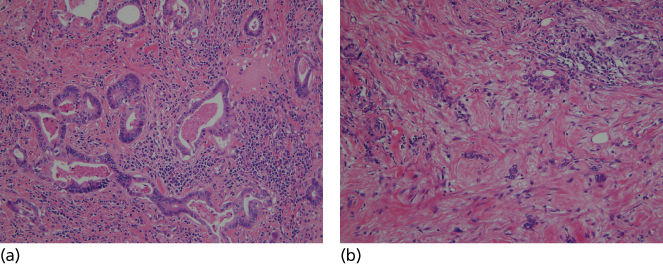
Figure 1 Photograph of histologic sections from intestinal (a) and diffuse (b) type gastric cancers.
Studies of gastrectomy specimens obtained from patients without clinical disease have shown early diffuse-type gastric cancer arising below normal-appearing epithelium.15 Tumor cells in this type appear to arise from the superficial layer of the lamina propria. An infiltrative growth pattern in diffuse-type gastric cancer often results in the absence of a mass. The cancer may be difficult to identify using endoscopy, but thickened gastric folds and a difficult to distend stomach are hallmarks of diffuse gastric cancer. Malignant cells can infiltrate well beyond the apparent tumor margin. In advanced cases, this leads to the condition known as linitis plastic (leather-bottle-like stomach) characterized by involvement of the entire stomach, rapid progression, resistance to therapy, and poor prognosis.
Pathogenesis and natural history
Molecular alterations
Multiple molecular alterations are important in the pathogenesis of gastric cancer (Table 1). Epigenetic phenomena include hypermethylation of promoter regions for many genes including CDH1, hMLH1, and p16, which may be involved in early carcinogenesis.21–27 Further, MSI caused by mutations in DNA repair genes has been reported in up to 39% of gastric cancers.28 Tumor suppressor genes such as p53, APC, MCC, and DCC are also mutated in gastric cancer, and the incidence often varies with histology and stage.29–31
Table 1 Molecular markers with clinicopathological correlations
| Marker | Involvement | Dominant histology | Clinical correlation |
| Epigenetic | |||
| Hypermethylation | Common | Both | — |
| MSI | 31–39% | Intestinal | Conflicting for survival, favorable or no difference |
| Tumor suppressor | |||
| p53 | 47–74% | Both (intestinal early event and diffuse late event) | Correlates with stage in diffuse-type cancer |
| APC | 8–34% | Intestinal | — |
| MCC | 24–33% | Diffuse | — |
| DCC | 12–49% | Intestinal | — |
| FHIT | 49–67% | — | Correlates with stage, conflicting for survival |
| Adhesion | |||
| CDH1 | 54–83% | Diffuse | — |
| α-Catenin | 83–92% | Diffuse | — |
| γ-Catenin | 91–100% | Diffuse | — |
| β-Catenin | — | Intestinal (GSK3β region) | Poor survival |
| CD44 | 31–72% | CD44v6-intestinal | CD44v6 in intestinal-type cancer correlates with inferior survival |
| Tyrosine kinases | |||
| EGFR | 35–81% | Both | Correlates with stage, conflicting for survival |
| HER2/neu | 10–38% | Intestinal | Poor survival |
| PDGF α | 42–45% | Both | Correlates with stage and poor survival |
| c-Met | 34–71% | Diffuse | Correlates with stage and peritoneal metastasis |
| Angiogenesis | |||
| VEGF | — | Intestinal | Correlates with hepatic metastasis |
| bFGF | — | Intestinal | Increased recurrence |
Loss of normal cellular adhesion is an important feature of human cancer development and prevalent in genetic alterations of gastric cancer. Diffuse-type gastric cancer is characterized by aberrant cellular adhesion with a pattern of infiltrative growth by a small cluster of or sometimes single tumor cells. The cadherin–catenin complex at the cell surface plays a critical role in cell adhesion and polarity, and up to 90% of gastric cancer cases have an abnormality in at least one component of the complex including CDH1 and α- and γ-catenin.32, 33 The CD44 transmembrane glycoprotein, expressed in 31–72% of gastric cancers, may modulate invasion and metastasis.34
Similar to other adenocarcinomas, alterations in cellular signaling pathways occur in gastric cancer and provide potential targets for biologic therapies. HER2/neu, an oncogene in the erbB family of membrane receptor tyrosine kinases, is overexpressed in 10–38% of gastric cancer cases and is associated with intestinal-type distal cancer and worse prognosis.30, 35 Expression of other cellular receptors in gastric cancer correlates with oncogenic behavior, such as associations between the transmembrane receptor EGFR (epidermal growth factor receptor) with invasion and c-Met with peritoneal metastasis.36 Angiogenesis appears essential for growth of gastric cancer and other solid tumors, and increased angiogenesis in gastric tumor specimens portends an unfavorable prognosis.37 Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are major regulators of angiogenesis, have prognostic value, and are potential targets for antiangiogenic therapy in patients with gastric cancer.38, 39 Expression of multiple transcription factors by gastric cancer cells, such as Sp1 and mTOR, promotes abnormal cell growth, survival, and angiogenesis.40, 41
Progression and patterns of metastasis
Gastric cancers metastasize in several ways. Adjacent organs such as the liver, diaphragm, pancreas, spleen, and colon (or its mesentery) may become involved by direct extension. Gastric cancers have a high tendency to spread through the lymphatic system to regional and distant nodes. Hematogenous metastatic disease is often found in the liver. Peritoneal metastatic disease is also common in the metastatic setting and may result in abdominal pain, bowel obstruction, cachexia, or all three. Japanese investigators have noted that histology and patient age may affect the pattern of spread of gastric cancer. In an autopsy study of 173 cases of gastric cancer, they found diffuse histology to be associated with peritoneal metastasis and intestinal histology to be associated with hepatic metastasis.42 They also found peritoneal metastasis to be more common in younger patients. In another study, case records of 216 patients with synchronous peritoneal or hepatic metastasis found at surgical exploration more commonly had poorly differentiated histology associated with peritoneal metastases and well to moderately differentiated histology associated with hepatic metastases.43
On a molecular level, expression of VEGF and its receptor KDR has been associated with liver metastasis.37, 44 Expression of VEGF-C, which can cause neogenesis of lymphatic vessels, is associated with lymph node metastasis.45, 46 In addition, dysregulation of cellular adhesion is likely central to the development of peritoneal metastasis. CD44H has been linked with increased gastric cancer cell adhesion to mesothelial cells and increased peritoneal metastasis in animal models.47 C-met amplification has also been linked with peritoneal metastasis.36, 48 Further translational research of the molecular biology of metastasis may improve our ability to predict sites of failure and refine therapeutic strategies.
- Molecular alterations of many genes and cellular pathways are involved in the pathogenesis of gastric cancer including modifications in methylation of promoters, MSI, cellular adhesion, growth factors, and angiogenesis
- Metastatic involvement of regional lymph nodes is very common
- Peritoneal surfaces and liver are other common sites of metastasis
Screening
Large-scale screening programs have been established in several countries with a high incidence of gastric cancer, including Japan, Korea, Venezuela, and Chile. Available screening tests include, among others, upper gastrointestinal endoscopy and radiologic studies using oral contrast agents such as barium. The method for most efficacious screening remains controversial, and there is no uniformity in terms of recommended age, interval, or type of screening exam. Comparisons between screening methods by large controlled trials are lacking. Screening of asymptomatic people in countries with lower risk of gastric cancer, such as the United States, is not feasible or cost-effective.
Importantly, symptoms of gastric cancer are often nonspecific, leading to diagnosis at an advanced stage. This is caused in large part because both the stomach and abdominal cavity are large and distensible. Early symptoms, such as vague discomfort, episodic nausea/vomiting, or anorexia are common in patients without cancer. Thus, physicians may not attribute such symptoms to gastric cancer for many months. In fact, patients may undergo several months of therapy for presumptive peptic ulcer disease before a diagnosis of gastric cancer. As such, more research is needed to develop better methods of early detection even in countries with lower incidence.
Diagnosis
Common symptoms of gastric cancer at diagnosis include abdominal pain and weight loss. Although anemia is also a frequent finding, overt upper gastrointestinal bleeding is much less common. Dysphagia may occur predominantly in patients with proximal cancer, whereas nausea and vomiting are more common in patients with nonproximal cancer. Early satiety can be especially prominent in patients with linitis plastica. Abnormal physical examination findings often indicate advanced disease, such as a palpable epigastric mass that may indicate a large locally advanced tumor. Jaundice usually indicates hepatic metastasis or metastatic lymphadenopathy in the portal region.
There are several aspects of appropriate clinical staging of gastric cancer that should be performed in a stepwise manner. Potentially helpful laboratory studies include a complete blood count, electrolytes, blood urea nitrogen, creatinine, alkaline phosphatase, transaminases, and bilirubin. Evaluation of tumor markers CEA, CA19-9, and CA125 may be considered. At the time of referral to surgeon or oncologist, upper endoscopy has typically been performed and made the diagnosis. Endoscopic findings should be reviewed by the primary treating surgeon with regard to tumor size and location, especially for gastroesophageal junction (GEJ) tumors as some may be more appropriately treated like distal esophageal cancers. Computed tomography (CT) scan of the chest, abdomen, and pelvis is performed to provide further information about the primary tumor and detect evidence for malignant lymphadenopathy or metastatic disease including carcinomatosis. The finding of even a small amount of ascites may indicate peritoneal disease.
Endoscopic ultrasound (EUS) is accurate for assessing T stage and enables needle biopsy of suspicious perigastric nodes and even some left liver masses. EUS may not always be needed when CT scan findings are suggestive of advanced cancer. EUS should be considered for patients entering neoadjuvant therapy trials and for assessment of small masses amenable to endoscopic mucosal resection (EMR) or early-stage cancers for which operation without neoadjuvant therapy is considered. The role of positron emission tomography (PET) for gastric cancer is evolving; there is some evidence PET can be used to evaluate response to therapy.49 Laparoscopy is controversial but considered essential by many for complete staging of gastric cancers. Laparoscopy results in upstaging in one-fifth to one-fourth of patients, primarily through detection of peritoneal metastases not seen on CT scans.50–52
TNM stage classification
Table 2 displays a portion of the current staging system for gastric cancer from the American Joint Committee on Cancer Staging Manual, seventh edition (Springer, New York 2010). This table applies to patients without metastatic disease (M1). In the current staging system, stages I to III are defined by various combinations of T (tumor) and N (nodal) stage without metastatic disease; prior versions of the system classified some patients with extensive nodal disease as stage IV. In the current system, stage IV disease is defined only by the presence of distant metastatic disease.
Table 2 AJCC stage grouping for gastric cancer without distant metastatic disease
| T stage | Nodal stage | |||
| N0 | N1 | N2 | N3 | |
| Tis | 0 | |||
| T1a/b | IA | IB | IIA | IIB |
| T2 | IB | IIA | IIB | IIIA |
| T3 | IIA | IIB | IIIA | IIIB |
| T4a | IIB | IIIA | IIIB | IIIC |
| T4b | IIIB | IIIB | IIIC | IIIC |
The following definitions are used to classify T and N stage:
- Tis—intraepithelial tumor with no invasion into lamina propria
- T1a—tumor invades lamina propria or muscularis mucosae
- T1b—tumor invades submucosa
- T2—tumor invades muscularis propria
- T3—tumor penetrates subserosal connective tissue without serosal invasion
- T4a—tumor invades serosa
- T4b—tumor invades adjacent organ or structure
- N1—regional nodal metastases, 1–2 nodes
- N2—regional nodal metastases, 3–6 nodes
- N3a—regional nodal metastases, 7–15 nodes
- N3b—regional nodal metastases, 16 or more nodes
- Early gastric cancer may be detected by screening in countries with such programs
- Possible symptoms of gastric cancer include abdominal pain, bleeding, dysphagia, nausea, anorexia, weight loss, and early satiety
- Clinical staging is based on upper endoscopy and cross-sectional imaging of the chest, abdomen, and pelvis (typically CT scan)
- Other potentially useful staging studies include laparoscopy, EUS and PET
Multidisciplinary care
For patients with small tumors and low histologic grade, EMR is gaining acceptance as the primary method for local therapy. Such therapy is predicated on these tumors having a very low incidence of node-positive disease.53 The incidence of nodal positivity with T1 tumors is approximately 10%, and other features of the primary tumor delineate patients with even lower risk. Tumors confined to the mucosa have a 1–3% incidence of nodal positivity versus a tumor with submucosal invasion with an incidence of up to 15%.54 Other factors that increase the incidence of nodal disease include poor differentiation, signet-ring cells, lymphatic invasion, and tumor size >2 cm.55 It is reasonable, therefore, to consider EMR for patients with small, well-differentiated T1 tumors confined to the mucosa. However, specialists at some high-volume centers have proposed expanded criteria for use of newer techniques using endoscopic submucosal dissection.56 Removal of larger even ulcerated masses has been done. In our opinion, this more aggressive approach requires careful histological evaluation of the resected specimen and confirmation of T1a (mucosal) disease.
Surgery
The choice of operation for gastric cancer depends on tumor location, histological type, and disease stage. Gastrectomy is the most widely used approach for invasive gastric cancer, and the most common techniques are total gastrectomy, distal subtotal, and proximal subtotal gastrectomy. Segmental resection is less commonly used for invasive gastric cancer but is very common and appropriate for other gastric malignancies such as gastrointestinal stromal tumors. Prospective and randomized studies reveal no survival advantage of total gastrectomy for tumors of the distal stomach compared to distal subtotal gastrectomy when all disease is removed with adequate margins. Both techniques are associated with similar rates of mortality (1–3%), complications, and 5-year survival (around 60%).57, 58 In most series, the quality of life after subtotal gastrectomy is superior to that after total gastrectomy.59, 60
Tumors of the proximal stomach and GEJ generally require more complex considerations for resection and reconstruction. Siewert’s classification is very useful and commonly used to describe GEJ tumors.61, 62:
- Type I: adenocarcinoma of the distal esophagus, which usually arises in Barrett’s esophagus and may infiltrate the GE junction from above
- Type II: true carcinoma of the cardia arising immediately at the GEJ within 1 cm above and 2 cm below the junction
- Type III: subcardial gastric carcinoma infiltrating the junction and distal esophagus from below
In patients with an advanced tumor involving the GEJ, the site of origin (esophagus or stomach) may be unclear. Patients with type I tumors are often treated as distal esophagus cancers with consideration of preoperative chemoradiation for more advanced tumors and surgical resection using a gastric conduit with anastomosis in the neck or chest. In some cases, the jejunum or colon is used instead of the stomach for the conduit. Type II and III tumors may be removed with either total gastrectomy or proximal subtotal gastrectomy often through a transabdominal approach depending on the local extent of the tumor.63, 64 Total gastrectomy is generally favored in the United States over proximal gastrectomy because reflux esophagitis is rare after Roux-en-Y esophagojejunostomy reconstruction compared to roughly one-third of patients who will have significant reflux after proximal subtotal resection.59, 65, 66 Further, proximal subtotal gastrectomy may fail to remove enough nodal tissue from the lesser curvature, a common site of nodal metastasis. However, some surgeons continue to advocate for proximal subtotal gastrectomy.64
The extent of lymph node dissection is one of the most controversial surgical topics in the management of gastric cancer. Radical lymph node dissection involves removal of lymph nodes beyond the usual field of gastrectomy. The Japanese Gastric Cancer Association defines extent of lymph node dissection using the designation “D.”67 Generally, D1 dissection includes perigastric lymph nodes. D2 dissection extends the lymphadenectomy to include nodes along the hepatic, left gastric, celiac, and splenic arteries (Figure 2). D3 dissection includes nodes along the porta hepatis and in the retropancreatic and periaortic regions.
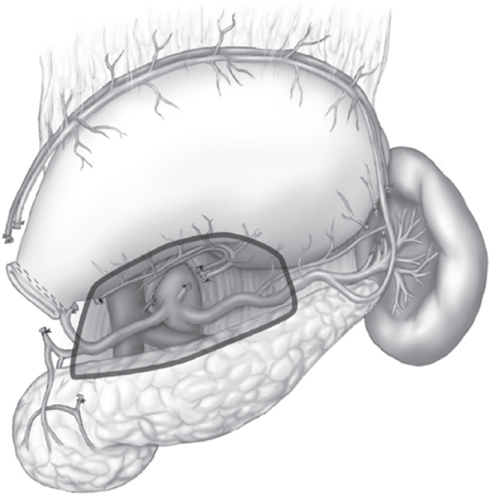
Figure 2 Area of critical modified D2 lymphadenectomy including celiac, hepatic, left gastric, and splenic artery lymph nodes (shaded area).
The largest prospective study examining the potential benefit of extended lymphadenectomy is the Dutch D1D2 lymphadenectomy trial which randomized more than 1000 patients (711 treated with curative intent) to D1 or D2 lymphadenectomy in the setting of gastrectomy for cancer, with surgical quality carefully controlled.68, 69 Operative morbidity and mortality rates were significantly greater in the D2 group than in the D1 group (43% and 10%, respectively, vs 25% and 4%, p < 0.01). However, the increase in mortality rate was associated with either male patients undergoing D2 dissection or patients undergoing splenectomy and distal pancreatectomy for complete nodal dissection. Patients who underwent D2 dissection with preservation of the spleen/tail of the pancreas had operative mortality similar to that of patients who underwent D1 dissection. After median follow-up of 15.2 years, the D2 lymphadenectomy group had a higher disease-specific survival (DSS) rate compared to the D1 group and lower rates of local (12% vs 22%, p = 0.02) and regional (13% vs 19%, p = 0.02) recurrence.70
A recent trial from the Italian Gastric Cancer Study Group evaluated the role of modified organ-preserving D2 lymphadenectomy.71 Patients with potentially curable gastric cancer were randomized (intraoperative) to D1 or D2 dissection. Partial pancreatectomy or splenectomy was done only when local invasion was suspected. A total of 267 patients were randomized, and morbidity and mortality were 12% and 3%, respectively, for the D1 group and 18% and 2% for the D2 group. Five-year DSS rates were 71% and 73% for the D1 and D2 groups; subgroup analysis revealed that in patients with node-positive disease the 5-year DSS rate was 61% in the D2 group versus 46% in the D1 group. Further, in patients with T-stage 2–4 and positive lymph nodes, the 5-year DSS was 59% in the D2 arm versus 38% in the D1 arm (Figure 3). The National Comprehensive Cancer Network (NCCN) gastric cancer panel has recommended consideration of modified D2 lymphadenectomy sparing distal pancreas and spleen in the setting of gastrectomy for cancer if available from an experienced surgeon at a high-volume center.72
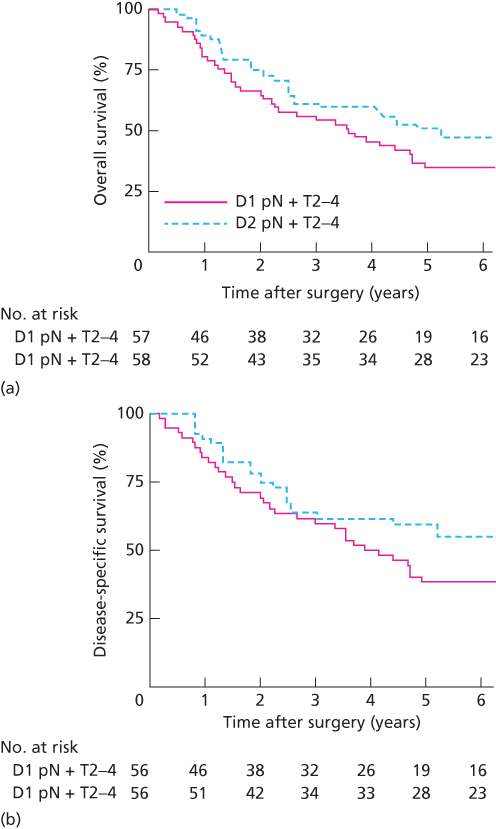
Figure 3 Kaplan–Meier curves of overall survival (a) and disease-specific survival (b) for patients with pathologic T2-4 and node-positive disease from the Italian Gastric Cancer Study Group D1D2 lymphadenectomy trial.71
Linitis plastica is an extremely virulent form of gastric cancer and is considered incurable by many clinicians. Some feel that patients with linitis plastica should never undergo gastrectomy since at best the 5-year survival is <10%.73 One approach to patients with linitis plastica is to evaluate with staging laparoscopy and, in the absence of metastatic disease, treat first with neoadjuvant therapy in the hope of selecting patients who do not develop metastatic disease for eventual gastrectomy. If a patient with linitis plastica has a positive margin of resection after operation, one should be cautious as to whether this deserves consideration or specific therapy given that isolated local recurrences are uncommon and rarely impact survival compared to the risk of metastatic disease. Recent evidence from the U.S. Gastric Cancer Collaborative suggests the traditional teaching that wide > 5 cm proximal margins are best for resection of distal cancers may not be necessary.74 In their retrospective cohort study combining data from seven academic medical centers, the proximal margin distance was not an independent factor associated with overall survival (OS). Rather, T and N-stage were the primary associated factors, and a 3 cm margin was adequate in most patients.
While studies mentioned earlier indicate multivisceral resection should not be done routinely during lymphadenectomy, resection of other organs is sometimes needed when involved by the primary tumor or grossly involved nodes. Investigators from Memorial Sloan-Kettering Cancer Center found that roughly one-third of 800 patients who underwent R0 resection of gastric cancer over 15 years required removal of at least one additional organ.75, 76 The operative mortality rate was 4% in these patients, similar to that reported in the Dutch trial for limited dissection and far lower than that for D2 dissection with adjacent organ resection. Interestingly, the likelihood of actual adjacent organ invasion was low (14%) after final pathological examination. The 5-year survival rate in these patients was 32% compared to 50% in patients who did not require multivisceral resection. These data support application of multivisceral resection when required to achieve R0 resection at centers where the operative mortality rate is low.
Minimally invasive approaches to the conduct of subtotal or total gastrectomy have increased in recent years using laparoscopy, computer-aided (robotic) surgery, or hybrid procedures. Prospective high-quality comparisons of these techniques to open surgery have not been done. There are multiple retrospective studies comparing them, which suggest that short- and long-term patient outcomes are improved or not adversely affected by minimally invasive techniques.77, 78 While operating time and cost may increase with these approaches, especially early in the surgeon’s learning curve, such costs may be balanced by other possible cost savings with shorter length of stay and more rapid overall recovery. However, all studies so far without randomization are subject to patient selection bias and, therefore, require caution when interpreting any possible benefits.
- EMR is considered with small T1a gastric cancers without adverse pathologic features
- Surgical resection is typically partial or total gastrectomy with upper abdominal (D1 or modified D2) lymphadenectomy
- Removal of other organs when needed due to local invasion is appropriate
- Use of laparoscopic and robotic techniques deserves further study
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








