Poorly Differentiated Neoplasms of Unknown Primary Site
If the pathologist cannot differentiate a general category of neoplasm (e.g., carcinoma, lymphoma, melanoma, sarcoma), the tumor is designated a poorly differentiated neoplasm. A more precise diagnosis is essential because many patients in this category have responsive tumors. Approximately 5% of all patients with CUP (4,000 patients annually) present with this diagnosis after a standard histologic evaluation. However, only a few remain without a defined lineage after specialized pathologic studies including IHC staining, electron microscopy, and, recently, MTP.4–7 (These techniques are discussed separately.) In reported series, 35% to 65% of poorly differentiated neoplasms were found to be lymphomas, which were highly responsive to specific therapy.4,5 Most of the remaining tumors were carcinomas, including poorly differentiated neuroendocrine tumors. Melanoma and sarcoma together account for less than 15% of all patients.
Poorly Differentiated Carcinoma
Poorly differentiated carcinomas (PDC) account for approximately 30% of CUP (about 25,000 patients annually). In approximately one-third of these patients, some features of adenocarcinomatous differentiation can be identified (poorly differentiated adenocarcinoma). Some patients have extremely responsive neoplasms, and therefore, a careful pathologic evaluation is crucial.
Histopathologic features that can differentiate chemotherapy-responsive tumors from nonresponsive tumors have not been identified.8 Even with a careful retrospective review of these tumors, responsive tumors of well-defined types (e.g., germ cell tumor, lymphoma) are only rarely identified.
All PDCs should undergo additional pathologic study for the purposes of (1) identifying other tumor types (e.g., lymphoma, sarcoma, melanoma) occasionally mistaken for carcinoma, (2) identifying neuroendocrine tumors, and (3) to determine the tissue of origin. Additional pathologic studies should include IHC stains, an MTP assay, and occasionally, electron microscopy and a karyotypic/cytogenetic analysis.
Adenocarcinoma
Well-differentiated and moderately differentiated adenocarcinomas are the most common tumors identified by light microscopy and account for 60% of CUP diagnoses (about 50,000 patients annually). These are the patients that many physicians associate with the entity of CUP. Typically, patients are elderly and have metastatic tumors at multiple sites. The sites of metastasis frequently determine the clinical presentation; common metastatic sites include the lymph nodes, the liver, the lung, and the bone.
The diagnosis of adenocarcinoma is based on light microscopic features, particularly the formation of glandular structures by neoplastic cells. All adenocarcinomas share histologic features, and the primary tumor site usually cannot be determined by a histologic examination. Certain histologic features are typically associated with a particular tumor type (e.g., papillary serous features with ovarian cancer and signet ring cells with gastric cancer). However, these features are not specific enough to be used as definitive evidence of their origin.
The identification of relatively cell-specific proteins by IHC staining has improved the ability to predict the tissue of origin in CUP patients.7,9,10 Panels of IHC stains are most useful and are often directed by clinical features (e.g., sites of metastases, gender). Molecular tumor profiling assays are relatively accurate and often provide additional diagnostic information. Both of these diagnostic modalities should be utilized in the pathologic evaluation of adenocarcinoma of unknown primary site.
Squamous Carcinoma
Squamous carcinoma represents approximately 5% of patients with CUP (about 4,000 patients annually). Approximately 90% of these patients have specific clinical syndromes for which effective treatment is available; therefore, an appropriate clinical evaluation is important.
A definitive diagnosis of squamous carcinoma is usually made by a histologic examination. On occasion, an MTP assay may diagnose the tissue of origin. An additional pathologic study with IHC and MTP should be considered in patients with poorly differentiated squamous carcinoma, particularly if the clinical presentation is atypical.
Neuroendocrine Carcinoma
Neuroendocrine carcinoma with widely varying clinical and histologic features account for approximately 3% of all CUP (about 3,500 patients annually). Improved pathologic methods for diagnosing neuroendocrine tumors have resulted in the recognition of an increased incidence and wider spectrum of these neoplasms.11
Two general subgroups can be routinely recognized by histologic features. Low-grade tumors share the same histologic features as carcinoids and islet cell tumors and may secrete bioactive substances. An MTP assay may point to the tissue of origin12,13; in some instances (e.g., pancreatic origin), defining the site of origin carries treatment implications. A second histologic group (variously described as small-cell carcinoma, atypical carcinoid, or poorly differentiated neuroendocrine carcinoma) has typical neuroendocrine features and a high-grade histology.
A third group cannot be recognized on a histologic examination, because neuroendocrine features are absent. These tumors appear histologically as poorly differentiated neoplasms or PDCs; an accurate identification requires IHC staining, an MTP assay, or occasionally, electron microscopy.
Immunohistochemical Tumor Staining
Immunohistochemical staining is the most widely available specialized technique for the classification of neoplasms.7–,22 Staining usually can be done on formalin-fixed, paraffin-embedded tissue, which broadens its applicability. Most IHC antibodies are directed at normal cellular proteins that are retained during neoplastic transformation. The ongoing development of antibodies to increasingly tissue-specific proteins makes this area of diagnostic pathology a dynamic and evolving field.
Several important questions can usually be answered by IHC staining. The correct lineage of poorly differentiated neoplasms can usually be identified (Table 113.1).7,9,10,14–16 In particular, lymphomas (common leukocyte antigen staining) and poorly differentiated neuroendocrine carcinomas (chromogranin, synaptophysin, and CD56 staining) can be recognized,10,15 and staining for germ cell tumors (octamer-binding transcription factor 4 [OCT4], placental alkaline phosphatase [PLAP]) may be diagnostic in an appropriate clinical situation.
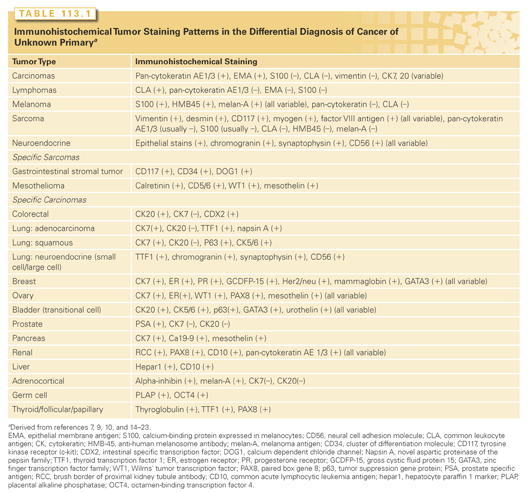
The ability of IHC staining to identify the origin of various neoplasms has improved, but in most cases, the staining results must be interpreted in the context of clinical and histologic features. An exception is the prostate-specific antigen (PSA) stain, which is very specific for prostate carcinoma.7 Stains suggestive of other primary sites are summarized in Table 113.1. The use of panels improves specificity7,9,10,19–22; several classic staining patterns have been described that are usually diagnostic of the tissue of origin (e.g., CK7+/CK20–/TTF-1+ in lung adenocarcinoma or CK7–/CK20+/CDX2+ in colorectal carcinoma). Four stains (CK7, CK20, thyroid transcription factor-1 [TTF1], CDX2) form the basis of several diagnostic patterns or suggest other possibilities and are appropriate initial stains on most CUP biopsies.
Several problems are associated with the IHC stains. Technical expertise is required to perform these tests accurately; interpretation is subjective and requires an experienced pathologist. False-positive and false-negative results can occur with any of these stains. For example, some carcinomas stain with vimentin, some sarcomas stain with cytokeratins, and a wide variety of carcinomas do not always stain in the expected patterns.7,22 Some classic staining patterns (see Table 113.1) can overlap with staining patterns of other carcinomas, forcing the pathologist to consider two or three possible primary sites. It is not feasible to routinely perform multiple unselected stains on biopsy specimens, and management of the tissue is becoming extremely important. Consideration of the clinical setting helps to direct the selection of stains and may narrow the spectrum of possibilities if patterns are not completely specific. For example, in a patient with mucin-positive adenocarcinoma and metastases to the liver, a CK20+/CK7–/CDX-2+ staining pattern provides strong evidence for colorectal carcinoma. The IHC findings may lead to additional diagnostic procedures; in the previous example, a colonoscopy should be performed and may identify the anatomical primary site.
In many cases, a single tissue of origin site cannot be identified with certainty even after an histologic examination, IHC staining, and correlation with clinical features. An electron microscopy or karyotypic analysis is occasionally helpful in this setting. However, the use of MTP assays allows for the identification of the tissue of origin in many cases where IHC is nondiagnostic.
Electron Microscopy
A diagnosis can be made by electron microscopy in some poorly differentiated neoplasms, but should be reserved for the study of neoplasms whose lineage is unclear after a routine light microscopy, IHC staining, and an MTP assay. Electron microscopy may be useful in undifferentiated sarcoma. Ultrastructural features such as neurosecretory granules (neuroendocrine tumors) or premelanosomes (melanoma) can suggest a particular tumor. Undifferentiated tumors can lose these specific ultrastructural features; therefore, the absence of a particular ultrastructural finding cannot be used to rule out a specific diagnosis. Electron microscopy is not able to distinguish among various adenocarcinomas and cannot usually identify a tissue of origin.
Karyotypic or Cytogenetic Analysis
The existence of specific chromosomal abnormalities is well characterized in several neoplasms. Most B-cell non-Hodgkin’s lymphomas are associated with tumor-specific immunoglobulin gene rearrangements, and typical chromosomal changes have been identified in some B-cell and T-cell lymphomas and in Hodgkin’s lymphoma.23,24 In rare instances, these studies or an MTP assay is necessary when a diagnosis of lymphoma cannot otherwise be established.
The recognition of specific chromosome 12 abnormalities in germ cell tumors (e.g., i[12p], del[12p], multiple copies of 12p) occasionally allowed for the identification of extragonadal germ cell tumors in young men with poorly differentiated carcinoma of unknown origin. Motzer et al.25 performed a karyotypic analysis on tumors in 40 young men with the extragonadal germ cell syndrome or midline carcinomas of uncertain histogenesis. In 12 of the 40 patients, abnormalities of chromosome 12 (e.g., i[12p]; del [12p]; multiple copies of 12 p) were diagnostic of a germ cell tumor. Other specific abnormalities were diagnostic of melanoma (two patients) and one patient each with lymphoma, peripheral neuroepithelioma, and desmoplastic small cell tumor. Of the germ cell tumors diagnosed on the basis of genetic analysis, five patients achieved a complete response to cisplatin-based chemotherapy. These data confirm the authors’ previously formulated hypothesis that some of these patients have histologically atypical germ cell tumors.
A few other nonrandom chromosomal rearrangements have been identified and occasionally can be useful in the diagnosis of CUP. Some examples include: t(11:22) in peripheral neuroepitheliomas, desmoplastic small round cell tumors, and Ewing’s tumor26–28; t(15:19) in children and young adults with carcinoma of midline structures of uncertain histogenesis29; chromosome 12 abnormalities in germ cell tumors30–32; t(2:13) in alveolar rhabdomyosarcoma; 3p deletion in small-cell lung cancer; 1p deletion in neuroblastoma; t(X:18) in synovial sarcoma; and 11p deletion in Wilms’ tumor. Epstein-Barr viral genomes in the tumor cells of CUP patients with cervical node metastases highly suggest nasopharyngeal primaries.33,34
However, most of these neoplasms can now be identified using methods that are more widely available (IHC, MTP assays), and karyotypic analyses should be reserved for patients with the histologic diagnoses of poorly differentiated neoplasm or PDC, in whom other studies fail to narrow the diagnostic spectrum.
Molecular Tumor Profiling Assays and Cancer Classification
Gene expression or molecular profiling of human neoplasms arose from a DNA microarray analysis described about 20 years ago,35,36 and subsequent studies have expanded genomic understanding of neoplasms.37–45 A pivotal study in cancer classification was reported by Golub et al.,37 and demonstrated for the first time that patterns of gene expression alone could discriminate acute myeloid leukemia from acute lymphoblastic leukemia. Other investigators demonstrated that numerous cancer types could be classified accurately by measuring the differential expression of specific gene sets.41–61 The basis of molecular profiling in recognizing specific cancer types is the identification of the genes responsible for the synthesis of proteins required for specific normal cellular functions or relatively specific cytoplasmic microRNAs in the many different normal cell types in humans. Cancer cells often retain some of the functional characteristics specific to their tissues of origin, and can be recognized by their gene expression profiles.59 Therefore, molecular profiling assays designed to determine the type of cancer measure gene expression dynamics in relation to cell lineage, rather than cancer-specific molecular abnormalities.
Patients with CUP represent a large heterogeneous group with clinically undefined anatomical primary tumor site and are ideal candidates for classification by molecular profiling.38 Several MTP assays (molecular cancer classifiers) have been validated in the identification of known cancers and studied in CUP.
Two such assays are commercially available.59,61 One of these is a 92-gene reverse transcription polymerase chain reaction (RT-PCR) mRNA assay59 (Cancer TYPE ID; bioTheranostics, Inc.), whereas the other uses microarray methodology61 to measure tissue-specific microRNAs (Cancer of Origin Test, Rosetta Genomics). A third microarray mRNA assay was previously also offered (Tissue of Origin Test), but is no longer available.49
Two studies of more than 100 tumors each have compared the accuracy of IHC and MTP assays in determining the tissue of origin of known primary cancers.62,63 These blinded studies usually had generous amounts of tissue to test and allowed the participating pathologists to do numerous IHC stains. The MTP assay diagnostic accuracy was superior to IHC, particularly when tumors were poorly differentiated, or when the IHC diagnosis was unclear following the first round of IHC stains. In addition, the molecular diagnosis required much less tumor tissue (two to three unstained slides). These data support a further evaluation of MTP assays in CUP, particularly when IHC staining is inconclusive or when limited biopsy tissue is available.
STUDIES OF MOLECULAR GENE EXPRESSION TUMOR PROFILING ASSAYS IN CANCER OF UNKNOWN PRIMARY DIAGNOSIS
Accuracy in Tissue of Origin Diagnosis
The determination of the tissue of origin in CUP patients has fundamental importance and has always been the goal of their evaluation. Improved IHC and MTP assays have the potential, when used in conjunction with other clinicopathologic data, to determine the tissue of origin in most CUP patients. However, in order to feel confident in the use of an MTP assay, three questions needed to be addressed: (1) Are they accurate in diagnosing known primary cancers? (2) Are they accurate in diagnosing the tissue of origin in CUP? (3) Are the outcomes of CUP patients improved by site-specific therapies directed by MTP diagnoses? Substantial evidence supports the accuracy of MTP in diagnosing known cancers and CUP, and some of these data are reviewed here. The third question has also been addressed and is discussed in a later section (see Site-Specific Treatment Directed by Results of Molecular Gene Expression Tumor Profiling Assays).
When applied to biopsy specimens from metastatic sites or primary tumors of known cancers, various MTP assays have correctly predicted the tissues of origins and/or primary sites in about 85% of patients.46–51,59–61 All these studies were blinded and used the known cancer type as the reference. The accuracy of the various MTP assays has not been directly compared.
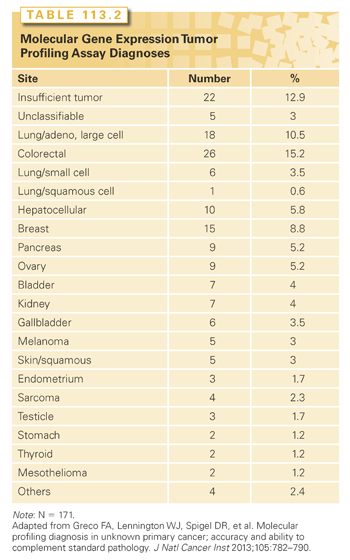
Accurate identification of the tissue of origin in CUP by MTP is difficult to validate, because the anatomical primary tumor site is unknown and only rarely becomes apparent during the subsequent clinical course of these patients. It would seem reasonable to assume that these assays have a similar accuracy rate in CUP because they have previously demonstrated in metastatic cancers of known types. This assumption is supported by the results of several studies in CUP patients (Table 113.2).46,47,53–58,64–68 Most studies of the accuracy of MTP assay diagnoses have been indirect, based on the correlation of the assay diagnosis with clinical features and other pathology studies (particularly IHC). In these studies, the MTP predictions correlated closely with the diagnoses suspected on the basis of standard clinical/pathologic findings. In a total of 698 CUP patients from a dozen studies using various MTP assays/platforms, an average of 80% of the molecular diagnoses were consistent with one of the suspected tissues of origin.46,47,53–58,64–68
The authors and associates have studied the initial version of the 92-gene RT-PCR MTP assay in CUP patients to determine the accuracy and ability to complement standard pathology.69 Three methods were used to assess the accuracy of the MTP assay, including a direct validation (gold standard reference) in patients who had their anatomical primary sites (latent primaries) found months to years after their initial CUP diagnoses. Two other indirect methods were used: (1) a comparison of the MTP and IHC diagnoses when IHC was able to predict a single tissue of origin, and (2) additional supportive directed IHC and clinical/histologic findings obtained after the MTP assay diagnoses.
A total of 171 CUP patients were evaluated: 151 prospectively seen from 2008 through 2010, and 20 others with latent primaries identified retrospectively from 501 patients seen from 2001 through 2008. The molecular diagnoses of these patients are listed in Table 113.2. Although the assay requires only 300 to 500 tumor cells, there was insufficient biopsy remaining (RNA) to perform the assay in 22 patients (12.9%). In 5 others (3%), the assay results were unclassifiable or not diagnostic of a single site. Of the 149 patients with sufficient tumor specimens, 144 (96%) were diagnosed with a single tissue of origin (23 tumor types).
Twenty-four patients (20 from retrospective group,58 and 4 from prospective group) had their latent primary sites detected at a median time of 12 months (range: 2.2 to 78.5 months) after their initial diagnosis/evaluation of CUP. In 75% (18 of 24 patients), the MTP assay diagnosis of their initial biopsies matched the latent primary tumor sites. In contrast, the IHC evaluation was successful in identifying the site of tumor origin in only 6 of 24 patients (25%).
The second method to assess the accuracy of MTP involved a comparison with IHC predictions in the 52 cases (30%) in which IHC suggested a single tissue of origin (Table 113.3). In 40 of these 52 patients (77%), the MTP and IHC diagnoses were identical. Others have reported similar results from five studies in a total of 65 patients (78% with identical IHC and MTP assay diagnoses) using various assays.54,55,57,67,70
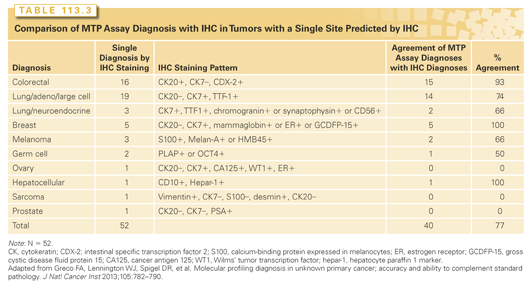
The third method to access MTP assay accuracy involved performing additional directed diagnostic studies not included in the initial evaluation (IHC studies, clinical testing, histology review) to support the MTP assay diagnoses. Fifty-four patients (32%) had MTP assay diagnoses that did not match any of the suggested IHC diagnoses; 35 of these patients had remaining biopsy specimens available for additional studies. In 74% of these patients (26 of 35), additional findings supported the accuracy of the MTP assay diagnoses (Table 113.4).
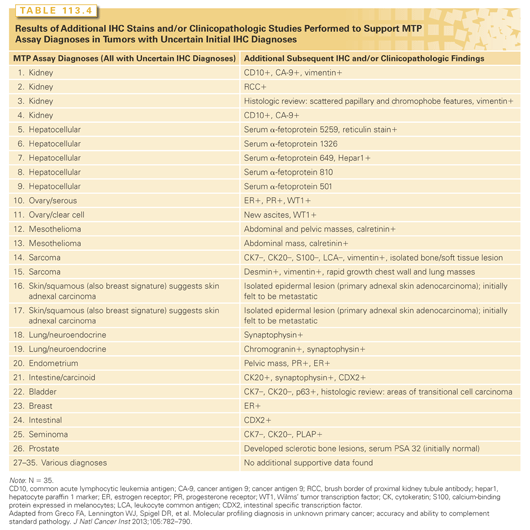
All three methods used in this study support the accuracy (75% to 80%) of the 92-gene RT-PCR assay in determining the tissue of origin in CUP. The accuracy is similar to that seen with MTP assays when tested in known cancer types. The aggregate data from many other studies in patients with CUP46,47,53–58,64–68 provide additional support of the value of molecular diagnoses. Molecular tumor profiling complements IHC, and usually provides a single diagnosis when IHC is inconclusive. When IHC predicted a single tissue of origin (as in 33% of patients in our series) the MTP assay prediction has high concordance and is probably not necessary.
As MTP is incorporated into the diagnostic evaluation of CUP patients, several potential pitfalls should be considered.69 First, tumor biopsy specimens are often small, and the medical oncologist and pathologist should use available tissue judiciously. Performing multiple IHC stains in a tumor that is difficult to classify can deplete the biopsy and preclude MTP. In some cases, a repeat biopsy should be considered. Second, MTP assay diagnoses are not 100% accurate, even in the identification of known cancer types. MTP predictions should always be considered in context with clinical features and results of other pathologic studies. If the MTP diagnosis is inconsistent with other findings, additional directed IHC stains or a clinical evaluation may help to support the diagnosis. Third, several neoplasms have overlapping gene expressions, and a misdiagnosis may occur in these circumstances (e.g., breast, salivary gland, and skin adnexal tumors have similar gene expression profiles). Fourth, any tumor types that are not included in the MTP assay panel cannot be diagnosed, and may be considered unclassifiable or misdiagnosed as a cancer with an overlapping gene expression profile.
Diagnosis in Poorly Differentiated Neoplasms
The use of modern IHC staining is usually effective at defining the tumor lineage in CUP patients with poorly differentiated neoplasms. In the uncommon tumor that defies classification, MTP usually clarifies the lineage and, in some cases, can identify the tissue of origin.71
From 2000 through 2012, 751 CUP patients were seen at the authors’ referral center,71 and 30 (4%) had no definitive lineage determined after extensive IHC (median of 18 stains; range: 9 to 51). The archival biopsies were tested with the 92-gene RT-PCR assay and when feasible, the additional evaluation was performed to support the molecular diagnoses (e.g., directed IHC not previously done, fluorescence in situ hybridization (FISH) for specific molecular abnormalities, gene sequencing, repeat biopsies, and correlation with clinical features). Four tumors were unclassifiable by MTP, and one additional tumor had insufficient biopsy material available (17%). A lineage diagnosis was made in 25 of 30 patients (83%), including carcinoma in 10 (germ cell: 3, neuroendocrine: 2, others: 5), sarcoma in 8 (mesothelioma: 3, primitive neuroectodermal tumor [PNET]: 1, others: 5), melanoma in 5, and hematopoietic neoplasm in 2 (lymphoma: 2). Additional directed IHC, genetic testing (BRAF, i12p), or repeat biopsies done after MTP confirmed the MTP diagnoses in 11 of 15 patients (73%). Earlier use of MTP in the diagnosis of these difficult cases would have resulted in the expedited identification of tumor lineage in almost all patients, and often in a prediction of the specific tissue of origin. Because this group of tumors contains germ cell tumors, lymphoma, neuroendocrine tumors, and melanomas, an accurate diagnosis is critical. Molecular tumor profiling appears superior to IHC in the diagnosis of these undifferentiated cancers.
Diagnosis of the Cancer of Unknown Primary Renal Cell Carcinoma Subset
The renal carcinoma subset of CUP has not been previously systematically addressed. A few CUP cases have been reported72,73 after recognition by pathologic features or MTP. The diagnosis of renal carcinoma is of practical importance, because these tumors are usually insensitive to cytotoxic chemotherapy, yet may often be treated with good control by a number of approved targeted/biologic drugs. When clear cell histology is seen in a CUP biopsy, the possibility of renal carcinoma is considered, and IHC may be diagnostic. However, histologies of adenocarcinoma or poorly differentiated carcinoma may not suggest renal carcinoma, and IHC stains to support the diagnosis of renal carcinoma may not be done.
In order to further characterize the renal cancer subset, 488 CUP patients seen from 2008 to 2012 had MTP of their biopsies using the 92-gene RT-PCR assay.74 In many of these patients, MTP results were available for patient management. A renal carcinoma diagnosis was made in 22 patients (4.5%), including the subtypes papillary in 8, clear cell in 7 and unknown in 7. None of these patients had a primary site detected by abdominal computed tomography, and the metastatic sites most often included retroperitoneal nodes (63%), mediastinal nodes (31%), lung (22%), and bone (18%). Only 1 biopsy had clear cell histology; 15 were PDC and 6 were adenocarcinomas (4 with papillary features). Because the histology did not suggest renal carcinoma in most patients, only three tumors (14%) had renal directed IHC (RCC, PAX8 stains) performed. However, additional IHCs performed to support the MTP assay diagnoses were typical of a renal origin in seven of nine tumors where a remaining biopsy was available. Sixteen patients received first-line treatment for advanced renal carcinoma; the objective response was 18% and the median survival was 13.4 months.
Renal cell carcinoma is a subset of CUP, which can be diagnosed by MTP assay and/or IHC. When the histologic examination does not identify clear cell features, the diagnosis of renal carcinoma may not be considered, and IHC staining for renal cancer may not be done. Papillary renal carcinoma was a relatively common subtype in the group studied. These patients are important to identify because they may benefit from renal cell–directed targeted drugs but not from empiric chemotherapy.
Diagnosis of the Cancer of Unknown Primary Colorectal Subset
These patients have been characterized recently and are a favorable subset. These data are discussed later (see section Treatment: Favorable Subsets: Colorectal Profile).
Summary
Molecular tumor profiling can predict the tissue of origin in a majority (about 95%) of patients with CUP when there is sufficient biopsy material available. These predictions are accurate in 75% to 80% of patients, as determined by several methods, including an evaluation of CUP patients who developed latent primaries. The correlation between IHC and MTP diagnoses is good (about 80%) when IHC predicts a specific tissue of origin; in patients with diagnostic IHC, MTP is not necessary. However, in the majority of patients, IHC is inconclusive and MTP provides valuable additional diagnostic information. The optimal identification and evaluation of several subgroups of potential therapeutic importance, including colorectal, renal cell, and poorly differentiated neoplasms now requires the use of MTP in conjunction with a standard pathologic evaluation. A specific diagnosis of some tissues of origin by MTP should trigger additional molecular analysis, because effective targeted therapy for patient subsets is becoming common. For example, a prediction of non–small-cell lung cancer, should prompt an analysis for treatable molecular alterations, including epidermal growth factor receptor (EGFR)-activating mutations and anaplastic lymphoma kinase (ALK) or ROS1 rearrangements.
CLINICAL FEATURES AND EVALUATION
Most patients with CUP develop signs or symptoms at the site of a metastatic lesion and are diagnosed with advanced cancer. The subsequent clinical course is usually dominated by symptoms related to metastases; a latent primary site becomes obvious in about 5% of patients during their lifetime. At autopsy, a primary site is identified in about 75% of patients.3,6 Primary sites in the pancreas, lung, colon/rectum, and liver account for approximately 60% of those identified. Primary sites in the breast, ovary, and prostate are uncommon in autopsy series, but data from MTP assay series suggest that the biliary tract, the urothelial tract, the breast, and the ovarian tissues of origin are more common than previously recognized.6,52
Although some clinical differences exist, there is substantial overlap between the clinical features of patients with adenocarcinoma and PDC. Patients with PDC have a somewhat younger median age and usually exhibit rapid tumor growth. These patients may also have a more frequent location of dominant metastatic sites in the mediastinum, retroperitoneum, and peripheral lymph nodes. The clinical evaluation of patients with these histologies should follow the same guidelines. Patients with neuroendocrine carcinoma and squamous carcinoma are discussed separately.
Clinical Evaluation
The recommended clinical evaluation for all patients is summarized in Table 113.5. In actuality, many of these procedures are usually done in the process of diagnosing CUP. The goal is to find the anatomical primary site or, if not possible, the tissue of origin. Although positron-emission tomography (PET) scanning may be useful in some patients,57 it should not be considered routine in the initial CUP evaluation; definitive data in large numbers of patients have not been published75 and PET was not superior to computed tomography (CT) in finding a primary site in one comparative study.76
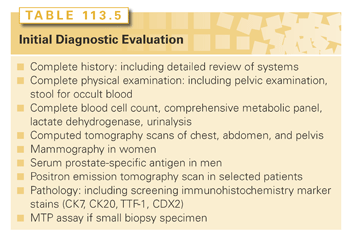
A further evaluation of patients should be directed by results of the initial clinical and pathologic evaluations. A focused evaluation may: (1) identify an anatomical primary site, (2) narrow the spectrum of possible tissues of origin, (3) identify specific favorable subsets of patients, or (4) identify the tissue of origin even if the anatomical primary site is undetectable.
Table 113.6 summarizes the additional evaluation indicated for several common clinical presentations. An additional focused evaluation should be triggered by either clinical findings or IHC results during the initial evaluation. An MTP assay is indicated when IHC or other testing is not conclusive. Panels of IHC stains and an MTP assay are complementary and when considered in conjunction with all other data provide a tissue of origin diagnosis in the majority of CUP patients.
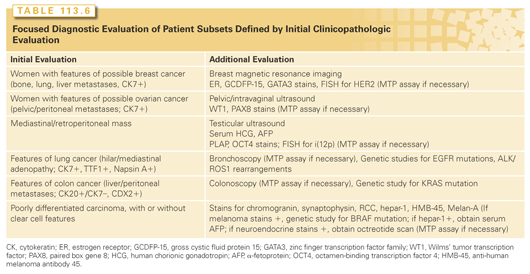
Neuroendocrine Carcinoma
Although the initial clinical evaluation is the same (see Table 113.5), patients with neuroendocrine carcinoma require special consideration in determining appropriate treatment. Of major importance is the separation of this group into tumors with low-grade histology (classic carcinoid) and indolent clinical course versus those with high-grade histology (small or large cell with neuroendocrine features) and an aggressive clinical course. Some of the high-grade carcinomas may not be recognized as neuroendocrine by H&E staining, but are usually diagnosed by IHC or MTP assay.
Low-grade neuroendocrine carcinomas, when presenting with an unknown primary site, most frequently involve the liver. Other metastatic sites include the lymph nodes (usually abdominal or mediastinal) and bone. Some are associated with various syndromes caused by the secretion of bioactive peptides (carcinoid syndrome, glucagonoma syndrome, VIPomas, Zollinger-Ellison syndrome). An additional clinical evaluation in these patients should include serum or urine screening for these substances. In addition to the evaluation listed in Table 113.5, an octreotide scan as well as an upper and lower gastrointestinal endoscopy should be performed, because some of these patients have detectable primary sites in the gastrointestinal tract. An MTP assay may diagnose the tissue of origin in some patients12,13; the identification of a pancreatic site of origin is potentially important because targeted drugs (sunitinib, everolimus) are useful in this setting.
High-grade neuroendocrine carcinomas of unknown primary site are usually found in multiple metastatic sites and rarely secrete bioactive peptides. Patients with small- or large-cell histology and a history of cigarette smoking should be suspected of having an occult lung primary and a fiber optic bronchoscopy should be considered. Patients with a positive tumor cell IHC stain for TTF1 should also be considered for a bronchoscopy. Extrapulmonary small-cell carcinomas arising from a variety of other primary sites (salivary glands, paranasal sinuses, esophagus, pancreas, colon/rectum, bladder, prostate, uterus, cervix) have been described and are occasionally identified during a clinical evaluation. A colonoscopy should be considered in patients with tumor IHC staining positive for CDX2.
The origin of these high-grade neuroendocrine carcinomas remains unclear. The tissue of origin may be determined in some patients by an MTP assay, but this knowledge now usually does not change therapy for most patients. Some patients with small-cell histology may have occult small-cell lung cancer. However, more than half of these patients have no smoking history, which makes this diagnosis unlikely. It has been speculated that these undifferentiated tumors share the same origin as the low-grade neuroendocrine tumors, and are at opposite ends of a spectrum of tumor biology. However, it now seems more likely that these high-grade neuroendocrine tumors have a different oncogenesis; many share the chromosomal abnormalities commonly seen in small-cell lung cancer (deletions of chromosomes 3p, 5q, 10q, and 17p), whereas no shared molecular abnormalities have been found with indolent carcinoid-type tumors.77,78
Anaplastic or atypical carcinoid tumors arising in the gastrointestinal tract are responsive to platinum-based chemotherapy, whereas carcinoid tumors with typical histology are usually resistant.79 A few reports of patients with extrapulmonary small-cell carcinomas of unknown primary site have also documented chemotherapy responsiveness and occasional long-term survival after systemic therapy.80,81 However, the term extrapulmonary small-cell carcinoma implies the existence of a known primary site; some CUP neuroendocrine tumors may have arisen from an occult extrapulmonary site, but are aptly described as neuroendocrine carcinoma of unknown primary site.
Squamous Carcinoma
Squamous carcinoma, as opposed to other histologies, often presents with isolated metastases in the cervical or inguinal lymph nodes. The cervical nodes are the most common metastatic site. Patients are usually middle aged or elderly, and frequently, they have abused tobacco or alcohol, although recently it has also been associated with human papilloma virus infection. When the upper or middle cervical nodes are involved, a primary tumor in the head and neck region should be suspected. The clinical evaluation should include an examination of the oropharynx, hypopharynx, nasopharynx, larynx, and upper esophagus by direct endoscopy, with a biopsy of any suspicious areas. CT of the neck better defines the disease in the neck and occasionally identifies a primary site. PET scanning in this subset is indicated, because it can frequently identify primary tumor sites.82 Detection of Epstein-Barr virus genome in the tumor tissue is highly suggestive of a nasopharyngeal primary site,33,34,83 particularly in poorly differentiated carcinomas. When the lower cervical or supraclavicular nodes are involved, a primary lung cancer should be suspected. A fiber optic bronchoscopy may identify a lung primary if other evaluations are unrevealing.84
An ipsilateral or bilateral tonsillectomy has been advocated as a diagnostic modality in patients with single nodal or bilateral nodal involvement, respectively.85 In one series of 87 patients who had tonsillectomy as part of their workup for cervical node presentations, 26% had a tonsillar primary identified.86 The identification of the primary site has several advantages in this group of patients, including the ability to develop a specific treatment plan, a reduction of the radiation therapy fields, more accurate assessment of prognosis, and easier follow-up.
Most patients with involvement of inguinal nodes have a detectable primary site in the anogenital area. Careful examination of the anal canal, vulva, vagina, uterine cervix, penis, and scrotum is important, with biopsy of any suspicious areas. The identification of a primary site in these patients is consequential because curative therapy is available for carcinomas of the vulva, vagina, cervix, and anus, even after it has spread to regional nodes.
Metastatic squamous carcinoma in areas other than the cervical or inguinal nodes usually represents metastasis from an occult lung cancer, but metastases from several other sites (the esophagus, skin, uterine cervix, and anal canal) are also possible. An MTP assay may be useful in the diagnosis of the tissue of origin and provides the basis of site-specific therapy.
The heterogeneous group of patients with CUP contains some patients who experience long-term survival after appropriate treatment and others for whom treatment makes little or no impact. Patients who have an anatomical primary site defined during their clinical evaluation do not have CUP and should be treated appropriately for their defined tumor type. A second group of patients can be identified as having favorable subsets and, in most, their tissues of origin may be presumed, even when the anatomical primary site is not identified (Table 113.7). The management of these subsets is detailed in this section. Most CUP patients (approximately 80%) do not fit into a favorable subset, even after an appropriate clinical and pathologic evaluation. Empiric chemotherapy has been the treatment standard for many years, and will be briefly reviewed. However, there is now ample evidence to support the use of site-specific therapy for most patients, guided by IHC and MTP predictions of the tissue of origin. These new data will also be reviewed.
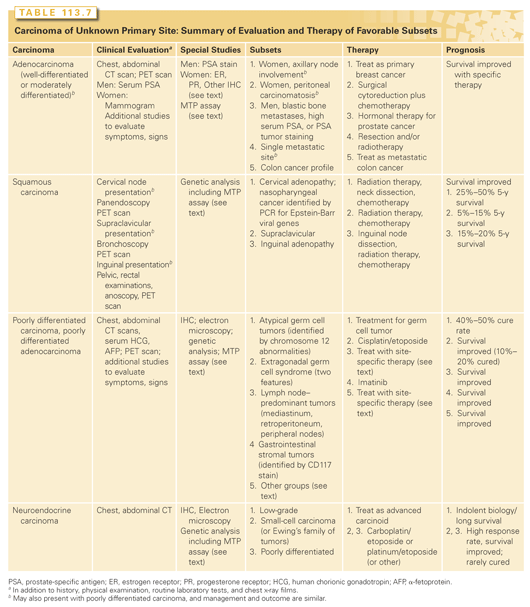
Favorable Subsets
Women with Peritoneal Carcinomatosis
Adenocarcinoma, particularly serous adenocarcinoma, causing diffuse peritoneal involvement, is typical of ovarian carcinoma, although carcinomas from the gastrointestinal tract, lung, or breast can occasionally produce this clinical syndrome. On occasion, women with diffuse peritoneal carcinomatosis have no primary site found in the ovaries or elsewhere in the abdomen at the time of laparotomy. These patients frequently have histologic features typical of ovarian carcinoma, such as papillary serous configuration or psammoma bodies, and also share clinical features, such as elevated serum cancer antigen 125 (CA-125) levels. These tumors are more common in women with a family history of ovarian cancer, and prophylactic oophorectomy does not always protect them from this tumor.87 Like ovarian carcinoma, the incidence of primary peritoneal carcinoma is increased in women with BRCA1/2 mutations.88
It is now clear that many of these carcinomas arise from the peritoneal surface (primary peritoneal carcinoma) or from the fimbriated end of the fallopian tubes.89,90 Many of these tumors have characteristic IHC findings (ovary pattern) or diagnostic MTP assays. Carcinomas arising from the peritoneal (mesothelial) surface or the uterine tubes share a common lineage (müllerian derivation) and biology with ovarian carcinoma. Support for this hypothesis has been strengthened by the demonstration of gene expression profiles nearly identical to ovarian carcinoma.58 Treatment of these women with standard ovarian carcinoma regimens (surgical cytoreduction followed by taxane/platinum chemotherapy) produces results similar to those seen in ovarian cancer.91–93 This entity has very rarely been seen in men.94
Women with Axillary Lymph Node Metastases
Breast cancer should be suspected in women who have metastatic carcinoma in an axillary lymph node.95 Men with occult breast cancer can present in this fashion, but these are very rare. The initial lymph node biopsy should be stained for IHC breast markers. When positive, these findings provide strong support of the diagnosis.96 An MTP assay may also be diagnostic in this setting.
If no other metastases are identified, these patients may have stage II breast cancer with an occult primary, which is potentially curable with appropriate therapy. Magnetic resonance imaging and PET have occasionally identified primary breast cancer even with normal mammography.97–99 A modified radical mastectomy has been recommended, even when physical examination and mammography are normal. An invasive breast primary has been identified after mastectomy in 44% to 80% of patients. Primary tumors are usually less than 2 cm in diameter and may measure only a few millimeters; occasionally in patients, only a noninvasive tumor is identified in the breast. Prognosis after primary therapy is similar to that of other patients with stage II breast cancer.95 Radiation therapy to the breast after axillary lymph node sampling and chemotherapy represents a reasonable alternative primary therapy.100 Either neoadjuvant or adjuvant systemic therapy is indicated in this setting, following guidelines established for the treatment of stage II breast cancer.
Women with metastatic sites in addition to the axillary lymph nodes, particularly when supported by IHC and/or a MTP assay diagnosis, should be managed as metastatic breast cancer. Hormone receptor and HER2 status are of particular importance in these patients because they may derive major benefit from hormonal therapy, chemotherapy, or HER2-targeted agents.
Men with Elevated Serum Prostate-Specific Antigen or Prostate-Specific Antigen Tumor Staining
Serum prostate-specific antigen (PSA) concentrations should be measured in men with adenocarcinoma of unknown primary site. These tumors can also be stained for PSA. Even when clinical features (i.e., metastatic pattern) do not suggest prostate cancer, a positive PSA (serum or tumor stain) is reason for a trial of androgen deprivation.101,102
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree