Prognostic Factors
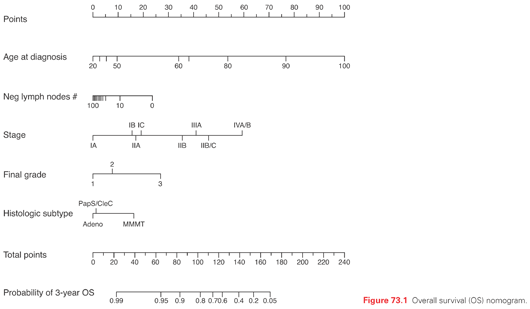
Several clinicopathologic factors have been identified in patients with endometrial carcinoma to help predict the prognosis and individualize the treatment plan.42,43 At Memorial Sloan Kettering Cancer Center (MSKCC), a nomogram was developed for predicting overall survival of women with endometrial cancer (n = 1,735) following primary therapy.44 Five prognostic factors (age, grade, histologic type, number of lymph nodes removed, and International Federation of Gynecology and Obstetrics [FIGO] 1988 surgical stage) were used to predict overall survival (OS) with high concordance probability (Fig. 73.1).
The adverse prognostic factors in endometrial cancer that are part of the staging system include depth of myometrial invasion, cervical stromal involvement, adnexal involvement, pelvic/para-aortic node involvement, extension into bladder or rectum, and distant spread.42,43 Other prognostic factors include age, grade, histologic type, and lymphovascular invasion (LVI). The adverse impact of older age is often explained away by pointing out that older patients tend to present with aggressive histologies and more advanced disease and are generally treated less aggressively. Age of ≥60 years, however, has been shown to be predictive of local-regional recurrence (hazard ratio [HR] 3.9; p = 0.0017) and death (HR: 2.66; p = 0.01) in a randomized trial limited to stage I and where patients with deep myometrial invasion and grade 3 were excluded.45 Further, the adverse impact of advanced age persists even when older patients are treated as aggressively as younger patients.46 It is unclear if the prognosis differs significantly between grade 1 and 2 patients. What is clear is the adverse impact of grade 3 on outcome. In the FIGO annual report,47 grade 3 was an independent predictor of poor survival on multivariate analysis within each stage: stage I (HR: 2.45), II (HR: 2.14), III (HR: 2.44), and stage IV (HR: 2.55). In that report, the 5-year survival rates based on histology were 83.2% for endometrioid adenocarcinoma, compared to 52.6% for serous and 62.5% for clear cell in 8,033 surgically staged patients.47 LVI is seen in about 15% of patients with clinically stage I (confined to uterus) endometrial cancer.42 Its presence causes a four- to six-fold increase in the rate of pelvic and para-aortic nodal metastases.42 This translates into more frequent relapses, including vaginal recurrences48,49 and a poorer outcome.50
On the basis of a prospective study of 366 patients with endometrial carcinoma, Bokhman51 postulated that there are two different pathogenetic types of endometrial carcinoma. Type I disease arises in women with obesity and signs of hyperestrogenism: anovulatory uterine bleeding, infertility, late onset of the menopause, and hyperplasia of the endometrium. The tumors tend to be well to moderately differentiated, with minimal invasion, and tend to carry good prognosis. Type II arises in women who lack signs of hyperestrogenism and tend to be older. The tumors are often poorly differentiated, with deep myometrial invasion and frequent occurrence of extrauterine spread.51 What is remarkable is that this clinical observation can now be confirmed at the molecular level. In a review by Dedes et al.,52 the most frequently altered molecular pathway in type I endometrial carcinomas is the phosphatidylinositol-3 kinase (PI3K)/phosphatase and tensin homolog (PTEN)/protein kinase B (AKT) pathway, whereas alterations in type II endometrial cancers involve the tumor suppressors p53 and/or p16. More recent genomics data seem to show that there are in fact four distinct types of endometrial cancer with different prognosis based on number of mutations (ultramutated versus hypermutated) and copy numbers (low versus high) observed.53
Staging
In 2009, the FIGO Committee on Gynecologic Oncology (Table 73.2) revised the 1988 staging for endometrial cancer.54 There were several modifications in the new staging system. Patients who used to be staged as IB (i.e., <50% myometrial invasion) are now considered IA. Those with ≥50% myometrial invasion are designated as stage IB. The discriminating power of the new FIGO staging system is being debated. Page et al.55 evaluated a total of 10,839 cases from 1998 to 2006 using the Surveillance, Epidemiology, and End Results program. The analysis demonstrated the usefulness of two divisions rather than three for stage I in the new FIGO staging system.55 In contrast, a study from MSKCC of 1,307 patients with FIGO 1988 stage I disease showed that the revised system for stage I did not improve its predictive ability over the 1988 system.56 Endocervical glandular involvement does not affect staging anymore; only patients with cervical stromal invasion are considered stage II. Having positive peritoneal cytology does not affect staging either. But it is important to continue to obtain peritoneal cytology because of its prognostic impact, especially in patients with other extrauterine features. Milgrom et al.57 reported on 196 patients with stage III (2009 FIGO staging system) endometrial cancer, where positive peritoneal cytology was associated with an increased hazard for relapse (HR 2.3; p = 0.002) and death (HR 2.9; p <0.001). Parametrial extension is now considered IIIB. Patients with stage IIIC are now subdivided into IIIC1 if pelvic nodes are involved and IIIC2 if para-aortic nodes are involved.
Treatment of Early-Stage (I-II) Disease
Surgery
The main treatment is simple hysterectomy/bilateral salpingo-oophorectomy (BSO), peritoneal cytology, and some form of regional lymph node assessment. Salpingo-oophorectomy is recommended since 5% of patients with clinically stage I (confined to uterus) endometrial cancer may have adnexal involvement.42 There is also the concern about synchronous ovarian cancer58; among all women with endometrial cancer, 3.1% have a synchronous ovarian cancer, but among women <50 years of age, the rate is as high as 9.4%. Differentiating between metastasis to ovary as opposed to synchronous ovarian cancer is not always easy. The use of genetic profiling may represent a powerful tool for use in clinical practice for distinguishing between metastatic and dual primaries in patients with simultaneous ovarian/endometrial cancer and predicting disease outcome.59 The revised staging system requires that cytology be obtained and be reported separately without changing the stage. Positive peritoneal cytology is seen in about 12% of patients with clinically stage I (confined to uterus) endometrial cancer.42 The simple hysterectomy could be done with an open approach (i.e., total abdominal hysterectomy [TAH/BSO]) or with a minimally invasive approach (i.e., laproscopic-assisted hysterotomy/BSO or robotic assisted hysterectomy/BSO). The GOG completed a trial where patients with clinical stage I to occult IIA uterine cancer were randomly assigned to laparoscopy (n = 1,696) or open laparotomy (n = 920), including hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. Laparoscopy had fewer moderate to severe postoperative adverse events than laparotomy (14% versus 21%, respectively; p <0.0001). Hospitalization of >2 days was significantly lower in laparoscopy versus laparotomy patients (52% versus 94%, respectively; p <0.0001). The conversion rate to laparotomy was 25.8%. The estimated 5-year OS was 89.8% for laparoscopy and 89.8% for laparotomy.60,61 In 2005, the US Food and Drug Administration (FDA) approved the da Vinci robotic system for gynecologic procedures. In a recent systematic review of robotic surgery in gynecology, investigators found that the proficiency plateau seems to be lower for robotic surgery than for conventional laparoscopy. The conclusion from this analysis was that the specific method of minimally invasive surgery, whether conventional laparoscopy or robotic surgery, should be tailored to patient selection, surgeon ability, and equipment availability.62
With regards to regional lymph node assessment, the options range from removing only grossly suspicious nodes to routine lymphadenectomy for all comers. Based on GOG 33 surgical-pathologic assessment,42 the rate of positive pelvic lymph node in patients who are otherwise clinically stage I (confined to the uterus) is 9% and the rate of positive para-aortic node is 6%. Two randomized trials addressed the role of lymphadenectomy in early-stage endometrial cancer. In both trials, patients with clinically stage I disease were randomized to pelvic lymphadenectomy versus none. Para-aortic node sampling was optional. In the first trial, 514 patients were randomized and with a median follow-up of 49 months, the 5-year disease-free and OS rates, in an intention-to-treat analysis, were similar between arms (81.0% and 85.9% in the lymphadenectomy arm and 81.7% and 90.0% in the no-lymphadenectomy arm, respectively).63,64
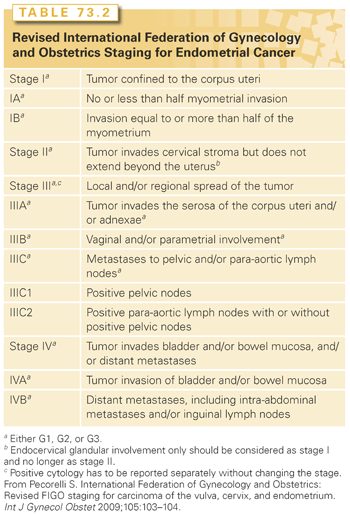
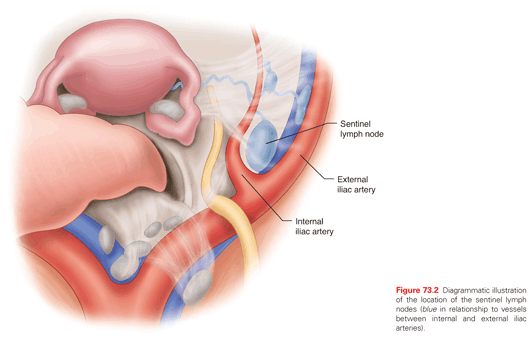
These two trials have been criticized extensively due to multiple flaws in design and to numerous study deviations. Nonetheless, most gynecologic oncologists agree that staging of apparent uterine-confined endometrial cancer is to help guide adjuvant therapy and potentially limit toxic additional therapy but by itself is not therapeutic. A middle ground approach between removing only grossly suspicious node to routine lymphadenectomy is emerging. It is sentinel lymph node (SLN) mapping (Fig. 73.2). In a prospective trial from France, regarding the role of SLN in early-stage endometrial cancer, at least one SLN was detected in 111 of the 125 eligible patients. Of the 111 patients, 19 (17%) had pelvic-lymph-node metastases. Three patients had false-negative results (two had metastatic nodes in the contralateral pelvic area and one in the para-aortic area), giving a negative predictive value of 97% (95% confidence interval = 91 to 99) and sensitivity of 84% (62% to 95%). SLN biopsy upstaged 10% of patients with low-risk and 15% of those with intermediate-risk endometrial cancer.65 Currently at MSKCC sentinel node mapping algorithm is utilized to further refine the utility of such technique.66 SLN mapping is for normal-appearing nodes. Any grossly enlarged or suspicious nodes must be evaluated irrespective of mapping. In fact such nodes, while being the sentinel nodes, often do not absorb the injected dye or radiocolloid (i.e. don’t map). Similarly, a failed mapping on a hemipelvis cannot be considered a “negative, healthy pelvic sidewall.” A failed mapping should be accompanied by a side-specific lymphadenectomy to include the iliac and obturator nodes (Fig. 73.3). In a recent study from MSKCC, 498 patients received a blue dye cervical injection for SLN mapping. At least one lymph node was removed in 95% of cases (474/498); at least one SLN was identified in 81% (401/498). SLN correctly diagnosed 40/47 patients with nodal metastases who had at least one SLN mapped, resulting in a 15% false-negative rate. However, after applying the algorithm mentioned previously, the false-negative rate dropped to 2%. Only one patient, whose lymph node spread would not have been caught by the algorithm, had an isolated positive right para-aortic lymph node with a negative ipsilateral SLN and pelvic lymph node dissection.67 In general, isolated para-aortic nodal metastasis in the absence of pelvic nodal metastasis is rare and only seen in 1% to 3% of cases.68 Further, there is no definitive, well-documented association between para-aortic nodal assessment and improved OS in endometrial cancer.69
In summary, minimally invasive simple hysterectomy/BSO (laproscopic or robotic), peritoneal cytology, and SLN mapping seems to provide the highest prognostic/therapeutic yield while maintaining a low morbidity profile.
Radiation
Indications and types of radiation for early-stage endometrioid adenocarcinoma are shown in Table 73.3. For patients with stage IA grade 1 and 2 endometrioid adenocarcinoma (no or <50% myometrial invasion), adjuvant radiotherapy (RT) is not routinely recommended. In a trial reported by Sorbe et al.,70 645 such patients were randomized after surgery to observation (n = 326) or intravaginal RT (n = 319). Surgery consisted of TAH/BSO (laproscopic was allowed), pelvic washing, and removal of enlarged nodes. The rate of vaginal recurrence was 3.1% in the observation arm compared to 1.2% for the intravaginal RT arm (p = 0.114). The rate of pelvic recurrence was 0.9% in the observation arm and 0.3% in the treatment arm (p = 0.326). There was no significance difference between the two arms in terms of OS.70 Since the data from this trial did not report outcome based on myometrial invasion, it is difficult to determine the true impact of intravaginal RT on patients with <50% invasion as opposed to those without any invasion. Therefore, while observation is still a valid option for patient with grade 1-2 and myometrial invasion that is <50%, age of patient and the presence of LVI should be taken into account. In the randomized trial by Sorbe et al.70 mentioned previously, patients with vaginal recurrences were significantly (p = 0.018) older (mean age: 68.6 years) compared to patients without vaginal recurrences (mean: 62.6 years). Mariani et al.48 reported on 508 patients (152 without any invasion) with stage I endometrial cancer treated with surgery alone. LVI significantly increased the vaginal relapse rate from 3% to 7% (p = 0.02). At MSKCC, patients who are ≥60 years old or have LVI are recommended to have intravaginal RT.
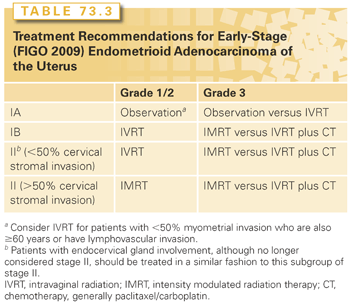
For patients with stage IA grade 3 endometrioid adenocarcinoma, those without myometrial invasion could be observed or treated with postoperative intravaginal RT, while patients with <50% invasion are often treated with adjuvant radiation. In PORTEC-1 trial, 715 patients with stage I (excluded were patients with <50% myometrial invasion grade 1 and patients with >50% grade 3) endometrial cancer were randomized after TAH/BSO to observation or pelvic RT.71 In the subset of patients with <50% myometrial invasion grade 3 endometrioid tumors (n = 37), the 5-year vaginal recurrence rate was 14% with surgery alone compared to 0% for those treated with pelvic RT. In contrast, none of 37 patients in this subset relapsed in the pelvis irrespective of the treatment.72 Horowitz et al.73 (n = 31) and Fanning et al.74 (n = 21) reported no vaginal or pelvic recurrence in their series of patients with <50% myometrial invasion grade 3 treated with postoperative intravaginal RT. Such data indicates that intravaginal RT is the preferred approach in these patients.
For patients with stage IB grade 1 and 2 endometrioid adenocarcinoma in PORTEC-1 trial, the 5-year vaginal recurrence for patients treated with surgery alone was 10% for those with grade 1 and 13% for grade 2. In contrast, the corresponding 5-year vaginal recurrence rates for patients treated with pelvic RT were 1% and 2%, respectively. The risk of pelvic recurrence at 5 years after surgery alone for these patients (>50% invasion) was 2% for grade 1 and 6% for grade 2 tumors.72 One of the important questions in patients with IB grade 1 and 2 is whether pelvic RT could be safely omitted. There are two randomized trials comparing pelvic RT to intravaginal RT. Neither trial required pelvic lymphadenectomy. In PORTEC 2 trial, 427 patients were randomized to pelvic RT (n = 214) or intravaginal RT (n = 213) after TAH/BSO. Inclusion criteria in this trial differed from that in PORTEC-1. For patients with stage I, they needed to be ≥60 years old and have either grade 3 tumors with <50% myometrial invasion or >50% invasion grade 1 and 2 tumors. Patients with endocervical mucosal involvement were also added. The 5-year vaginal recurrence rates were 1.8% in the intravaginal RT arm and 1.6% in the pelvic RT arm (p = 0.74). The pelvic recurrence was 3.8% in the intravaginal RT arm compared to only 0.5% in the pelvic RT arm (p = 0.02). There was no significant difference in disease-free or OS between the two arms.75 The other trial reported by Sorbe et al.,76 patients with stage I endometrioid adenocarcinoma with at least one of the following risk factors (grade 3, ≥50% myometrial invasion, or DNA aneuploidy) were randomized to adjuvant intravaginal RT (n = 263) or pelvic plus intravaginal RT (n = 264). The vaginal recurrence rate was 2.7% (7/263) in the intravaginal RT alone arm compared to 1.9% (5/264) in the pelvic RT arm (p = 0.555). Pelvic recurrence rate, however, was different: 5.3% in the intravaginal RT alone arm compared to 0.4% in the pelvic RT arm (p = 0.0006). There was no significant difference in OS between the two arms (90% versus 89%, respectively).76 The data from these trials indicate that the omission of pelvic RT in favor of intravaginal RT did not significantly increase the risk of vaginal recurrence. The reported risk of pelvic recurrence after lymphadenectomy or pelvic RT in patients with >50% invasion grade 1 and 2 tumors is about 1.5% on average.73,77–79 But that is not a sufficient reason to recommend routine lymphadenectomy or pelvic RT for two reasons. First, neither pelvic RT nor lymphadenectomy have been shown to improve survival in patients with early-stage endometrial cancer. Second, in the PORTEC-2 trial,75 most patients with pelvic recurrences had simultaneous distant metastasis. The 5-year rate of isolated pelvic recurrence was only 1.5% in the intravaginal RT arm compared to 0.5% for pelvic RT (p = 0.3). At MSKCC, most of these patients (IB, grade 1 and 2) undergo SLN mapping, and if the nodes are pathologically negative, they receive intravaginal RT alone.
Patients with stage IB grade 3 endometrioid adenocarcinoma were not enrolled in PORTEC 1 or 2 trials, because it was felt that omitting pelvic RT when lymphadenectomy was not performed could not be justified. In the registry study reported by Creutzberg et al.,72 99 patients with ≥50% myometrial invasion grade 3 were treated with postoperative pelvic RT. The 5-year rate of vaginal recurrence was 5%, pelvic recurrence 8%, and distant relapse 31%. Very few investigators would recommend surgery alone for these patients. In fact, an argument could be made that pelvic RT might be needed even after a negative lymphadenectomy especially for older patients and those with LVI. In a GOG 99 trial, 190 patients with stage I-II (patients without myometrial invasion were excluded) underwent TAH/BSO, pelvic washing, and pelvic/para-aortic lymph nodes sampling and then were randomized to observation versus pelvic RT.80 At 2 years, there was a statistically significant difference in the rates of relapse in favor of the adjuvant pelvic RT arm (3% versus 12%, p = 0.007). There was, however, no significant difference in 4-year OS (92% RT versus 86% with surgery alone, p = 0.557). The benefit of pelvic RT was predominantly seen in a subset of patients designated “high-intermediate risk (HIR)” based on advancing age, grade 2 or 3 tumors, outer third myometrial invasion, and LVI. Those on the pelvic RT arm demonstrated a somewhat lower overall death rate when compared to those on the observation arm (hazard ratio = 0.73, 90% confidence interval = 0.43 to 1.26) in the HIR subgroup.80 AT MSKCC, patients with >50% myometrial invasion grade 3 who are HIR per GOG 99 would be offered postoperative pelvic RT even in the setting of negative lymphadenectomy. If they are not HIR, then intravaginal RT could be considered but only with adequate pelvic lymphadenectomy and/or SLN mapping.
Patients with endocervical mucosal invasion are no longer considered stage II per the FIGO 2009 staging system. However, there are no data to indicate that observation is safe; in fact such patients were randomized to either pelvic RT or intravaginal RT in PORTEC 2 trial.75 At MSKCC, these patients are treated with postoperative intravaginal RT. Having cervical involvement, especially stromal invasion, increases the risk of lymph node involvement. In the GOG 33 surgical-pathologic assessment study, the rate positive pelvic lymph node was 8% when the cervix-isthmus was not involved versus16% when it was involved.42 For patients with stage II (cervical stromal invasion) omitting pelvic RT could be justified, in the setting of negative lymphadenectomy, based on an average rate of vaginal recurrence of 1.47% and a similar pelvic recurrence rate of 1.47% reported in the literature for patients who underwent lymphadenectomy and intravaginal RT.49,73,81,82 However, patients in these series were highly selected. At MSKCC, intravaginal RT alone as opposed to pelvic RT is considered only for stage II patients who meet the following criteria: grade 1 and 2 endometriod histology, depth of cervical stromal invasion of <50%, and adequate lymphadenectomy/SLN mapping. Patients with stage II grade 3 or those with cervical stromal invasion >50% are treated with pelvic RT.
It is clear from the data mentioned thus far that if postoperative radiation is needed in early-stage endometrioid adenocarcinoma, the preferred approach is intravaginal RT rather than pelvic RT in most cases. Intravaginal RT targets the most likely site of recurrence in patients with early-stage disease (about 80% of recurrences are vaginal) and its morbidity profile is better than pelvic RT (Fig. 73.4). In the PORTEC 2 trial randomizing patients to pelvic RT versus intravaginal RT, the rate of grade 1 and 2 acute gastrointestinal toxicity was 53% versus 12% (p <0.001) in favor of intravaginal RT.75 Furthermore, the increased risk of secondary malignancies after pelvic RT for patients who are <60 years old has to be taken into consideration.83
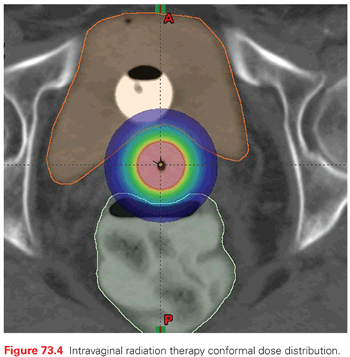
In summary, intravaginal RT provides the best therapeutic ration for most patients with early-stage endometrial cancer. At MSKCC, intravaginal RT is given as an outpatient treatment using a high dose rate Ir-192. The dose is 18-21 Gy given in three fractions with 1 to 2 weeks between fractions.
Chemotherapy
Adjuvant chemotherapy is not routinely recommended for patients with stage I-II endometrioid adenocarcinoma. In the PORTEC-2 trial,75 where only the high-risk patients from PORTEC-1 were included, the 5-year rate of distant relapse was 8.3% in the intravaginal RT arm and 5.7% in the pelvic RT arm (p = 0.46). In contrast, for patients with deep myometrial invasion grade 3 tumors in the PORTEC registry (all received pelvic RT), the 5-year rate of distant relapse was 31%, indicating the need for effective systemic therapy in this group of patients.72 The results of recently completed trial for early-stage high-risk patients with endometrial cancer (GOG249), where patients were randomized to pelvic RT versus intravaginal RT plus three cycles of paclitaxel/carboplatin, are eagerly awaited. At MSKCC, intravaginal RT and chemotherapy are offered as an option for patients with endometrioid adenocarcinoma stage IB grade 3 and stage II grade 3. There are more data in the literature on adjuvant chemotherapy in patients with serous endometrial cancer and to a lesser extent clear cell carcinoma. In a study from MSKCC, 41 patients with stage I-II serous cancer (those with no residual disease in hysterectomy were excluded) were treated with postoperative intravaginal RT and six cycles of paclitaxel/carboplatin. Of the 41 patients, 85% completed treatment as prescribed. With a median follow-up time of 58 months, the 5-year disease-free and OS rates were 85% and 90%, respectively. The 5-year actuarial recurrence rates were 9% in the pelvis, 5% in the para-aortic nodes, and 10% at distant sites. None of the patients developed vaginal recurrence.84
Treatment of Stage III Disease
There are three randomized trials comparing adjuvant chemotherapy to adjuvant radiation alone. The Japanese Gynecology Oncology Group trial randomized 385 patients with stage I (deep invasion)-III endometrial cancer patients to pelvic RT (193 patients) or to chemotherapy (192 patients). Chemotherapy consisted of cyclophosphamide (333 mg/m2), cisplatin (50 mg/m2), and doxorubicin (40 mg/m2) every 4 weeks for three cycles or more. There was no significant difference in progression-free survival (PFS; p = 0.726) or OS (p = 0.462) between the two groups.85 The trial from Italy had similar design and inclusion criteria, where 340 patients were randomized to RT or to chemotherapy. With a median follow-up of 95.5 months, the 5-year disease-free survival was 63% in both arms (p = 0.44) and the 5-year overall survival rates were 69% in the radiation arm compared to 66% in the chemotherapy arm (p = 0.77). Again, there was no significant difference in outcome despite using five cycles of cyclophosphamide, cisplatin, and doxorubicin.86 In GOG 122, 396 patients with stage III-IV disease were randomized to whole abdomen radiation (n = 202) versus doxorubicin/cisplatin (n = 194) for eight cycles. Progression-free survival was the primary end point of this study. With a median follow-up of 74 months, there was significant improvement in both PFS (50% versus 38%; p = 0.007) as well as OS (55% versus 42%; p = 0.004), respectively, in favor of chemotherapy.87
There are two trials comparing adjuvant RT to chemotherapy plus RT. One trial from Finland,88 randomized 156 patients with stage I-IIIA (FIGO 1988) to RT only or RT combined with three cycles of cisplatin (50 mg/m2), epirubicin (60 mg/m2), and cyclophosphamide (500 mg/m2) chemotherapy (n = 84). The disease-specific overall 5-year survival was 84.7% in the RT arm versus 82.1% in the chemoradiation arm (p = 0.148). The second trial by Hogberg et al.89 is a report on two trials. In the MaNGO trial portion, there were 157 patients; 76 were randomized to pelvic RT (45 Gy) and 80 to chemoradiation. The chemotherapy consisted of three cycles of doxorubicin (60 mg/m2) and cisplatin (50 mg/m2). The 5-year PFS was 61% in the RT group compared to 74% for chemoradiation but that difference was not significant (p = 0.1). The 5-year OS was also not significant (73% versus 78%, respectively, p = 0.41). In the Nordic Society of Gynaecological Oncology/European Organization for Research and Treatment of Cancer (EORTC) trial portion, 383 patients were randomized to RT (n = 191) versus chemoradiation (n = 187). The type of chemotherapy varied and only a handful of patients were stage III. The 5-year PFS was better for the chemoradiation arm (79% versus 72% for RT, p = 0.04) but OS was not significantly better (83% versus 76%, respectively, p = 0.1).
Unlike in cervical cancer where the addition of chemotherapy to radiation has been clearly demonstrated in several large-scale randomized trials, the superiority of chemotherapy over radiation alone in endometrial cancer is not conclusive. In fact, even the results from GOG 120 trial are not that definitive. The chemotherapy arm has more patients with stage IIIC disease, therefore PFS and OS were adjusted accordingly. Without that adjustment, there would be no difference between the two arms.87 The crude rate of relapse in the radiation arm was 54% compared to 50% in the chemotherapy arm, indicating the need for improvements in both arms of the study. Therefore, the question is no longer chemotherapy versus radiation, but rather what is the optimal way of combining the two modalities? Greven et al.90 reported the results of RTOG 9708 phase 2 study on 44 patients with stage I-III endometrial cancer who were treated with pelvic radiation and intravaginal RT given concurrently with cisplatin (50 mg/m2) on day 1 and 28 of radiation followed by four cycles of cisplatin (50 mg/m2) and paclitaxel (175 mg/m2). The 4-year disease-free and OS was 85% and 81%, respectively. In the subset of patients with stage III disease (66% of patients), the corresponding rates were 72% and 77%, respectively.90 Currently, the GOG is comparing chemoradiation (similar to RTOG 9708) to six cycles of carboplatin/paclitaxel in patients with stage III disease. The PORTEC 3 randomized trial is comparing chemoradiation to RT alone. Until the results of these trials are available, the decision on whether to give chemoradiation or chemotherapy alone should be based on risk factors. Patients with stage III endometrial cancer represent a heterogenous group of patients. In a report from MSKCC on 192 patients with stage III (FIGO 2009) endometrial cancer, three factors emerged as independent predictors of relapse and death from endometrial cancer.91 These factors were >50% myometrial invasion, positive peritoneal cytology, and aggressive histology defined as grade 3 endometrioid, serous, clear cell, or undifferentiated. The 5-year relapse rate for patients with no risk factors was 13%, one risk factor 27%, and two or more risk factors 62% (p <0.001). The corresponding 5-year rates of death from endometrial cancer were 11%, 20%, and 56% (p <0.001). Therefore, for patients with stage III with two or more risk factors, especially positive peritoneal cytology in the setting of serous endometrial cancer, full systemic chemotherapy (six cycles of paclitaxel/carboplatin) is preferred over chemoradiation. For those with no more than one risk factor (lymph node involvement only, for example), using an approach similar to RTOG 9708 seems feasible. In a report from MSKCC, 40 patients with stage III disease were treated as such. Of the 40 patients, 78% completed chemoradiation followed by four cycles of paclitaxel/carboplatin. With a median follow-up of 49 months, the 5-year rate of freedom from relapse and OS were 79% and 85%, respectively.92 When using radiation in stage III endometrial cancer, the preferred approach is intensity modulated (IM)RT because of its favorable morbidity profile. In a report from MSKCC, 46 patients with stage I-III (78% stage III) endometrial cancer were treated with postoperative radiation using IMRT. With a median follow-up of 52 months, the 5-year disease-free survival was 88% and OS was 97%. Chemotherapy was given to 30/46 patients; 5 had grade 3 leukopenia, 8 grade 2 anemia, and 2 grade 2 thrombocytopenia.93
In summary, patients with stage III endometrial cancer are very heterogenous; the treatment recommendations thus need to be individualized. Concurrent chemoradiation followed by four cycles of paclitaxel/carboplatin seems to provide excellent outcome for most patients. The preferred form of radiation at MSKCC is pelvic/extended-field IMRT to 50.4 Gy in 28 fractions. Patients who are at high risk for peritoneal relapse should be treated with full systemic therapy.
Treatment of Distant Recurrence
Patients with distant recurrence are not generally curable, and treatment choices should take both efficacy and toxicity into account, particularly as many patients with recurrent endometrial cancer are elderly.
Endocrine Therapy
Progestins have been used in the management of recurrent endometrial cancer since the 1961 report by Kelley and Baker94 describing a response rate of 29% with various progestational agents, generally parenterally administered hydroxyprogesterone caproate. Subsequent trials generally have used medroxyprogesterone acetate or megestrol acetate (MA), which are available in pill form (Table 73.4).95–99 Results in unselected patient groups show limited activity with short median PFS, and a recent Cochrane analysis found no evidence that hormonal therapy as a single agent or in combination prolonged overall or 5-year disease-free survival.100 Nonetheless, some patients will have their disease controlled for several years on hormonal therapy with minimal toxicity. In early reports, patients with well-differentiated tumors, a long disease-free interval, and lung metastases were noted to have the best results. Subsequent work suggested that tumors expressing estrogen receptors (ER) and/or progesterone receptors (PR) had the highest chance of response to progestin therapy, and some more recent trials of endocrine therapy have been limited to tumors expressing ERs or PRs.95,101
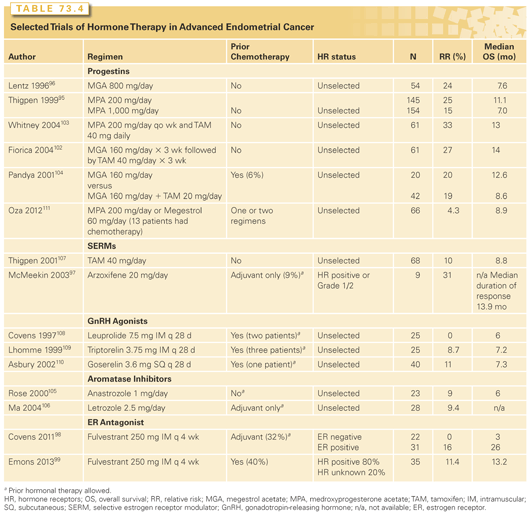
Sequentially alternating tamoxifen and medroxyprogesterone acetate or MA was tested based on the hypothesis that the antiestrogen therapy could upregulate the progesterone receptor.102,103 Response rates were promising, in the range of 30% in a chemotherapy-naïve population; however, median PFS, as with single agent progestin therapy, was short (approximately 3 months). A small randomized trial of MA alone or with concomitant tamoxifen showed no difference between the two regimens.104 Phase 2 trials with other agents, including aromatase inhibitors,105,160 tamoxifen,107 and luteinizing hormone-releasing hormone (LHRH) agonists,108–110 have mostly yielded lower response rates than seen with progestins, although results may not be truly comparable, as some of these more recent trials were conducted in patients who had prior chemotherapy or hormonal therapy. It seems likely that results would be inferior in a pretreated population. A recent randomized phase 2 trial comparing the mTOR inhibitor ridaforolimus to control treatment (control treatment consisting of progestin therapy with either medroxyprogesterone [200 mg/day] or megestrol [60 mg/day] in 53 patients and chemotherapy in 13 patients) in women with one or two prior chemotherapy regimens reported a response rate of only 4.3% for control therapy.111
Preclinical data have suggested that inhibiting the PI3K/Akt pathway reverses progestin resistance in endometrial cancer,112 and attempts have been made to improve results of endocrine therapy by combining it with an mTOR inhibitor (analogous to the FDA-approved use of everolimus with exemestane in patients with breast cancer). GOG conducted a randomized phase 2 trial testing the mTOR inhibitor temsirolimus with or without a regimen of MA alternating with tamoxifen in patients with no more than one prior chemotherapy regimen. Unfortunately, the combination of temsirolimus with MA/tamoxifen resulted in an unacceptable rate of venous thrombosis (seven events in 22 patients), and the combination arm was closed to accrual after the first stage. Three of twenty-one patients had a response (14%), which is in the range of reported with temsirolimus alone (see the following).113 A phase 2 open-label single arm study of the combination of everolimus and letrozole enrolled 28 patients who had received one or two prior chemotherapy regimens114 and showed a somewhat more promising objective response rate of 21%.
In summary, hormonal therapy should be considered for women with low grade ER-positive tumors, particularly if they have a long interval from diagnosis to recurrence. Commonly used regimens in the United States are MA 80 mg twice a day and MA 80 mg twice a day for 3 weeks alternating with tamoxifen 20 mg twice a day for 3 weeks.
Cytotoxic Chemotherapy
First-Line Chemotherapy. Most women with recurrent or stage IV endometrial carcinoma should be assessed for treatment with cytotoxic chemotherapy. Current combination chemotherapy regimens can yield response rates of 50% to 60% with median OS about 12 to 15 months.
Historically, doxorubicin showed reproducible activity in multiple phase 2 and phase 3 studies of patients with chemotherapy-naïve disease (Table 73.5),115–118 with response rates in the range of 20% to 30%. Single-agent cisplatin119 and carboplatin120,121 therapy subsequently produced similar results. As the combination of doxorubicin plus cisplatin (AP) appeared more active than single agent doxorubicin,115,122 it became the standard for use in both adjuvant and metastatic disease settings. Taxanes were next found to have meaningful activity and, indeed, remain the class of agents that have produced the highest response rates in the setting of pretreated disease (see Table 73.5).123 In a phase 3 trial of women with recurrent or stage IV endometrial cancer, the triplet of paclitaxel, cisplatin, and doxorubicin (TAP) produced a superior response rate (57% versus 34%), PFS (median, 8.3 versus 5.3 months), and OS (median, 15.3 versus 12.3 months; p = 0.037) when compared to AP.124 TAP is the only treatment proven to produce a survival benefit in women with recurrent or stage IV endometrial disease. However, a subsequent phase 3 trial comparing TAP to AP as adjuvant therapy after surgery and radiation for stage III and resected stage IV disease showed no difference between the two regimens, although the subset of women with gross residual disease had a 50% reduction in the risk of recurrence or death with the addition of paclitaxel.125
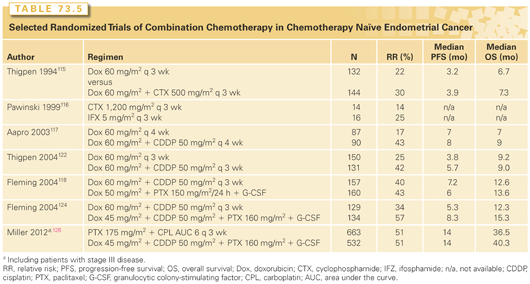
The TAP regimen required growth factor support and produced grade 2 or higher neurotoxicity in 25% to 40% of patients. As phase 2 studies had shown good tolerability and activity with paclitaxel/carboplatin (TC), a 1,300-woman phase 3 noninferiority trial for those with stage III, IV, or recurrent disease was conducted comparing TC to TAP. Preliminary results showed no significant difference in median PFS (14 months in each arm) or median OS (32 months for TC, 38 months for TAP; not statistically significant). Regarding toxicity, study treatment was discontinued due to toxicity in 18% of subjects on TAP and 12% on TC.126
One interesting alternative option for front-line therapy might be the combination of liposomal doxorubicin/carboplatin. Considering the well-reproduced activity of free doxorubicin, single-agent liposomal doxorubicin produced a disappointingly low response rate of 11.5% in patients with chemotherapy-naïve endometrial cancer.127 Oddly, almost the same level of activity was reported128 in women with pretreated disease (9.5%), raising the question of whether some unknown adverse selection factors were operating in the front-line trial (e.g., since upfront combination chemotherapy was already established at the time, perhaps less fit patients elected to participate in the trial of single-agent liposomal doxorubicin). The Multicentre Italian Trials in Ovarian Cancer and Gynecologic Malignancies Group conducted a single arm phase 2 trial of pegylated liposomal doxorubicin plus carboplatin in the front-line therapy of advanced endometrial cancer and reported a response rate of 59.5% and a median survival of 80.1 weeks.129 While the combination of carboplatin/liposomal doxorubicin has performed well compared to TC in a randomized trial for women with ovarian cancer,130 it has not been compared to other options in women with endometrial cancer. Nonetheless, the carboplatin/liposomal doxorubicin regimen, which produces minimal alopecia and neuropathy, might be a reasonable choice, particularly for patients with contraindications to taxane therapy.
In summary, TC therapy every 3 weeks is the usual first-line chemotherapy treatment in the United States for women with stage IV endometrial cancer. Issues of duration of therapy, optimal treatment after adjuvant TC (which is increasingly used, particularly for women with serous carcinomas), and of paclitaxel scheduling (weekly versus every three weeks) remain to be addressed.
Second (and Later)-Line Chemotherapy. The efficacy of second-line cytotoxic chemotherapy remains very limited. Women who have a prolonged disease-free interval after prior carboplatin/taxane therapy can be retreated with a similar regimen. Nagao et al.131 reviewed 262 patients, 49% of whom had a platinum-free interval of ≥12 months, and 51% of whom had a platinum-free interval of ≥12 months. Over half received their initial chemotherapy regimen in the adjuvant setting. They found response rates to retreatment with a platinum-based regimen for platinum-free intervals of <6 months, 6 to 11months, 12 to 23 months, and ≥24 months to be 25%, 38%, 61%, and 65%, respectively, suggesting that the general concept of “platinum sensitivity” in selection of second-line therapy can be applied to endometrial cancer as well as ovarian cancer. However, trials of second-line treatments for endometrial cancer have not traditionally been prospectively stratified along these lines (which may contribute to some of the variability seen in response rates of small phase 2 studies in endometrial cancer to agents such as pegylated liposomal doxorubicin).
Results of selected trials with standard available cytotoxic agents in patients who have received prior chemotherapy are shown in Table 73.6.132–149 Taxanes showed good activity in the days before taxane-containing therapy was the standard first-line approach.123 Neither doxorubicin nor platinum agents have generally produced high response rates when used second-line. Other agents such as topotecan and gemcitabine have likewise shown minimal efficacy in previously treated populations.150
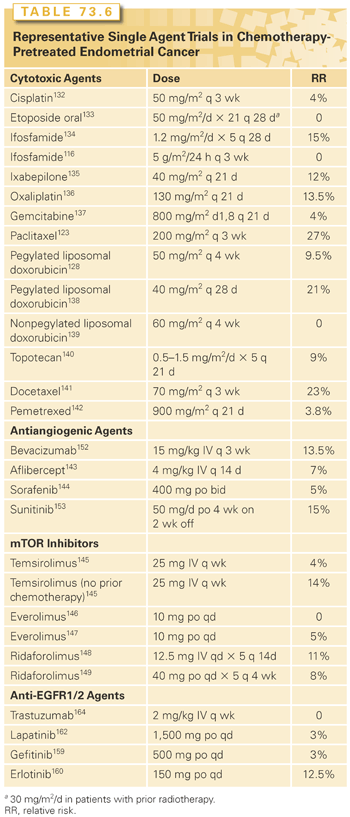
In summary, women with a prolonged disease-free interval from their front-line platinum therapy can be retreated with a platinum-containing combination. However, in general, response rates to second-line cytotoxic therapy (and noncytotoxic agents) are low.
Predictors of Response to Cytotoxic Therapy. As discussed previously, endometrial carcinoma is composed of several biologically different subsets, including endometrioid (high and low grade), serous, and clear cell. The proportions of the subtypes with advanced recurrent/disease different than in early-stage disease, where low-grade tumors are most common. These histologic subtypes have been shown to have very different genetic makeup, but to date, differences in response to standard front-line cytotoxic therapy have not been observed. McMeekin et al.151 published results showing that histologic subtype was not an independent predictor of response to doxorubicin/cisplatin/doxorubicin–based regimens. The overall response rate to treatment was 42% (endometrioid 44%, serous 44%, clear cell 32%). The effect of grade was not considered in this analysis.151 A primary endpoint of the 1,300 patient GOG 209 trial comparing TC to TAP is to determine whether ER status of the tumor has any effect on chemotherapy outcomes, and these data should be available soon.
An integrated genomic analysis of serous and endometrioid endometrial carcinomas by the The Cancer Genome Atlas has revealed that 19.6% of histologically classified high-grade endometrioid endometrial carcinomas had genomic profiles that resemble those of serous carcinomas. Four molecular subgroups were described: a POLE ultramutated subgroup, a hypermutated/microsatellite-unstable subgroup, a copy number-low microsatellite-stable group, and a copy number-high subgroup.53 Hopefully, improved classifications will eventually help select those tumors most likely to respond to conventional agents as well as identifying targets for novel therapies.
Therapeutic Agents in Development
Antiangiogenic Agents. Antiangiogenic therapies have shown some activity in the treatment of endometrial cancer (see Table 73.6). Bevacizumab, an antivascular endothelial growth factor monoclonal antibody, produced a response rate of 13.5% in patients with one or two prior cytotoxic regimens, and 40% of subjects were progression-free at 6 months.152 A front-line randomized phase 2 GOG trial randomly assigning patients to TC with either bevacizumab or temsirolimus or to carboplatin plus ixabepilone with bevacizumab has completed accrual and results should be available soon.
Other antiangiogenic agents tested have generally not appeared quite as active, although a preliminary report suggested that sunitinib produced a 15% response rate.153 Trials of multiple other multitargeted antiangiogenic agents, including cediranib, brivanib, cabozantanib, nintedanib, lenvatinib, and AMG 386 (trebananib), are ongoing or completed but not yet reported.
Phosphatidylinositol-3 Kinase/mTOR Pathway. Endometrial cancers harbor the highest rates of PI3K pathway alterations reported to date. Germline mutations in PTEN, which deactivates the PI3K-AKT signaling pathway, are the etiology of Cowden syndrome, for which endometrial cancer is a diagnostic criterion. PTEN mutations are extremely common in sporadic type I endometrial cancer. There has therefore been widespread interest in testing PI3K pathway inhibitors in endometrial cancer.
Numerous trials involving the rapamycin analogs have been published, and in general modest activity is seen in patients with chemotherapy-naïve disease, but response rates in patients with prior therapy are low (see Table 73.6). The randomized phase 2 trial comparing ridaforolimus progestins as second-line therapy reported improved PFS with ridaforolimus (3.6 versus 1.9 months), but no objective responses in the ridaforolimus arm.111 Nonetheless, sporadic patients treated with mTOR inhibitors have prolonged responses, and these responses are seen across histologic subtypes, including endometrioid, serous, and clear cell cancers. Several studies have explored the association between molecular biomarkers and response. No association between clinical benefit and any marker, including PTEN mutation, PIK3CA mutation, PTEN protein expression or mutation, or stathmin protein expression, has been observed. One analysis noted that K-Ras mutation was associated with decreased PFS and OS.154
Trials of newer PI3K pathway agents, including pan-PI3K, dual PI3K/mTOR, AKT, and catalytic mTOR inhibitors, are underway or have been completed, and results are expected soon. A preliminary report of a phase 2 trial of the allosteric AKT inhibitor, MK-2206, noted a 5.5% response rate with no clear association between PIC3CA mutation and response.155
Combinations of mTOR inhibitors with other agents are also being explored. Combinations of mTOR inhibitors with hormonal therapy and chemotherapy are described previously (see “Endocrine Therapy” and “Antiangiogenic Agents”). Two trials have combined temsirolimus with bevacizumab. In previously treated patients, the combination produced a 24.5% response rate, but there was significant toxicity, with two gastrointestinal-vaginal fistulas, one grade 3 epistaxis, two intestinal perforations, and one grade 4 thrombosis/embolism. Three patient deaths were possibly treatment related.156
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








