Seminoma
The microscopic appearance of seminoma is characterized by sheets of neoplastic cells with abundant cytoplasm, round, hyperchromatic nuclei and prominent nucleoli (Fig. 70.1). A prominent lymphocytic infiltrate is common, such that it is sometimes confused with lymphoma until the surface immunophenotype has been determined. Most seminomas do not produce serum tumor markers, but the presence of syncytiotrophoblastic giant cells in a minority of cases accounts for modest elevations of serum human chorionic gonadotropin (hCG).21 Seminomas never produce alpha-fetoprotein (AFP), and patients whose tumors have the histologic appearance of seminoma and whose serum AFP is elevated should be considered to have a mixed NSGCT, even if a nonseminomatous histologic pattern cannot be identified.22 Exceptions are cases in which there is another explanation for the elevated AFP, such as liver disease or a chronic nonspecific elevation.

Immunohistochemistry is usually positive for placental alkaline phosphatase (PLAP), negative for CD30, AFP, and epithelial membrane antigen, and either negative or weak/focally positive for cytokeratin.23
Histologic variations of seminoma such as “anaplastic” or “atypical” seminoma are of no known clinical relevance. Spermatocytic seminoma, however, is the one variant of seminoma that has a different natural history and is even of uncertain relation to other germ cell tumors. Spermatocytic seminoma usually occurs in older individuals and has low metastatic potential.24 Orchiectomy is the only treatment required. Unlike all other germ cell tumors, spermatocytic seminoma is not associated with ITGCN.
Nonseminomatous Histologies
Germ cell tumors may be composed of a single histology or multiple histologic patterns.19 Through poorly understood processes of mutation and differentiation, a single clone beginning as ITGCN can develop into an undifferentiated neoplasm (seminoma), or to a primitive zygotic neoplasm (embryonal carcinoma).25 Further differentiation from embryonal carcinoma results in somatically differentiated tumors (teratoma) or extraembryonal-differentiated tumors (yolk sac and choriocarcinoma).26 Nonseminomatous histologies are found in 55% of germ cell tumors. Male germ cell tumors that contain any histologic cell type other than seminoma or syncytiotrophoblasts are collectively referred to as NSGCT.
Embryonal Carcinoma
Embryonal carcinoma is the most undifferentiated type of germ cell tumor and is thought to be pluripotent. Cells are characterized by indistinct borders and scant cytoplasm, which can be arranged in solid sheets or in glandular or tubular structures (Fig. 70.2). On immunohistochemical staining, embryonal carcinoma can be positive for cytokeratin, CD30, PLAP, AFP, and hCG. Modest elevations of serum AFP and/or hCG can be seen, and frequently it is marker-negative. Lactate dehydrogenase (LDH) concentration in the serum is an important prognostic factor in metastatic embryonal carcinoma that is marker negative.
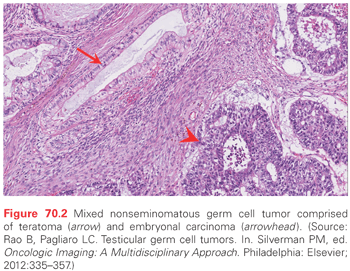
Choriocarcinoma
Choriocarcinoma is composed of both cytotrophoblasts and syncytiotrophoblasts.27 The cells strongly express hCG. The clinical presentation of a choriocarcinoma-predominant or pure choriocarcinoma tumor often includes very high serum hCG levels, widespread hematogenous metastases, and tumor hemorrhage (Fig. 70.3). Syncytiotrophoblasts and syncytiotrophoblastic giant cells can be associated with other germ cell histologies, so the presence of cytotophoblasts is required for the diagnosis.

Yolk Sac Tumor
Yolk sac tumor (endodermal sinus tumor) is commonly seen as a component of mixed NSGCT. Pure yolk sac tumors represent a significant proportion of mediastinal germ cell tumors, but are rarely seen in adult testicular cancer. Histologic patterns include papillary, solid, glandular, hepatoid, macrocystic, and microcystic types. Perivascular arrangements of epithelial cells can be seen in yolk sac tumor and are known as glomeruloid or Schiller-Duval bodies. Immunostains are diffusely positive for AFP and may also be positive for cytokeratin, SALL4, glipican-3, PLAP, and CD117. Yolk sac tumor is associated with high serum levels of AFP.
Teratoma
Teratoma arises from a pluripotent malignant precursor (embryonal carcionoma or yolk sac tumor) and contains somatic cells from at least two germ cell layers (ectoderm, endoderm, or mesoderm). Teratoma is commonly seen as a component of adult mixed NSGCT (see Fig. 70.2). A small percentage (2% to 3%) of postpubertal male germ cell tumors appear to have teratoma as the only histologic type, but these are always assumed to harbor a minor component of pluripotent NSGCT and are treated the same as a mixed NSGCT.
Immature teratoma shows partial somatic differentiation, whereas mature teratoma has terminally differentiated tissues such as cartilage, skeletal muscle, or nerve tissue, and frequently forms cystic structures. Although these cells can resemble normal tissues, teratoma is a low-grade malignancy and if untreated will grow until it is unresectable. Moreover, teratomas can give rise to secondary somatic malignancy, such as rhabdomyosarcoma, poorly differentiated carcinoma, or primitive neuroectodermal tumor.28 These typically display the biology of their de novo counterparts and are treated accordingly.29,30
Teratoma does not produce elevated serum AFP or hCG. Patients with elevated serum AFP and/or hCG should be assumed to have a nonteratoma germ cell tumor component, unless the elevation can be otherwise explained.
Mechanism of Germ Cell Transformation
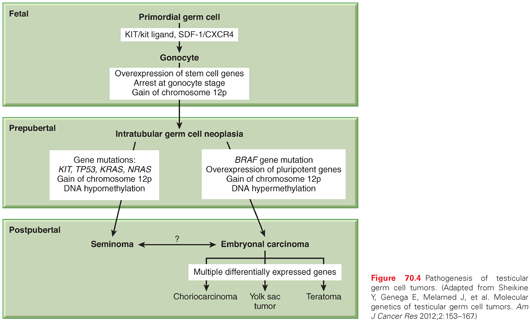
ITGCN is thought to derive from malignant transformation of primordial germ cells or gonocytes during fetal development.31 Primordial germ cells migrate from the proximal epiblast (yolk sac) through the hindgut and mesentery to the genital ridge, and become gonocytes. The precise molecular events underlying transformation to ITGCN are not well understood. The most consistent genetic finding in germ cell tumors is a gain of material from chromosome 12p. The majority of NSGCT and seminomas contain i(12p), an isochromosome comprised of two fused short arms of chromosome 12. The remaining i(12p)-negative germ cell tumors also have a gain of 12p sequences in the form of tandem duplications that may be transposed elsewhere in the genome.
Gain of 12p sequences has been found in ITGCN, indicating that it is an early event in testicular cancer pathogenesis. The acquisition of i(12p) is not thought to be the initiating event, however, because it is preceded by polyploidization.32 Overexpressed genes on 12p are likely to be important, and there are candidate genes on 12p including several that confer growth advantage (KRAS2, CCND2 [cyclin D2]), and others that establish or maintain the stem cell phenotype (NANOG, DPPA3, GDF3). The exact genes that are critical to this step have not yet been identified.
Seminomas are usually hypertriploid, whereas NSGCT is more commonly hypotriploid.33 Other chromosome regions were found to have nonrandom gains or losses in germ cell tumors with less frequency than 12p. Single gene mutations are uncommon in germ cell tumors. The most commonly mutated genes are BRAF, KIT, KRAS, NRAS, and TP53.11 The KIT/kit ligand (KITLG) pathway has special relevance for gonadal development. The biologic function of this pathway is broad and includes development of hematopoietic cells, melanocytes, and germ cells.34 KITLG is essential for primordial germ cell survival and motility, as are the chemokine SDF-1 (CXCL12) and its receptor CXCR4.11 Immunohistochemical markers found on primordial germ cells and gonocytes (PLAP, CD117 [KIT], OCT3/4 [POU5F1]) are also found on ITGCN, suggesting a transformation from these cells during fetal development (Fig. 70.4). The biallelic expression of imprinted genes in germ cell tumors has been reported, showing that they likely arose from primordial germ cells where the genomic imprinting is temporarily erased.31
Somatic mutations in KIT as well as increased copy number have been described in 9% of testicular germ cell tumors and 20% of seminomas.4 The somatic alterations in KIT found in germ cell tumors are predicted to upregulate pathway activity. KITLG plays a role in determining skin pigmentation and has undergone strong selection in European and Asian populations. Difference in the frequency of risk alleles for KITLG between European and African populations may provide an explanation for the difference in germ cell tumor incidence between Caucasian and African Americans.
There is evidence that epigenetic regulation of gene expression plays a role in the pathogenesis of germ cell tumors. The DNA methylation patterns are different among histologic types. Global hypomethylation is more common in seminomas than in NSGCT.31 In a study of 16 germ cell tumors, the methylation of CpG islands in NSGCT was similar to that observed in other tumor types, whereas it was virtually absent in seminomas.35 Aberrant promoter methylation is generally associated with absent or downregulated expression of the methylated genes. This can result, for example, in the silencing of tumor suppressor genes.31 Methylation has also been correlated with germ cell tumor differentiation. The more differentiated tumors (yolk sac tumor, choriocarcinoma, teratoma) were consistently hypermethylated, whereas seminoma and ITGCN were hypomethylated.36 Some of the observed methylation patterns may reflect normal development rather than germ cell tumor pathogenesis.
Pluripotency of Embryonal-Like Differentiation in Germ Cell Tumors
Embryonal carcinoma has a six-gene signature (DNMT3B, DPPA4, GAL, GPC4, POU5F1, TERF1), which was detected in three of five independent studies.11 All six of these genes are involved in establishing and maintaining pluripotency. SOX2 encodes a transcription factor essential for maintaining self-renewal of embryonic stem cells and is upregulated in embryonal carcinoma. Two additional genes that encode transcription factors associated with stem cell pluripotency, NANOG and POU5F1, are upregulated in both seminoma and embyronal carcinoma.26 Lineage differentiation takes place in NSGCT, mimicking the development of a normal zygote. Thus, embryonal carcinoma can be thought of as the transformed counterpart of embryonic stem cells, displaying self-renewal, pluripotency, and lineage differentiation.
Familial Predisposition to Germ Cell Tumors
Approximately 1.4% of patients with newly diagnosed germ cell tumor report a family history of germ cell tumor.34 First-degree male relatives of patients with testicular cancer have a 6- to 10-fold increased risk of developing testicular cancer. These observations point to the likely existence of a hereditary germ cell tumor subset. The inheritable effect is mild, and the most common number of affected relatives in a family is two. The age at diagnosis among familial cases is 2 to 3 years younger than sporadic cases, and there is a higher incidence of bilateral tumors.
The gr/gr deletion on chromosome Y, common among infertility patients and associated with a two- to threefold increased risk of testicular cancer, was studied as a candidate region for hereditary risk.4 The frequency of gr/gr variant is low, however, such that it could only account for a small component of hereditary risk. Genome-wide association studies (GWAS) for testicular cancer have been hampered by relatively small sample sizes. Nevertheless, five loci of interest were identified in a GWAS study from the United Kingdom, on chromosomes 1, 4, 5q31.3, 6, and 12p22, including confirmatory data for the chromosome 6 locus. The single nucleotide polymorphism (SNP) associations on chromosomes 1 and 4 were not convincingly replicated in an independent US study, whereas the associations on chromosomes 5 and 12 were confirmed. The strongest associated SNPs were at 12q22 within the KITLG gene, for which there is a >2.5 risk of disease per major allele. For the chromosome 5 and 6 loci, a 1.5-fold increased risk was identified per major allele. The loci on chromosome 5 implicate the gene SPRY4, which encodes an inhibitor of the mitogen-activated protein kinase pathway. The locus on chromosome 6 falls within the intron of BAK1, which promotes apoptosis. The location of a disease-associated SNP near or within a gene does not definitively implicate that gene, yet all three of the genes identified in GWASs are directly or indirectly associated with the KIT/KITLG pathway.11 These genes may be responsible for an estimated 15% of genetic predisposition to germ cell tumors, whereas the genetic basis of the other 85% of familial aggregations remains unexplained.
Cisplatin Sensitivity and Acquired Resistance in Adult Germ Cell Tumors
Exquisite sensitivity to chemotherapy distinguishes germ cell tumors from most other cancers. Levels of p53 protein are elevated in all germ cell tumors except teratoma.31 To maintain genomic integrity, embryonic stem cells have a high sensitivity to DNA damage, suggesting that the high levels of wild-type p53 seen in germ cell tumors may be intrinsic to their germ cell nature. TP53 gene mutations are uncommon in germ cell tumors, however, and the role of acquired TP53 mutations in the emergence of chemotherapy resistance has not been established.11 Moreover, levels of p53 protein assessed by immunohistochemistry were not associated with outcome. While mutations in TP53 have been observed in about 7% of seminomas, its significance in germ cell tumors remains unclear.
Mutations of BRAF including V600E mutations have been detected in a small percentage of NSGCT. BRAF mutations were most prevalent in mediastinal primary tumors, late relapse, and cisplatin-resistant NSGCT, suggesting a role in chemoresistance. Further study is needed to define the prognostic significance of BRAF mutation and whether the V600E mutation can be therapeutically targeted. A high ratio of proapoptotic Bax to antiapoptotic Bcl-2 was found in invasive germ cell tumors and may explain the rapid apoptotic response to DNA-damaging drugs; however, the Bax:Bcl-2 ratio was not associated with treatment outcome.31 Finally, the emergence of cisplatin resistance may be intimately associated with the expression of genes and pathways responsible for lineage differentiation in NSGCT, in other words, intrinsic to the germ cell biology rather than the result of specific mutations.
SALL4 is expressed in almost all germ cell tumors and has been reported to be positive in ITGCN, classic seminoma, spermatocytic seminoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratoma.37,38 OCT3/4 is variably expressed in ITGCN, classic seminoma, embryonal carcinoma, and yolk sac tumor. Spermatocytic seminoma, choriocarcinoma, and teratoma are usually negative for OCT3/4. CD117 (KIT) helps highlight ITGCN and classic seminoma. CD30, SOX2, and keratin are helpful in the diagnosis of embryonal carcinoma, whereas SALL4 and Glypican-3 are often positive in yolk sac tumor.39 In tumors of unknown primary or those presenting as a retroperitoneal or mediastinal mass, SALL4, OCT3/4, CD117, SOX2, CD30, and low-molecular-weight keratins all may be useful in distinguishing germ cell tumors from non–germ cell tumors.
The most widely used system for staging testicular cancer is the tumor, node, metastasis classification endorsed by the American Joint Commission on Cancer and the International Union Against Cancer (Tables 70.2 and 70.3).40 An important distinction for germ cell tumors is the inclusion of “S” classification (S0 to S3), signifying serum tumor marker elevation. There are three stage groupings of tumor, node, metastasis/serum tumor marker elevation classifications whereby, in general, stage I disease is confined to the testis, stage II is confined to the retroperitoneal lymph nodes with serum tumor markers in the good-prognosis range (S0 to S1), and stage III includes metastases that extend beyond the retroperitoneum or are extranodal in location. Stage III NSGCT also includes any patient with serum tumor markers in the intermediate- or poor-prognosis range (S2 to S3).

Patterns of Metastasis
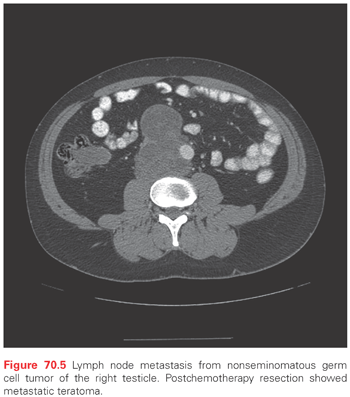
Testicular cancers can undergo both lymphatic and hematogenous dissemination. The lymphatics arising from the testicle accompany the gonadal vessels in the spermatic cord. Some follow the gonadal vessels to their origin while others diverge and drain into the retroperitoneum. The landing zone for metastasis from the right testicle is in the interaortocaval lymph nodes just inferior to the renal vessels (Fig. 70.5). The landing zone from the left testicle is in the para-aortic lymph nodes just inferior to the left renal vessels (Fig. 70.6). Large volume disease tends to progress in retrograde fashion to the aortic bifurcation and below, along the iliac vessels.41

Seminoma
Seminoma can spread extensively through the lymphatic system to include retroperitoneal, retrocrural, mediastinal, supraclavicular, and cervical lymph nodes, often in the absence of hematogenous metastasis.42 Metastasis to lungs (stage IIIA) is common; metastasis to nonpulmonary organs (stage IIIB) is less common.43 Serum tumor markers do not affect the stage (except in stage IS) or prognosis in seminoma. Hematogenous metastasis to extrapulmonary organs (e.g., bone) in seminoma carries an intermediate prognosis. There is no stage IIIC or poor-prognosis designation in seminoma.44
Nonseminomatous Germ Cell Tumors
Similar to seminoma, lymphatic spread is the most common and usually the earliest type of dissemination in NSGCT. Stage groupings depend on both the anatomic extent of disease and serum tumor markers.40 Stage IIIB is distinguished from stage II or IIIA on the basis of tumor markers being in the intermediate-prognosis range (S2). Stage IIIC NSGCT carries a poor prognosis, and more often than seminoma it involves multiple organs such as liver and brain.44
Embryonal carcinoma, in some cases, exhibits hematogenous metastasis to lungs or nonpulmonary viscera without clinical involvement of retroperitoneal lymph nodes.45 Computed tomography (CT) of the chest is necessary for complete staging workup of tumors that have a high percentage of embryonal carcinoma.
Serum Tumor Markers
Serum tumor markers are an important part of the staging system for germ cell tumors. Three markers, namely AFP, hCG, and total LDH, are considered for establishing the correct prognostic classification (good, intermediate, or poor prognosis).44 Markers should be assessed after orchiectomy and before the start of chemotherapy. Markers that are elevated prior to orchiectomy and then normalize appropriately have no prognostic significance. Markers that do not normalize in a patient without any other clinical evidence of metastatic disease are considered stage IS.40 In the absence of residual disease, the expected half-life of postoperative serum tumor marker decline is 2 to 3 days for hCG and 5 to 7 days for AFP.
Elevated AFP has special significance in seminoma because it is only produced by NSGCT. Germ cell tumors that histologically appear to be pure seminoma with elevated serum AFP are given the clinical diagnosis of NSGCT, and are treated as such.22 HCG can be elevated in either seminoma or NSGCT, but has prognostic significance only for NSCGT.
Total LDH concentration prior to chemotherapy functions as a prognostic factor in NSGCT and helps to determine stage. In seminoma with bulky metastases, LDH can be markedly elevated but has no prognostic significance.
There are several potential causes of spurious elevation of tumor markers. AFP is not cancer specific; it may be elevated in the presence of liver disease or as a nonspecific chronic elevation. Stability of AFP over time suggests a benign etiology. HCG is cancer specific in men, but it is not specific to germ cell tumors. It can be associated with other neoplasms and with exposure to cannabis products. Patients should be questioned about the use of marijuana. A positive hCG test can also occur as a laboratory artifact in patients with low serum testosterone, owing to the increased secretion of luteinizing hormone and its sequence similarity to hCG.46,47
Clinical Staging
The most commonly used methods for detecting metastatic disease are serum tumor markers and CT scan.15 Positron emission tomography (PET) scan can be helpful in the staging evaluation of a seminoma patient by distinguishing hypermetabolic lymph node metastases from reactive lymph nodes. PET is not as useful in NSGCT, where CT scan with oral and intravenous contrast is the preferred technique for detecting retroperitoneal adenopathy.
Pathologic Staging
The T classification is determined by pathology of the orchiectomy specimen.40 The presence of lymphovascular invasion (LVI) or invasion through the tunica albuginea with involvement of the tunica vaginalis are pT2, invasion of the spermatic cord is pT3, and involvement of the scrotum constitutes pT4 (see Table 70.2).
Prophylactic retroperitoneal lymph node dissection (RPLND) is performed for surgical staging in some patients with clinical stage I NSGCT. In such cases, if no metastatic germ cell tumor is found, the stage is pathologic stage I; when disease is found, it is designated pathologic stage II.
Factors Affecting Outcome
In clinical stage I NSGCT, the presence of LVI (pT2) is associated with an approximately 50% risk of recurrence with surveillance alone. A high percentage of embryonal carcinoma has also been associated with high risk in some series, but embryonal carcinoma is often seen together with LVI and has not been validated as an independent risk factor.48
In clinical stage I seminoma, tumor size ≥4 cm is associated with approximately 30% risk of recurrence with surveillance alone. Involvement of rete testis has not been validated as a risk factor, although it is often mentioned.49
The classification of patients with metastatic germ cell tumors as good, intermediate, or poor prognosis, based on serum tumor markers and extent of disease, was proposed in 1997 by The International Germ Cell Cancer Collaborative Group (Table 70.4).44 In this system, the presence or absence of nonpulmonary, extranodal metastasis was validated as an independent prognostic factor for progression-free survival. For NSGCT, the degree of marker elevation and mediastinal primary (versus testis or retroperitoneal primary) were also validated as prognostic factors. The threshold values for tumor markers (hCG, AFP, and LDH) have been incorporated into the American Joint Commission on Cancer/International Union Against Cancer staging system as the “S” classification. These prognostic groupings are used to make treatment decisions and are discussed in the following sections.

MANAGEMENT OF CLINICAL STAGE I DISEASE
Virtually all patients with clinical stage I germ cell tumors survive (cancer specific survival 98% to 99%).49 In general, patients managed with observation have the same life expectancy as those who receive adjuvant intervention. Treatment decisions must therefore be based on considerations of cost, burden of therapy, and patient preference.48
Seminoma
Clinical stage I seminoma is grouped into high-risk and average-risk based on tumor size, but similar options exist for all patients with stage I seminoma, regardless of risk.48,49 Stage I is a common presentation, accounting for approximately 70% of patients diagnosed with pure seminoma.
Surveillance
The average risk of recurrence with surveillance for stage I seminoma is 15% to 20%. Recurrences are temporally distributed over a 10-year period.50 High-risk tumors have a recurrence probability of 30% to 35%, whereas tumors <4 cm without rete testis involvement may have a risk as low as 12%.51 Observation is heavily dependent on CT scanning because the region at highest risk is in the retroperitoneum, and most seminomas do not secrete tumor markers.
Adjuvant Chemotherapy
Carboplatin is a simple and apparently safe form of adjuvant chemotherapy that is very similar in efficacy to prophylactic radiotherapy. In a randomized trial of carboplatin given as a single infusion (area under curve equals 7) versus radiotherapy to the para-aortic lymph nodes, there was no significant difference in progression-free survival (94.7% and 96%, respectively).52,53 There was only one death from seminoma (N = 1,447) reported at a median follow-up of 6.5 years. Recurrences after adjuvant carboplatin were frequently retroperitoneal in location, meaning that follow-up CT scans are mandatory. There has been no reported evidence of second malignant neoplasms (SMN), and there have been fewer contralateral germ cell tumors reported in the patients treated with chemotherapy versus radiotherapy at medium-term follow-up. Two courses of carboplatin have also been shown to be effective in the adjuvant setting, and in its 2014 guidelines the National Comprehensive Cancer Network (NCCN) endorsed one or two courses as a standard of care for clinical stage I seminoma, regardless of the estimated risk of recurrence.54 Observation is also standard and is the preferred option.
Prophylactic Radiotherapy
Treatment of para-aortic lymph nodes to a dose of 20 Gy was associated with excellent local control approaching 100%. A randomized trial of 20 Gy versus 30 Gy showed no difference in rate of recurrence.55 Omission of ipsilateral iliac lymph nodes from the treatment field resulted in less toxicity (infertility, gastrointestinal effects) and minimal loss of efficacy. The recurrence rate after prophylactic radiotherapy for clinical stage I seminoma is about 4%, and most of those patients survive with additional treatment (combination chemotherapy). Recurrences tend to occur below the radiation field (pelvic lymph nodes) or above it (lungs). Radiotherapy is contraindicated for patients with horseshoe kidney or inflammatory bowel disease.
Radiotherapy was once popularized because it reduced the number of CT scans that were necessary for follow-up, with a net reduction in the cost of treatment.56 There is, however, a different type of cost with radiotherapy, and that is the risk of SMN.57 Studies of testicular cancer survivors 20 or more years after treatment have revealed an increase in midline cancers such as gastrointestinal and genitourinary malignancies.58 This has brought about a reassessment of prophylactic radiotherapy, specifically, whether it is warranted when 80% of seminoma patients will be treated unnecessarily, there is a risk of SMN, and there is no survival benefit. While it continues to be offered, the use of prophylactic radiotherapy for seminoma is declining.59
Nonseminomatous Germ Cell Tumor
Clinical stage I NSGCT is broadly categorized as high risk and average risk. Although there are many differences in presentation, including tumor size, histology, and tumor marker pattern, only LVI has been validated as a risk factor. NSGCT with LVI has a recurrence rate of approximately 50% with observation.60 Most recurrences are seen within 2 to 3 years of the orchiectomy.50 The average risk is 30%, and most patients with LVI-negative (“good risk”) stage I NSGCT probably have a risk of recurrence <30%. The risk of recurrence for LVI-negative stage I NSGCT with predominantly embryonal carcinoma histology, however, may be higher (30% to 50%).61
Surveillance
Approximately two-thirds of relapses among patients on surveillance are in retroperitoneal lymph nodes and one-third occur in lungs or by marker elevation alone. Recurrences in nonpulmonary viscera are rare. Most relapses occur within 2 to 3 years of orchiectomy, and patients need to remain on follow-up for at least 5 years. The ability to cure systemic disease with cisplatin-based chemotherapy in those who relapse makes observation an attractive option. It avoids unnecessary therapy for about two-thirds of patients.
Criteria for selecting patients for observation have been suggested in the literature and in the NCCN guidelines.54 Selection based on whether the patient is “reliable” (i.e., likely to be compliant with follow-up) was once the prevalent view, but this has been largely replaced by a more objective risk-adapted approach. Observation is the standard of care for patients with stage IA NSGCT. The alternative is nerve-sparing RPLND, which is an unnecessary procedure for the majority of these patients. There is no subgroup of stage IA for whom RPLND is preferred, but patients who choose observation should agree to be compliant with follow-up and to receive chemotherapy in the event of recurrence. Patients with stage IA embryonal carcinoma–predominant tumors are probably the least likely to benefit from RPLND because of the tendency for hematogenous spread and associated risk of recurrence in lungs.61,62
Most patients with LVI-negative, clinical stage I NSGCT are candidates for observation. There is less of a consensus on management of patients with pT2-pT4 tumors (including LVI-positive). A minority of patients with pT2 tumors may choose observation, but they must understand that the risk of recurrence is 50% and they should agree (as in stage IA) to comply with follow-up. The preferred treatment for LVI-positive or advanced T classification NSGCT is adjuvant chemotherapy.
Adjuvant Chemotherapy
Primary chemotherapy for NSGCT consists of bleomycin, etoposide, and cisplatin (BEP) (Table 70.5).63,64 There are several published studies using either one or two courses of adjuvant BEP or similar regimens in patients with clinical stage I NSGCT.65–68 A phase II study by the Medical Research Council found 98% relapse-free survival after two courses of bleomycin, vincristine, and cisplatin, although chemotherapy-induced neurotoxicity (CIN) remained a problem.68 Two courses of BEP are similarly effective in preventing recurrence with less CIN; however, etoposide causes transient myelosuppression and is associated with a low but real risk of treatment-induced leukemia. The toxicity of two courses of BEP makes it unacceptable for stage IA NSGCT, but reasonable for stage IB where the relapse rate on observation is 50%.48 One limitation of adjuvant chemotherapy is the continuing risk of growing teratoma syndrome. Only RPLND can remove foci of teratoma from the retroperitoneum, where they may persist and grow following chemotherapy.69

One strategy for lowering the toxicity of adjuvant chemotherapy is by shortening treatment to one course of BEP. Two European studies provide data collected prospectively; in one study, patients were randomized to a single course of BEP or RPLND (none were observed), and in a second study, the patients with LVI-negative tumors were offered surveillance or one course of BEP, while patients with LVI-positive tumors were offered one or two courses of adjuvant BEP.66,67 Both of these studies showed a <5% recurrence rate after one course of BEP and an acceptably low rate of growing teratoma. The 2014 NCCN guideline endorses either one or two courses of adjuvant BEP for stage IB NSGCT.54
Retroperitoneal Lymph Node Dissection
RPLND performed in clinical stage I NSGCT is done to accurately stage the patient (as pathologic stage I or stage II) and remove all viable disease. RPLND is curative for teratoma, which has been reported in 21% to 30% of cases with viable disease.70 Mortality from RPLND is <1%; minor complications include prolonged ileus, wound infection, and lymphocele. Major complications (hemorrhage, ureteral injury, chylous ascites, pulmonary embolus, wound dehiscence, bowel obstruction) are rare. A notable long-term morbidity is sympathetic nerve damage leading to failure of ejaculaton.71
For optimal outcomes, RPLND should be performed at a referral center with an experienced surgeon. A bilateral infrahilar RPLND includes the precaval, retrocaval, paracaval, interaortocaval, retroaortic, preaortic, paraortic, and common iliac lymph nodes.
There are two types of nerve-sparing RPLND: the modified template RPLND and nerve-dissection bilateral RPLND.70,72,73 The template dissection helps the surgeon avoid regions where the risk of metastasis may be less. The nerve-dissection technique identifies and preserves both sympathetic chains, postganglionic sympathetic fibers, and the hypogastric plexus, which are necessary for anterograde ejaculation. The reported incidence of retrograde ejaculation with this technique is <5%. Another innovation is the robot-assisted RPLND, in which robotic instruments are inserted through a series of trocar entry sites and controlled remotely by the surgeon.74–76 Patients have less pain, shorter hospitalization time, rapid recovery, and smaller surgical scars.
The relapse rate following RPLND is variable. It may be as low as 4% with a low-risk patient and an experienced surgeon. Most series include patients who also received adjuvant chemotherapy, typically with two courses of BEP, for pathologic stage II disease. Thus, the success of RPLND comes at the expense of double therapy for some patients. The LVI-positive and other high-risk patients have higher reported failure rates (10% to 14%) with prophylactic RPLND alone.62 The greatest advantage of RPLND is the early control of teratoma when it exists. The randomized trial of RPLND versus one course of BEP found a recurrence rate of 8.3% in the RPLND arm and 1.1% in the BEP arm.66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








