The blood supply to the stomach is extensive and is based on vessels arising from the celiac axis (see Fig. 46.1). The right gastric artery arises from the hepatic artery proper (50% to 68%), left hepatic artery (29% to 40%), or from the common hepatic artery (3.2%). The left gastric artery originates from the celiac axis directly (90%) and may arise from the common hepatic artery (2%), splenic artery (4%), or aorta or from the superior mesenteric artery (3%). Both right and left gastric arteries course along the lesser curvature. Along the greater curvature are the right gastroepiploic artery, which originates from the gastroduodenal artery at the inferior border of the proximal duodenum (rarely from the superior mesenteric artery), and the left gastroepiploic artery (highly variable artery), branching from the distal (72%), inferior, middle splenic artery laterally. The short gastric arteries (vasa brevia, five to seven separate vessels) arise directly from the splenic artery or the left gastroepiploic artery. The posterior (dorsal) gastric artery (17% to 68%) may arise from the splenic artery to supply the distal esophagus, cardia, and fundus. The preservation of any of these vessels in the course of a subtotal gastrectomy for carcinoma is not necessary, and the most proximal few centimeters of remaining stomach are well supplied by collateral flow from the lower segmental esophageal arterial arcade. The rich submucosal blood supply of the stomach is an important factor in its ability to heal rapidly and produce a low incidence of anastomotic disruption following radical gastric resection. The venous drainage of the stomach tends to parallel the arterial supply. The venous efflux ultimately passes through the portal venous system, and this is reflected in the fact that the liver is the primary site for distant metastatic spread.
The lymphatic drainage of the stomach is extensive, and distinct anatomic groups of perigastric lymph nodes have been defined according to their relationship to the stomach and its blood supply. There are six perigastric lymph node groups. In the first echelon (stations 1 through 6) are the right and left pericardial nodes (stations 1 and 2). Along the lesser curvature are the lesser curvature nodes (station 3) and the suprapyloric nodes (station 5). Along the greater curvature, the gastroepiploic nodes or greater curvature nodes (station 4), and the subpyloric nodes (station 6). In the second echelon (stations 7 through 12) are the nodes along named arteries, which include the left gastric, common hepatic, celiac, splenic hilum, splenic artery, and hepatoduodenal lymphatics (stations 7 through 12, respectively), which drain into the celiac and periaortic lymphatics. The third echelon (stations 13 through 16) contains the posterior to pancreatic head, superior mesenteric artery, middle colic artery, and para-aortic lymphatics (stations 13 through 16, respectively). Proximally are the lower esophageal lymph nodes; extensive spread of gastric cancer along the intrathoracic lymph channels may be manifested clinically by a metastatic lymph node in the left supraclavicular fossa (Virchow’s node) or left axilla (Irish’s node). Tumor spread to the lymphatics in the hepatoduodenal ligament can extend along the falciform ligament and result in subcutaneous periumbilical tumor deposits (Sister Mary Joseph’s nodes).
Approximately 95% of all gastric cancers are adenocarcinomas. The term gastric cancer refers to adenocarcinoma of the stomach. Other malignant tumors are rare and include squamous cell carcinoma, adenoacanthoma, carcinoid tumors, small cell carcinoma, mucinous carcinoma, hepatoid adenocarcinoma, oncocytic (parietal gland) carcinoma, sarcomatoid carcinoma, lymphoepithelioma-like carcinoma, adenocarcinoma with rhabdoid features, gastric carcinoma with osteoclastlike giant cells, neuroendocrine tumor, gastrointestinal stromal tumor, or leiomyosarcoma.8 Although no normal lymphoid tissue is found in the gastric mucosa, the stomach is the most common site for lymphomas of the gastrointestinal tract. The increased awareness of association between mucosa-associated lymphoid tissue lymphomas and Helicobacter pylori may explain, in part, the rise in incidence,9 although the incidence of mucoas-associated lymphoid tissue gastric lymphomas is decreasing likely because of effective treatment against H. pylori.10
In terms of pathogenesis, two new concepts are worth mentioning: bone marrow participation in gastric carcinogenesis and gastric cancer stem cells. It has been hypothesized that the gastric epithelial cells acquiring abnormal phenotype (resembling intestinal epithelium) originate from gastric stem cells localized to the only cell replication zone of the gastric glands (i.e., the isthmus). However, Houghton et al.,11 Stoicov et al.,12,13 and Li et al.14 demonstrated in a rodent model of Helicobacter-induced gastric cancer that the entire cancer mass was derived from cells originating in the bone marrow. This interesting phenomenon was observed by other authors studying solid cancers in patients receiving bone marrow transplantation.15 Recent evidence proposes the existence of cancer stem cells or stemlike cancer cells in various cancers. Although controversial, cancer stem cells are defined as cancer cells with the exclusive ability to initiate tumors, metastasize, and self-renew tumors. In gastric cancer, several investigators suggested the existence of gastric cancer stem cells (i.e., CD44+) and side population cells.16,17 These cells showed relative resistance to chemotherapy and radiation, and exclusive ability to initiate tumors. These important observations might lead to novel approaches to the diagnosis and treatment of gastric cancer in the next decade.
Several staging schemas have been proposed based on the morphologic features of gastric tumors. The Borrmann classification divides gastric cancer into five types depending on macroscopic appearance. Type I represents polypoid or fungating cancers, type II encompasses ulcerating lesions surrounded by elevated borders, type III represents ulcerated lesions infiltrating the gastric wall, type IV includes diffusely infiltrating tumors, and type V gastric cancers are unclassifiable cancers.18 The gross morphologic appearance of gastric cancer and the degree of histologic differentiation are not independent prognostic variables. Ming19 has proposed a histomorphologic staging system that divides gastric cancer into either a prognostically favorable expansive type or a poor prognosis infiltrating type. Based on an analysis of 171 gastric cancers, the expansive-type tumors were uniformly polypoid or superficial on gross appearance, whereas the infiltrative tumors were almost always diffuse. Grossly ulcerated lesions were divided between the expansive or infiltrative forms. Broder’s classification of gastric cancer grades tumors histologically from 1 (well-differentiated) to 4 (anaplastic). Bearzi and Ranaldi20 have correlated the degree of histologic differentiation with the gross appearance of 41 primary gastric cancers seen on endoscopy. Ninety percent of protruding or superficial cancers were well differentiated (Broder’s grade 1), whereas almost half of all ulcerated tumors were poorly differentiated or diffusely infiltrating (Broder’s grades 3 and 4).
The most widely used classification of gastric cancer is by Laurén.21 It divides gastric cancers into either intestinal or diffuse forms. This classification scheme, based on tumor histology, characterizes two varieties of gastric adenocarcinomas that manifest distinctively different pathology, epidemiology, genetics, and etiologies. The intestinal variety represents a differentiated cancer with a tendency to form glands similar to other sites in the gastrointestinal tract, but in particular the colon type; hence the intestinal type. In contrast, the diffuse form exhibits very little cell cohesion with a predilection for extensive submucosal spread and early metastases. Although the diffuse-type cancers are associated with a worse outcome than the intestinal type, this finding is not independent of tumor, node, and metastasis (TNM) stage. The molecular pathogenesis of these two distinct forms of gastric cancer is also different. Although the intestinal type represents H. pylori–initiated multistep progression with less defined progressive genetic alterations, the diffuse type main carcinogenic event is loss of expression of E-cadherin (CDH1 gene). E-cadherin is a molecule involved in cell-to-cell adhesion; loss of its expression leads to noncohesive growth, hence the diffuse type. In tumors that display both intestinal and diffuse phenotypes, the CDH1 mutation and loss of E-cadherin function are observed only within the diffuse phenotype.22
Carcinomas of the stomach can spread by local extension to involve adjacent structures and can develop lymphatic metastases, peritoneal metastases, and distant metastases. These extensions can occur by the local invasive properties of the tumor, lymphatic spread, or hematogenous dissemination. The initial growth of the tumor occurs by penetration into the gastric wall, extension through the wall, within the wall longitudinally,23 and subsequent involvement of an increasing percentage of the stomach. The two modes of local extension that can have major therapeutic implications are tumor penetration through the gastric serosa, where the risk of tumor invasion of adjacent structures or peritoneal spread is increased, and lymphatic involvement. Zinninger24 has evaluated spread within the gastric wall and has found a wide variation in its extent. Tumor spread is often through the intramural lymphatics or in the subserosal layers.23 Local extension can also occur into the esophagus or the duodenum.25 Duodenal extension is rare (0.5% to 1.8% of all resected cases),26 portrays poor prognosis, and is principally through the muscular layer by direct infiltration and through the subserosal lymphatics, but is not generally to any great extent.27 Extension into the esophagus occurs primarily through the submucosal lymphatics.28
Local extension does not occur solely by radial intramural spread but also by deep invasion through the wall to adjacent structures23 (omentum, spleen, adrenal gland, diaphragm, liver, pancreas, or colon). Many studies report that 60% to 90% of patients had primary tumors penetrating the serosa or invading adjacent organs and that at least 50% had lymphatic metastases. In the largest series reporting on 10,783 patients with gastric cancer from Korea, 57% of the patients had lymph node metastasis, and the average number of involved lymph nodes was five.29,30 Of the 1,577 primary gastric cancer cases admitted to Memorial Sloan Kettering Cancer Center (MSKCC) between July 1, 1985, and June 30, 1998, 60% of the 1,221 resected cases had evidence of serosal penetration and 68% had positive nodes. Lymph node metastases were found in 18% of pT1 lesions, and 60% of pT2 lesions after R0 resection in 941 patients. The highest incidence of lymphatic metastasis was seen in tumors diffusely involving the entire stomach. Tumors located at the gastroesophageal junction also had a high incidence relative to other sites.31
The pattern of nodal metastases also varies depending on the location of the primary site. In a study, reporting on 1,137 patients with early gastric cancer (EGC), tumors located in the upper, middle, and lower third of the stomach had 12%, 10%, and 8% nodal involvement, respectively. The most common nodal station metastases for the upper, middle, and lower third of the stomach were stations 3 (lesser curvature), 3/4/7 (lesser/greater curvature/left gastric artery), and 3/4/6 (lesser/greater curvature/infrapyloric), respectively.32 Earlier studies that included more advanced gastric cancers showed that the left gastric artery nodes were at increased risk for nodal metastases independent of tumor location.32,33
Gastric cancer recurs in multiple sites, locoregionally and systemically. Patterns of failure are variable. These differences are likely related to the patient cohorts evaluated, the time at which failure was determined, and the method of determination of failure patterns. Recent series from the MSKCC and Korea do shed light on modern patterns of failure.31,34 In the report from MSKCC, recurrence patterns of 1,038 patients who underwent R0 gastrectomy with D2 lymphadenectomy (61%) were analyzed; complete data on recurrence were available in 367 (74%) of 496 patients who experienced recurrence. The locoregional area was involved in 199 (54%) patients. Distant sites were involved in 188 (51%) patients, and peritoneal recurrence was detected in 108 (29%) patients. More than one site of recurrence was detected: distal, peritoneal, and locoregional recurrences in 9 (2.5%); locoregional and peritoneal in 34 (9.3%); locoregional and distant in 61 (16.6%); and distant and peritoneal in 15 (4.1%) patients. On multivariate analysis, peritoneal recurrence was associated with female gender, advanced T stage, and distal and diffuse type tumors; locoregional recurrence was associated with proximal location, early T stage, and intestinal type tumors. In the study from Korea, recurrence patterns were analyzed in 2,038 patients who were treated with potentially curative gastrectomy.34 Of 508 patients who developed recurrence, 33% involved locoregional sites, 44% were peritoneal, and 38% were distant. At time of presentation, 35% of patients presented with distant metastasis, with 4% to 14% having liver metastases.35,36
CLINICAL PRESENTATION AND PRETREATMENT EVALUATION
Signs and Symptoms
Because of the vague, nonspecific symptoms that characterize gastric cancer, many patients are diagnosed with advanced-stage disease. Patients may have a combination of signs and symptoms such as weight loss (22% to 61%)37; anorexia (5% to 40%); fatigue, epigastric discomfort, or pain (62% to 91%); and postprandial fullness, heart burn, indigestion, nausea, and vomiting (6% to 40%). None of these unequivocally indicates gastric cancer. In addition, patients may be asymptomatic (4% to 17%).38 Weight loss and abdominal pain are the most common presenting symptoms at initial encounter.39–41 Weight loss is a common symptom, and its clinical significance should not be underestimated. Dewys et al.42 found that in 179 patients with advanced gastric cancer, >80% of patients had a >10% decrease in body weight before diagnosis. Furthermore, patients with weight loss had a significantly shorter survival than did those without weight loss.40
In some patients, symptoms may suggest the presence of a lesion at a specific location. Up to 25% of the patients have history/symptoms of peptic ulcer disease.39 A history of dysphagia or pseudoachalasia may indicate the presence of a tumor in the cardia with extension through the gastroesophageal junction.43 Early satiety is an infrequent symptom of gastric cancer but is indicative of a diffusely infiltrative tumor that has resulted in loss of distensibility of the gastric wall. Delayed satiety and vomiting may indicate pyloric involvement. Significant gastrointestinal bleeding is uncommon with gastric cancer; however, hematemesis does occur in approximately 10% to 15% of patients, and anemia in 1% to 12% of patients. Signs and symptoms at presentation are often related to spread of disease. Ascites, jaundice, or a palpable mass indicate incurable disease.40 The transverse colon is a potential site of malignant fistulization and obstruction from a gastric primary tumor. Diffuse peritoneal spread of disease frequently produces other sites of intestinal obstruction. A large ovarian mass (Krukenberg’s tumor) or a large peritoneal implant in the pelvis (Blumer’s shelf), which can produce symptoms of rectal obstruction, may be palpable on pelvic or rectal examination.37,44 Nodular metastases in the subcutaneous tissue around the umbilicus (Sister Mary Joseph’s node) or in peripheral lymph nodes such as in the supraclavicular area (Virchow’s node) or axillary region (Irish’s node) represent areas in which a tissue diagnosis can be established with minimal morbidity.45 There is no symptom complex that occurs early in the evolution of gastric cancer that can identify individuals for further diagnostic measures. However, alarming symptoms (dysphagia, weight loss, and palpable abdominal mass) are independently associated with survival; increased number and the specific symptom is associated with mortality.40,41
Screening
A list of risk factors associated with gastric cancer is outlined in Table 46.1. These factors might be use for risk stratification in screening programs. Mass screening programs for gastric cancer have been most successful in high-risk areas, especially in Japan.46 A variety of screening tests have been studied in Japanese patients, with a sensitivity and specificity of approximately 90%.40 Screening typically includes serology for H. pylori, the use of double-contrast barium radiographs, or upper endoscopy with risk stratification (OLGA staging system for gastric cancer risk46,47).

Ohata et al.48 reported on 4,655 asymptomatic patients with an average age of 50 years old who were followed for 7.7 years. Atrophic gastritis was identified using pepsinogen and H. pylori testing: 2,341 (52%) were H. pylori–positive with nonatrophic gastritis, 967 (21%) were H. pylori–negative without atrophic gastritis, 1,316 (28%) were H. pylori–positive with atrophic gastritis, and 31 (0.7%) had severe atrophic gastritis. The rates of gastric cancer development per population per year were 107/100,000 for H. pylori–positive with nonatrophic gastritis, 0/100,000 for H. pylori–negative without atrophic gastritis, 238/100,000 for H. pylori–positive with atrophic gastritis, and 871/100,000 for severe atrophic gastritis. Thus the number of endoscopies needed to detect one cancer was 1/1,000, 0/1,000, 1/410, and 1/114, respectively. Similar data were reported on 6,985 patients by Watabe et al.49 Surveillance in endemic populations is clinically important because EGC has a very high cure rate with surgical treatment. However, the fact that gastric cancer remains one of the top causes of death in Japan indicates the limitations of a mass-screening program when the entire population at risk is not effectively screened. However, more recent studies indicate that for surveillance programs to be effective and feasible from an economical perspective, they should be instituted only in high-risk populations (>20/100,000 incidence of disease) and include the following components: detection and eradication of H. pylori, serum pepsinogen (pepsinogen I/II ratio), endoscopy with biopsy, and risk stratification before and after H. pylori eradication using a system such as the OLGA staging system for gastric cancer risk. Such programs are expected to avoid long-term repeated screening of approximately 70% of the population who are at low risk of developing gastric cancer.46,50–52 A US study found that screening and eradication of H. pylori in Japanese Americans is cost-effective in preventing gastric cancer.53 These findings were confirmed by two studies from the United Kingdom.54,55
Tumor Markers
Most gastric cancers have at least one elevated tumor marker, but some benign gastric diseases show elevated serum tumor markers as well. Tumor markers in gastric cancer continue to have limited diagnostic usefulness, with their role more informative in follow-up after primary treatment. The most commonly used markers are serum carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9, CA 50, and CA 72-4. There is wide variation in the reported serum levels of these markers; positive CEA and CA 19-9 levels varied from 8% to 58% and 4% to 65%, respectively. Overall, the sensitivity of each serum tumor marker alone as a diagnostic marker of gastric cancer is low. However, when the levels are elevated, it does usually correlate with stage of disease. Combining CEA with other markers, such as CA 19-9, CA 72-4, or CA 50, can increase sensitivity compared with CEA alone.56–62
In a large study evaluating serum CEA, α-fetoprotein, human chronic gonadotropin-β, CA 19-9, CA 125, as well as tissue staining for HER2 in gastric cancer patients, only human chronic gonadotropin-β level >4 IU/L and a CA 125 level ≥350 U/mL had prognostic significance. Elevated serum tumor marker levels in gastric cancer before chemotherapy may reflect not just tumor burden but also biology of disease.
Endoscopy
Endoscopy is the best method to diagnose gastric cancer as it visualizes the gastric mucosa and allows biopsy for a histologic diagnosis. Chromoendoscopy helps identify mucosal abnormalities through topical mucosal stains. Magnification endoscopy is used to magnify standard endoscopic fields by 1.5- to 150-fold. Narrow band imaging affords enhanced visualization of the mucosal microvasculature. Confocal laser endomicroscopy permits in vivo, three-dimensional microscopy including subsurface structures with diagnostic accuracy, sensitivity, and specificity of 97%, 90%, and 99.5%, respectively.63–65
Endoscopic ultrasound (EUS) is a tool for preoperative staging and selection for neoadjuvant therapy. It is used to assess the T and N stage of primary tumors. A study of 225 patients from MSKCC found that the concordance between EUS and pathology was lower than expected. The accuracy for individual T and N stage were 57% and 50%, respectively. However, the combined assessment of N stage and serosal invasion identified 77% of the patients at risk of disease-related death after curative resection.66 Other investigators compared the accuracy of EUS with that of multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) and found that the overall accuracy was 65% to 92% (EUS), 77% to 89% (MDCT), and 71% to 83% (MRI) for T stage, and 55% to 66% (EUS), 32% to 77% (MDCT) for N stage, respectively. The corresponding sensitivity and specificity for serosal involvement were 78% to 100% (EUS), 83% to 100% (MDCT), and 89% to 93% (MRI) for T stage, and 68% to 100% (EUS), 80% to 97% (MDCT), and 91% to 100% (MRI) for N stage, respectively.65,67
Computed Tomography
Once gastric cancer is suspected, a triphasic CT with oral and intravenous contrast of the abdomen, chest, and pelvis is imperative. In a study of 790 patients who underwent MDCT prior to surgery, the overall accuracy in determining T stage was 74% (T1 46%, T2 53%, T3 86%, and T4 86%), and for N staging it was 75% (N0 76%, N1 69%, and N2 80%). The sensitivity, specificity, and accuracy for lymph node metastasis were 86%, 76%, and 82%, respectively.68 MDCT with thin-sliced multiplanar reconstruction (MPR) and water filling is increasingly used. The accuracy rate for advanced gastric cancer was 96% and for EGC it was 41%. An improvement on axial CT and MPR-MDCT was the addition of staging with three-dimensional MPR-MDCT. The detection rate for MPR with virtual gastroscopy was 98%. MPR-MDCT with combined water and air distention is superior to conventional axial imaging.69
Magnetic Resonance Imaging
MRI is not used routinely in preoperative staging of gastric cancer. Several studies have demonstrated that CT and MRI are comparable in terms of accuracy and understaging.70,71 However, MRI is a useful modality to further characterize liver lesions identified on preoperative CT staging workup.
Positron Emission Tomography
Whole-body 2-[18F]-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) is being applied increasingly in the evaluation of gastrointestinal malignancies. In gastric cancer, approximately half of the primary tumors are FDG-negative; the diffuse (signet cell) subtype was most likely to be non-FDG avid, likely because of decreased expression of the glucose transporter-1 (Glut-1).72 In patients with non-FDG–avid primary tumor, FDG-PET/CT is not useful.72–76 PET/CT was tested as a tool to predict response to neoadjuvant chemotherapy. Ott et al.77 reported 90% 2-year survival in patients with PET-defined response (<35% decrease standardized uptake value [SUV]) versus 25% for patients not responding to PET. PET response could be detected as early as 14 days. At least 60% of the patients were PET-nonresponding patients and thus could have been spared further chemotherapy. Authors of the MUNICON trial reported on patients who were PET nonresponders by day 14 after cisplatin and fluorouracil (5-FU) (CF) neoadjuvant chemotherapy, and subsequently were sent for surgery, and patients who were PET responders and continued 3 months of neoadjuvant therapy before surgery. The PET-responding patients had a survival benefit (hazard ratio [HR] = 2.13; p <0.15). In PET-nonresponding patients, stopping the chemotherapy did not affect long-term survival.78 Recent studies, including one large meta-analysis, showed that in terms of diagnostic accuracy and lymph node staging EUS, MDCT, MRI, and PET/CT are comparable modalities. There were no significant differences between mean sensitivities and specificities.79,80 Even in patients whose tumors were FDG-avid, FDG-PET/CT scans did not identify occult peritoneal disease (0 of 18), but did identify extraperitoneal M1 disease in nine patients with bone (n = 2), liver (n = 4), and retroperitoneal lymph node (n = 3) involvement. In patients with FDG-avid tumors, PET may be useful in detecting metastatic disease and follow-up for recurrence. Interestingly, the presence of Glut-1– and FDG-avid gastric cancers may be associated with decreased OS.72 The role of PET/CT in the primary staging of gastric cancer remains to be established; its role might be better defined in advanced disease.
In a prospective study of 113 patients who were clinically staged as locally advanced but nonmetastatic gastric cancer (T3-T4, Nx or N+, M0), investigators found that FDG-PET/CT did identify occult metastatic disease in about 10% of patients. In this study, FDG-PET/CT did not identify occult peritoneal disease, suggesting a necessary role for laparoscopy in preoperative staging of locally advanced gastric cancer. A cost evaluation was also performed, and it suggested that if FDG-PET/CT is included as part of the staging algorithm, that would result in an estimated cost savings of approximately $13,000 US dollars per patient.81
Staging Laparoscopy and Peritoneal Cytology
Staging laparoscopy with peritoneal lavage should be an integral part of the pretreatment staging evaluation of patients believed to have localized gastric cancer. Current noninvasive modalities used in preoperative staging of gastric cancer have sensitivities significantly lower than 100%, particularly in cases of low-volume peritoneal carcinomatosis.82–84 Current CT techniques cannot consistently identify low-volume macroscopic metastases that are ≤5 mm in size. Laparoscopy directly inspects the peritoneal and visceral surfaces for detection of CT-occult, small-volume metastases. Staging laparoscopy also allows for assessment of peritoneal cytology and laparoscopic ultrasound. Laparoscopic staging is done to spare nontherapeutic operations and for potential stratification in various trials.85
The rate of detection of CT-occult M1 disease by laparoscopy depends on the quality of CT scanning and interpretation.86 Muntean et al.87 reported on 98 patients with primary gastric cancer: 45 underwent staging laparoscopy with subsequent surgery and 53 went directly to surgery. An unnecessary laparotomy was avoided in 38% of the patients. The overall sensitivity and specificity were 89% and 100%, respectively. Nonetheless, even high-quality MDCT is insufficiently sensitive for detection of low-volume extragastric disease and thus CT, EUS, and laparoscopy are complementary staging studies.88,89
The value of peritoneal cytology as a preoperative staging tool in patients with gastric cancer who are potential candidates for curative resection by EUS and CT has been examined by several investigators. Bentrem et al.90 reported on 371 patients who underwent R0 resection, 6.5% of whom had positive cytology after staging laparoscopy. Median survival of patients with positive cytology was 14.8 versus 98.5 months for patients with negative cytology findings (p <0.001). Positive cytology predicted death from gastric cancer (relative risk = 2.7; p <0.001) and is tantamount to M1 disease. Several groups confirmed these findings and concluded that staging laparoscopy with peritoneal cytology can change the management of gastric cancer in 6.5% to 52% of patients.83,91–94
Laparoscopy can be performed as a separate staging procedure prior to definitive treatment planning or immediately prior to planned laparotomy for gastrectomy. When performed as a separate procedure, laparoscopy has the disadvantage of the additional risks and expense of a second general anesthetic. However, separate procedure laparoscopy allows the additional staging information including cytology acquired at laparoscopy to be reviewed and discussed with the patient and in multidisciplinary treatment group prior to definitive treatment planning. Laparoscopic ultrasound (LUS) and “extended laparoscopy” are techniques that may increase the diagnostic yield of laparoscopy. Preliminary results reveal conflicting data on the added benefit of LUS and extended laparoscopy.95–97 Further prospective studies will be required to evaluate the cost-benefit relationship of LUS and extended laparoscopy in the routine or selective workup of patients with gastric cancer.
Although laparoscopic staging is thought to detect CT-occult metastatic disease in approximately 40% of patients and spares nontherapeutic operations in approximately one-third of patients with gastric cancer, one needs to remember that tumor biology, not staging, will eventually guide outcomes. Clearly, not all patients benefit from preoperative laparoscopic staging; therefore, future studies should address the issue of selective laparoscopy based on noninvasive staging (i.e., patients with T1 tumors). Staging laparoscopy with or without cytology should be considered only if therapy will be altered consequent to information obtained by laparoscopy.82
STAGING, CLASSIFICATION, AND PROGNOSIS
For patients with surgically treated gastric adenocarcinoma, both pathologic staging (American Joint Committee on Cancer [AJCC]/International Union Against Cancer [UICC] or Japanese system) and classification of the completeness of resection (R classification) should be done. Although not formal components of the stage grouping, the AJCC recommends collection of additional prognostic factors: tumor location, serum CEA and CA 19.9, and histopathologic grade and type.98
American Joint Committee on Cancer/International Union Against Cancer Tumor, Node, Metastasis Staging
The AJCC/UICC TNM staging system for gastric cancer is outlined in Table 46.2.98 The AJCC/UICC stage-stratified survival rates of 10,601 patients treated by surgical resection from the Surveillance, Epidemiology, and End Results program 1973 to 2005 public-use file diagnosed in years 1991 to 2000 are shown in Fig. 46.2. Several definitions in the most recent version of the AJCC (2010) differ from the previous version (2002). Tumors arising at the EGJ including Siewert type I or arising in the stomach ≤5 cm from the EGJ and crossing into the EJG including Siewert types II and III are staged using the TNM system for esophageal adenocarcinoma. Gastric tumors lying ≤5 cm from the EGJ but that do not cross the EGJ into the esophagus are staged as gastric cancer.98
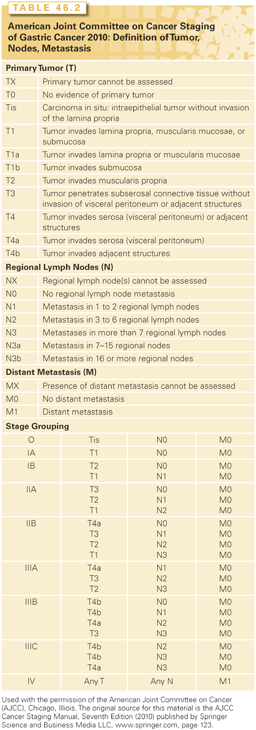
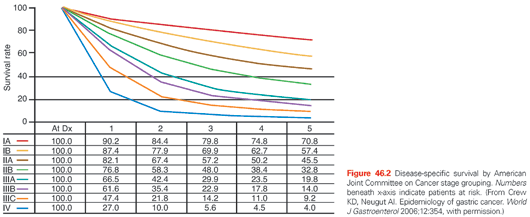
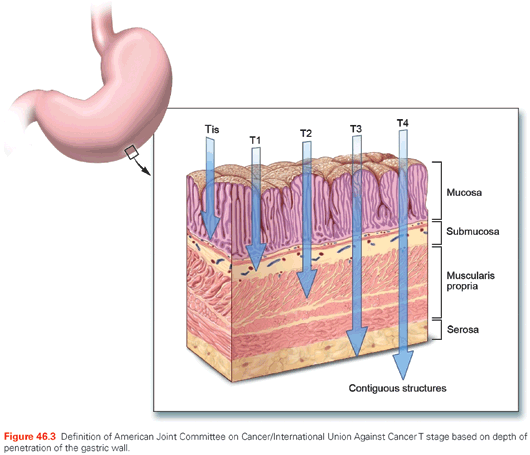
In the AJCC/UICC staging system, tumor (T) stage is determined by depth of tumor invasion into the gastric wall and extension into adjacent structures (Fig. 46.3). The relationship between T stage, the overall stage, and survival is well defined (see Fig. 46.2). Nodal stage (N) is based on the number of involved lymph nodes, a criterion that may predict outcome more accurately than the location of involved lymph nodes.99,100 Tumors with 1 to 2 involved nodes are classified as pN1, 3 to 6 involved nodes are classified as pN2, and those with 7 or more involved nodes are classified as pN3 (N3a has 7 to 15 nodes and N3b has ≥16 nodes). The use of numerical thresholds for nodal classification has gained increasing acceptance, although the extent of lymphadenectomy and rigor of pathologic assessment may affect results.101,102 The nodal numerical threshold approach is based on observations that survival decreases as the number of metastatic lymph nodes increases,103,104 and that survival significantly decreases at three or more involved103 lymph nodes and again at seven or more involved lymph nodes.30,105
Given the reliance on numerical thresholds for nodal staging, it is extremely important that adequate number of lymph nodes are retrieved surgically and examined pathologically. However, recent reports document poor compliance with AJCC staging primarily because the number of lymph nodes removed and/or examined (≤15) was insufficient.104,106 Positive peritoneal cytology is classified as M1. Ratio-based lymph node classification (number of positive nodes over number of total nodes resected and evaluated) is an alternative to the threshold-based system currently utilized by the AJCC/UICC staging systems. It may minimize the confounding effects of regional variations in the extent of lymphadenectomy and pathologic evaluation on lymph node staging and thereby reduce stage migration.107–109 Sun et al.108 evaluated the ratio between metastatic and examined lymph nodes (RML) in a group of 2,159 patients who underwent curative gastrectomy. The anatomic location, number of positive lymph nodes (AJCC/UICC), and RML were analyzed for staging accuracy and relationship to survival. RML was an independent prognostic factor for survival and reduced stage migration. These findings were confirmed by several investigators reporting on approximately 2,000 patients treated by R0 gastrectomy.107,109–112
Japanese Staging System
The most recent Japanese Classification for Gastric Carcinoma was published in 1998.113–116 The Japanese classification and staging system is more detailed than the AJCC/UICC staging system and places more emphasis on the distinction between clinical, surgical, pathologic, and “final” staging (prefixes “c,” “s,” “p,” and “f,” respectively). For example, a surgically treated and staged patient with locally advanced, nonmetastatic gastric cancer might be staged as pT3, pN2, sH0, sM0, stage f-IIIB (where H0 denotes no hepatic metastases and the “f” prefix denotes final clinicopathologic stage). The Japanese classification system also includes a classification system for EGC (Fig. 46.4).
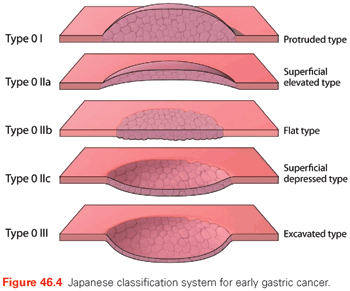
In the combined superficial types, the type occupying the largest area should be described first, followed by the next type (e.g., IIc + III). Type 0I and type 0IIa are distinguished as follows: type 0I, the lesion has a thickness of more than twice that of the normal mucosa; type 0IIa, the lesion has a thickness up to twice that of the normal mucosa.
Similar to the AJCC/UICC staging system, primary tumor (T) stage in the Japanese system is based on the depth of invasion and extension to adjacent structures, as outlined in Table 46.3.117 However, the assignment of lymph node (N) stage involves much more rigorous pathologic assessment than is required for AJCC/UICC staging. The Japanese system extensively classifies 18 lymph node regions into four N categories (N0 to N3) depending on their relationship to the primary tumor and anatomic location.118 Most perigastric lymph nodes (nodal stations 1 through 6) are considered group N1. Lymph nodes situated along the proximal left gastric artery (station 7), common hepatic artery (station 8), celiac axis (station 9), splenic artery (station 11), and proper hepatic artery (station 12) are defined as group N2. Para-aortic lymph nodes (station 16) are defined as group N3. However, some lymph nodes, even perigastric nodes for specific tumor locations, can be regarded as M1 disease (i.e., involvement of station 2 in the case of antral tumors). This is because their involvement in antral tumors is rare and portrays a poor prognosis.117
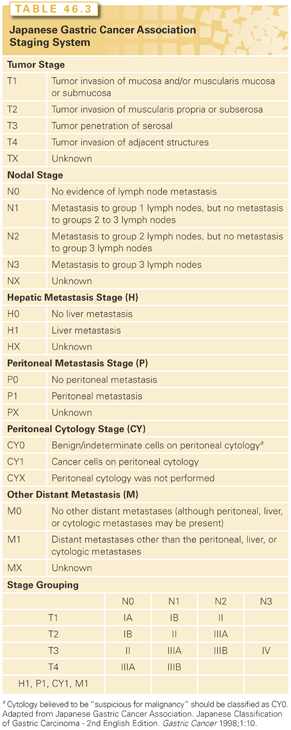
The Japanese staging system also includes elements not included in the AJCC/UICC system (see Table 46.3). These are macroscopic descriptions of the tumor (EGC subtype or Borrmann type for more advanced tumors), extent of peritoneal metastases (classified as P0-1), extent of hepatic metastases (H0-1), and peritoneal cytology findings (CY0-1). Recent comparison of the Japanese and AJCC/UICC staging systems in 731 patients suggests that both are comparable.119 However, older studies suggest that the AJCC/UICC system more accurately estimates prognosis.101
Classification of Esophagogastric Junction Cancers
EGJ cancers (i.e., tumors with a definitive component involving the EGJ) are no longer classified by the AJCC as gastric cancers per se. They are briefly reviewed here for historical reasons. Siewert and Stein120 classified adenocarcinomas of the EGJ (Siewert classification) into three distinct clinical entities that arise within 5 cm of the EGJ: type I arises in the distal esophagus and may infiltrate the EGJ from above, type II arises in the cardia or the EGJ, and type III arises in the subcardial stomach and may infiltrate the EGJ from below. The assignment of tumors to one of these subtypes is based on morphology and the anatomic location of the epicenter of the tumor. The Siewert classification has important therapeutic implications.121 The lymphatic drainage routes differ for type I versus types II and III lesions. The lymphatic pathways from the lower esophagus pass both cephalad and caudad. In contrast, the lymphatic drainage from the cardia and subcardial regions is caudad. Thus, the Siewert classification provides a practical means for choosing among surgical options. For type I tumors, esophagectomy is required, whereas types II and III tumors can be treated by transabdominal gastrectomy.121,122
Resection Classification
The R classification system indicates the amount of residual disease left after tumor resection.123 R0 indicates no gross or microscopic residual disease, R1 indicates microscopic residual disease (positive margins), and R2 signifies gross residual disease. The R classification has implications for individual patient care and clinical research. Results of clinical trials that include surgery should include information on R status. Readers should be aware of the dual use of the “R” terminology in the gastric cancer literature. Prior to 1995, the Japanese staging and treatment vernacular included an “R level,” which described the extent of lymphadenectomy. The latter is now classified by “D” (for dissection) level.
Kattan et al.103 have developed a nomogram for predicting individual patient 5-year disease-specific survival using established prognostic factors derived from a population of 1,039 patients with gastric cancer treated by R0 surgical resection without neoadjuvant therapy at a single institution (nomograms@mskcc.org). Clinicopathologic factors incorporated in the nomogram include age and gender, primary tumor site, Laurén classification, numbers of positive and negative lymph nodes resected, and depth of invasion. This nomogram was subsequently validated by several authors. Peeters et al.124 found that the nomogram prognosticates better then the AJCC staging system. Novotny et al.125 validated the nomogram in 862 patients from Germany and the Netherlands; Strong et al.126 compared outcomes using the nomogram in 711 patients from the United States and 1,646 patients from Korea. This tool may be useful for individual patient counseling regarding the use of adjuvant therapy, follow-up scheduling, and clinical trial eligibility assessment, and is available for personal handheld computer devices at www.nomograms.org.
Recently, a large retrospective cohort study of 1,273 patients who underwent resection revealed that having a positive family history of gastric cancer (defined as a self-reported history of cancer in first-degree relatives) was associated with significant reduction in disease-free survival (DFS; p = 0.012), relapse-free survival (p = 0.006), and OS (p = 0.005) when compared with those who did not have a family history of gastric cancer. The improvement in outcomes was more pronounced among patients with stage III or IV gastric cancer, with significant adjusted HRs for DFS (HR = 0.49; 95% confidence interval [CI] = 0.29 to 0.84), relapse-free survival (HR = 0.47; 95% CI = 0.30 to 0.87), and OS (HR = 0.47; 95% CI = 0.26 to 0.84), respectively.127
TREATMENT OF LOCALIZED DISEASE
Stage I Disease (Early Gastric Cancer)
Classification of Early Gastric Cancer and Risk for Nodal Metastases
The Japanese Research Society for Gastric Cancer has classified EGCs based on endoscopic criteria first established for the description of T1 tumors. The current classification system is used for both in situ and invasive tumors and categorizes tumors based on endoscopic findings as follows: protruded, type 0I; superficial elevated, type 0IIa; flat, type 0IIb; superficial depressed, type 0IIc; and excavated, type 0III (see Fig. 46.4). The English language version of the Japanese EGC classification contains excellent color photos of these subtypes.128 This classification system is important in describing patients treated by newer gastric-sparing approaches for EGC, such as endoscopic mucosal resection (EMR).114–116,129 The risk for lymph node metastasis is important when evaluating treatment options for patients with EGC. The frequency and anatomic distribution of nodal disease are related to the depth of tumor invasion. In a Japanese series of >5,000 patients who underwent gastrectomy with lymph node dissection for EGC, none of the 1,230 patients with well-differentiated intramucosal tumors <3 cm in diameter (regardless of ulceration) had lymph node metastases.130 None of the 929 patients with EGC without ulceration had nodal metastases, irrespective of tumor size. In contrast, in the subset of >2,000 patients with tumors that invaded the submucosa, the frequencies of lymph node involvement for tumors ≤1.0 cm, 1.1 to 2.0 cm, 2.1 to 3.0 cm, and >3.0 cm were 7.9%, 13.3%, 15.6%, and 23.3%, respectively. Thus, once tumors penetrate into the submucosa, the risk for nodal metastasis increases with tumor size.131–137 The estimates of the frequency of nodal disease in EGC are based on conventional light-microscopic histologic assessment. However, the use of more sensitive techniques such as serial sectioning of individual lymph nodes, immunohistochemistry, or reverse transcriptase-polymerase chain reaction may increase the frequency of detection of occult micrometastatic disease.138 The clinical significance of micrometastasis remains unknown.
Endoscopic Mucosal Resection
A subset of patients with EGC can undergo an R0 resection without lymphadenectomy or gastrectomy. The Japanese have popularized EMR for EGC. This approach involves the submucosal injection of fluid to elevate the lesion and facilitate complete mucosal resection under endoscopic guidance. Most centers reporting significant experience with EMR are in Japan. There is less experience with EMR in Western countries. Only patients with tumors that have extremely low metastatic potential should be offered EMR. These are generally well-differentiated, superficial type IIa or IIc lesions, <3 cm in diameter, and located in an easily manipulated area. Tumors invading the submucosa are at increased risk for metastasizing to lymph nodes and are not usually considered candidates for EMR.
Recently, Bennett et al.139 reviewed the available data reporting on EMR for EGC. No randomized controlled trial (RCT) reported on EMR. The indications for EMR are well-differentiated lesions, size ≤20 mm in elevated type, ≤10 mm in depressed type, no ulceration, and limited to the mucosa.140 The incidence of lymph node metastases for such lesions is approximately 1%. Complete resection of selected EGCs can be accomplished in a majority of cases (73.4% to 98%).139,141,142
Two retrospective reports indicated that EMR can reduce the risk of local residual tumor, and repeat EMR is an effective option.143,144 Recurrence after EMR varies with type of EGC. Ida et al.145 reported on 412 patients: 8 of 199 (4.0%) had recurrence of lesions size 20 to 40 mm; 5 patients were retreated by EMR and 3 underwent open surgery; and none had lymph node metastases. Patients (0/305) with lesions ≤20 mm did not have recurrence. Complication rates associated with EMR are low. Bleeding and perforation are reported in 0% to 20.5% and 0% to 5.2 % of patients, respectively.141,146–149 The reported morbidity and mortality rates in patients undergoing open surgery (n = 256) versus EMR (n = 56) were 0.8% and 7.8% versus 0% and 16%, repsectively.139,150
Fukase et al.151,152 reported on the long-term outcomes of patients undergoing open surgery (n = 116) versus EMR (n = 59). For patients age 65 year or under, the 5- and 10-year survival rates were 100% and 91.7% versus 92.8% and 92.8%, with open surgery and EMR, respectively. For patients older than 65 years of age, the 5- and 10-year survival rates were 100% and 75% versus 80.8% and 80.8%, respectively. The differences were not statistically significant. Similar results were reported by Park et al.153 and Kim et al.154
There are emerging variations of EMR techniques, including the cap suction and cut versus a ligating device. As outcome studies accumulate demonstrating favorable survival, EMR is emerging as the definitive management of selected EGCs and is not just reserved for patients in whom gastrectomy cannot be considered. However, RCTs are needed to establish an outcome advantage over open surgery.140,155–165
Limited Surgical Resection
Given the low rate of nodal involvement for patients with EGC, limited resection may be a reasonable alternative to gastrectomy for some patients. There are no well-accepted pretreatment criteria for selection of patients for limited resection. Based on available pathology studies, patients with small (<3 cm) intramucosal tumors and those with nonulcerated intramucosal tumors of any size may be candidates for EMR or limited resection. Surgical options for these patients may include gastrotomy with local excision. This procedure should be performed with full-thickness mural excision (to allow accurate pathologic assessment of T status) and is often aided by intraoperative gastroscopy for tumor localization. Formal lymph node dissection is not required in these patients.
Gastrectomy
Gastrectomy with lymph node dissection should be considered for patients with EGC who cannot be treated with EMR or limited surgical resection, and/or patients who have intramucosal tumors with poor histologic differentiation, or size >3 cm, or who have tumor penetration into the submucosa or beyond. Gastrectomy with lymph node dissection allows for adequate pathologic staging and local therapy for these patients at increased risk of nodal metastasis. Dissection of level I lymph nodes is a reasonable minimum standard at this time for higher risk EGCs. The roles for nodal “sampling” without formal node dissection (D0 dissection) and sentinel lymph node (SLN) mapping and biopsy in the treatment of EGC remain undefined at this time.
Stage II and Stage III Disease
Surgery
Surgical resection of the primary tumor and regional lymph nodes is the cornerstone of treatment for patients with localized gastric cancer. However, for stage II and III disease, surgery is necessary but often not sufficient for cure. The general therapeutic goal is to achieve a microscopically complete resection (R0). A complete discussion of all the technical details of gastric resection and reconstruction is beyond the scope of this chapter. However, specific surgical issues of oncologic significance are addressed here, including the extent of gastrectomy, extent of regional lymph node dissection, and role of partial pancreatectomy and splenectomy. Additional technical details can be found in surgical atlases and the section “Technical Treatment-Related Issues.”
Extent of Resection for Mid- and Distal Gastric Cancers. The extent of gastrectomy required for satisfactory primary tumor treatment depends primarily on the gross and microscopic status of surgical margins. For most clinical situations, a 5-cm grossly negative margin around the tumor and microscopically negative surgical margin (R0) are the treatment goals. When gastrectomy is performed with curative intent, frozen-section assessment of proximal and distal resection margins should be used intraoperatively to improve the likelihood that an R0 resection has been attained. Three relatively small prospective RCTs have compared total gastrectomy with partial (subtotal) gastrectomy for distal gastric cancer.166–168 Overall morbidity, mortality, and oncologic outcome were comparable in each of these RCTs. When the general oncologic goal of an R0 resection can be achieved by a gastric-preserving approach, partial gastrectomy is preferred over total gastrectomy. This is particularly relevant for distal gastric cancers, for which a gastric-preserving R0 approach may minimize the risks of specific sequelae of total gastrectomy such as early satiety, weight loss, and the need for vitamin B12 supplementation.
Extent of Resection for Proximal Gastric Cancer. There are many choices for surgical management of adenocarcinomas arising at the EGJ or in the proximal stomach (Siewert types II and III). Many abdominal surgeons have advocated transabdominal approaches with resection of the lower esophagus and proximal stomach or total gastrectomy. Surgeons trained in thoracic surgery have frequently advocated a combined abdominal and thoracic procedure (often termed esophagogastrectomy) with an intrathoracic or cervical anastomosis between the proximal esophagus and the distal stomach, or a procedure termed transhiatal (or blunt) esophagectomy (THE), which involves resection of the esophagus and EGJ with mediastinal dissection performed in a blunt fashion through the esophageal hiatus of the diaphragm. When THE is performed for adenocarcinoma of the EGJ, gastrointestinal continuity is restored by low cervical anastomosis of the stomach (usually advanced through the esophageal bed in the posterior mediastinum) to the cervical esophagus. Selection among the options has been dependent primarily on individual surgeon training and experience.
The optimal surgical procedure for patients with localized tumors of the EGJ and proximal stomach is a matter of considerable debate. A recently completed Dutch RCT compared transthoracic esophagogastrectomy (TTEG, with abdominal and thoracic incisions) with THE in 220 patients with adenocarcinoma of the esophagus and EGJ.169 Although this trial was designed for patients with esophageal cancer, 40 (18%) of the patients had adenocarcinomas of the EGJ (Siewert type II), and the operations evaluated are among those considered for patients with Siewert type II or III cancers. Perioperative morbidity was higher after THE, but there was no significant difference in in-hospital mortality compared with TTEG. Although median overall, disease-free, and quality-adjusted survival did not differ significantly between the groups, there was a trend toward improved OS at 5 years with TTEG. These results are judged equivocal, and there is currently no consensus on the optimal surgical approach for patients with Siewert type II tumors.170 Until longer follow-up of the Dutch trial is available and/or additional RCTs are performed, the surgical approach to these patients will continue to be individualized and determined by a constellation of factors including surgeon factors (training and experience), patient factors (age, comorbid conditions, and functional status), and tumor factors (pretreatment T and N stage).
Extent of Lymphadenectomy. There has been intense debate surrounding the extent of lymphadenectomy. It involves at least two important issues: (1) adequate staging in terms of the number of lymph nodes resected surgically and examined pathologically, and (2) adequate therapy (i.e., do some forms of lymphadenectomy result in better outcomes?).171–182
Single-institution reports suggest that the number of pathologically positive lymph nodes is of prognostic significance,100,102,180,183–185 and that removal and pathologic analysis of at least 15 lymph nodes is required for adequate pathologic staging.99,186 Indeed, the current AJCC staging system accounts for these issues and therefore requires analysis of ≥16 lymph nodes to assign a pathologic N stage.187 The possible therapeutic benefit of extended lymph node dissection D2 versus D1 dissection has been the focus of six RCTs, which are summarized in Table 46.4.167,188–193 These trials were performed because retrospective and prospective nonrandomized evidence suggested that extended lymph node dissection may be associated with improved long-term survival.194 The RCTs tested the hypothesis that removal of additional pathologically positive lymph nodes (not generally removed as part of a standard lymph node dissection) improves survival. The larger RCTs attempted to follow what are referred to as the “Japanese rules” for lymph node classification and dissection that govern the extent of nodal dissection required based on anatomic location of the primary tumor.195 Using these Japanese definitions, the RCTs compared limited lymphadenectomy of the perigastric lymph nodes (D1 dissection) to en bloc removal of second-echelon lymph nodes (D2 dissection). At least two of the completed trials are underpowered for their primary end point, OS.167,188 The trials from the Medical Research Council (MRC) of the United Kingdom189 and the Dutch Gastric Cancer Group190 have received the most attention and discussion.
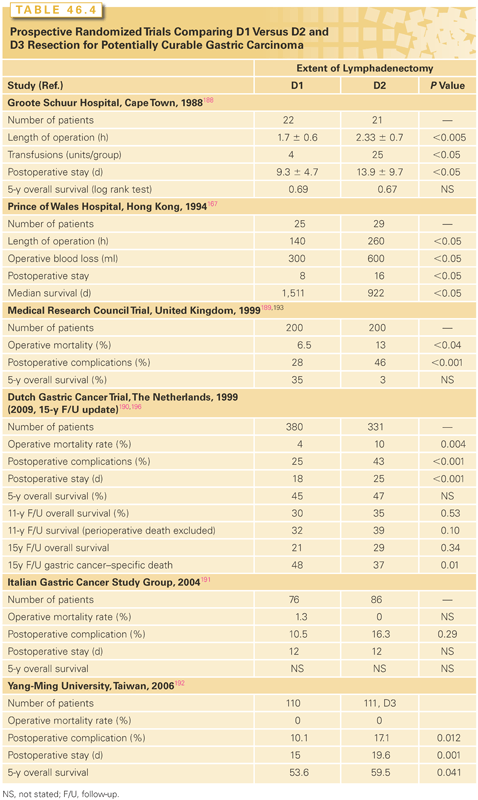
The MRC trial registered 737 patients with gastric adenocarcinoma; 337 (46%) patients were ineligible by staging laparotomy because of advanced disease, and 400 (54%) patients were randomized at the time of laparotomy to undergo D1 (200) or D2 (200) lymph node dissection. Postoperative morbidity was significantly greater in the D2 group (46% versus 28%; p <0.001), and in-hospital mortality rates were also significantly higher in the D2 group than in the D1 group (13% versus 6%, p <0.04).189 The most frequent postoperative complications were related to anastomotic leakage (D2 26% versus D1 11%), cardiac complications (8% versus 2%), and respiratory complications (8% versus 5%). The excess morbidity and mortality seen in the D2 group were thought to be related to the routine use of distal (left) pancreatectomy and splenectomy. Partial pancreatectomy and splenectomy were performed to maximize clearance of lymph nodes at the splenic hilum, primarily for patients with proximal tumors; however, many surgeons now believe that adequate lymph node dissection can be performed with pancreas- and spleen-preserving techniques. Long-term follow-up analysis of patients in the MRC trial demonstrated comparable 5-year OS rates of 35% and 33% in the D1 and D2 dissection groups, respectively. Survival based on death from gastric cancer as the event was also similar in the D1 and D2 groups (HR = 1.05; 95% CI = 0.79 to 1.39), as was recurrence-free survival (HR = 1.03; 95% CI = 0.82 to 1.29). The authors concluded that classic Japanese-style D2 lymphadenectomy (with partial pancreatectomy and splenectomy) offered no survival advantage over D1 lymphadenectomy.
The Dutch Gastric Cancer Group conducted a larger RCT with optimal surgical quality control comparing D1 to D2 lymph node dissection for patients with gastric adenocarcinoma that was updated in 2010 after 15-year follow-up.196 Between 1989 and July 1993, 1,078 patients were entered, of whom 996 patients were eligible; 711 patients were randomized to D1 dissection (n = 380) or D2 dissection (n = 331). To maximize surgical quality control, all operations were monitored.197 Initially, this oversight was done by a Japanese surgeon who trained a group of Dutch surgeons, who in turn acted as supervisors during surgery at 80 participating centers. Notwithstanding the extraordinary efforts to ensure quality control of the two types of lymph node dissection, both noncompliance (not removing all lymph node stations) and contamination (removing more than was indicated) occurred, thus blurring the distinction between the two operations and confounding the interpretation of the oncologic end points.196,198 The postoperative morbidity rate was higher in the D2 group (43% versus 25%; p <0.001), the reoperation rate was also higher at 18% (59/331) versus 8% (30/380), and the mortality rate was also significantly higher in the D2 group (10% versus 4%; p = 0.004). Patients treated with D2 dissection also required a longer hospitalization.199 As in the MRC trial, partial pancreatectomy and splenectomy were performed en passant in the D2 group. Five-year survival rates were similar in the two groups: 45% for the D1 group and 47% for the D2 group (95% CI for the difference = −9.6% to 5.6%). The subset of patients who had R0 resections, excluding those who died postoperatively, had cumulative risks of relapse at 5 years of 43% with D1 dissection and 37% with D2 dissection (95% CI for the difference = −2.4% to 14.4%).
The Dutch investigators concluded that there was no role for the routine use of D2 lymph node dissection in patients with gastric cancer. At 15-year follow-up, 174/711 (25%) patients were alive, all but one without recurrence. The OS was 21% (82/711) and 29% (92 patients) for the D1 and D2 groups, respectively (p = 0.34). Interestingly, gastric cancer–specific death was higher in the D1 group 48% (182/380) versus 37% (123/331). Local recurrence was higher in the D1 group 22% (82/380) versus 12% (40/331), and regional recurrence 19% (73/380) versus 13% (43/331). The authors concluded that after 15 years of follow-up, D2 lymphadenectomy is associated with lower locoregional recurrence and gastric cancer–specific death rates than D1 lymphadenectomy. D2 resection is also associated with higher postoperative mortality, morbidity, and reoperation rates. Examining the results after 15-year follow-up and given the data regarding gastric cancer–specific mortality, local recurrence, and regional recurrence, the authors revised their original conclusion: “Because spleen-preserving D2 resection is safer in high-volume centers, it is the recommended surgical approach for patients with potentially curable gastric cancer.”196
Degiuli et al.191 reported on the Italian Gastric Cancer Study Group experience with a prospective randomized trial comparing pancreas-sparing D1 versus D2. There were 76 patients randomized to undergo D1 and 86 D2 resections. Complication rates were higher in the D2 group: 16.3% versus 10.5%. Postoperative mortality was higher in the D1 group: 1.3% versus 0% in the D2 group. Thus far, no survival data are available. The authors concluded that, in experienced hands, the morbidity and mortality can be as low as shown by Japanese surgeons.
Wu et al.192 reported on a randomized trial comparing D1 versus D3 dissections.199 There were no operative deaths, and morbidity was only 12%. At median follow-up of 94.5 months, D3 showed better overall 5-year survival of 59.5% (95% CI = 50.3 to 68.7) versus 53.6% (95% CI = 44.2 to 63.0; p = 0.041), and a trend toward better DFS at 5 years: 40.3% versus 50.6% (p = 0.197). Only 13% had pancreas or splenic resection as compared with 23% in the Dutch trial. The authors concluded that D3 as compared to D1 offers survival benefit. As far as the authors of this chapter understand, this is the first RCT to demonstrate survival advantage for more extensive lymphadenectomy (D3). As such, it requires careful examination. Roggin and Posner200 have critically reviewed the work by Wu et al.192 One controversial element of this trial was the use of OS versus gastric cancer–specific survival; 17/111 (15%) of the reported deaths were not related to tumor recurrence, resulting in very small survival benefit.
Interpretation of the existing level 1 evidence is encumbered by a number of issues that have been discussed in detail elsewhere.171,172 The primary concerns relate to whether (1) the increased operative mortality associated with protocol-mandated partial pancreatectomy and splenectomy for patients with proximal tumors undergoing D2 dissection prevented identification of a potential therapeutic impact of extended lymph node dissection, and (2) the phenomena of noncompliance and contamination led to homogenization of the operative procedures to such an extent that the fundamental hypothesis was not tested. Owing to these interpretation issues, the question of a possible therapeutic benefit of D2 dissection remains unsettled.
Many Japanese gastric surgeons have considered the caveats associated with the MRC and Dutch trials and believe that, notwithstanding inherent patient selection and stage migration biases,171,196,198,201,202 the existing retrospective data provide sufficient proof of a clinical benefit of D2 dissection. On this basis, D2 dissection has been adopted as the standard of care for patients with localized, higher-risk gastric cancer in many centers in Japan and some specialized centers in the West.203 The Japanese Clinical Oncology Group (JCOG-9501) has investigated an even more aggressive surgical approach in an RCT evaluating standard D2 versus D2-plus (para-aortic node dissection [PAND]) in the management of completely resected (R0) T2–4 gastric cancer.204,205 Between July 1995 and April 2001, 523 patients from 25 institutions were registered. Patients were randomized intraoperatively to undergo D2 lymphadenectomy alone (263 patients) or D2 lymphadenectomy plus PAND (260 patients). The primary end point was OS. Postoperative morbidity was higher in the PAND group (28% versus. 21%; p = 0.07), and mortality was similar at 0.8% in each group. Five-year OS for patients undergoing PAND was 70.3% versus 69.2% (HR = 1.03; p = 0.85). There was no significant difference in recurrence-free survival. The authors concluded that, as compared to D2 lymphadenectomy, PAND when added to D2 lymphadenectomy does not improve survival rates.
Another Japanese study compared D2 with extended PAND (D4).206 This trial randomized patients to undergo gastrectomy with D2 (n = 135) or D4 (n = 134) lymphadenectomy. The 5-year survival rates were 52.6% versus 55%, respectively (p = 0.8). The authors concluded that prophylactic D4 dissection is not recommended. In an RCT, a Western group from Poland investigated D2 dissection versus extended D2 dissection defined according to the Japanese Gastric Cancer Association classification.207 They randomized 275 patients with gastric cancer to gastrectomy with D2 (n = 141) versus D2 + lymphadenectomy (n = 134). The overall postoperative morbidity and mortality were similar and did not differ statistically. Survival data are not available at this time.207 Thus, the limits of radical surgery have been reached in Japan and the pendulum has swung back toward D2 dissection in clinical settings in which this can be safely performed.201
In summary, lymph nodes should be considered as indicators that the gate was opened rather than as the gate keepers for cure.208 None of the prospective RCT trials executed in the West demonstrated survival advantage for more extensive lymphadenectomy. However, none of these studies were powered enough to detect single-digit difference in 5-year OS. Several non-a priori planned subgroup analyses were done and showed some survival advantage for certain subgroups. These analyses cannot be used to form evidence-based medicine, but should be used to form hypotheses for further RCT studies. In high-volume specialty centers, spleen- and pancreas-preserving D2 dissection is performed safely, and can potentially result in decreased gastric cancer–specific mortality based on 15 years of follow-up from the Dutch study (D2 37% versus 48%; p = 0.01).196
A recently published RCT from the multicenter Italian Gastric Cancer Study Group compared D1 to D2 lymphadenectomy for gastric adenocarcinoma with primary outcome of OS over median follow-up of 8.8 years.209 There was no significant difference in OS (5-year OS = 66.5% versus 64.2%), operative morbidity (12% versus 17.9%), and mortality (3% versus 2.2%) in patients undergoing D1 and D2 gastrectomy; however, subgroup analysis suggested a trend toward improved disease-specific survival with D2 gastrectomy in patients with advanced T-stage (pT2-4) and node-positive disease (5-year disease-specific survival: D1 versus D2 = 38% versus 59%; p = 0.055).
A systematic review and meta-analysis of eight RCTs encompassing over 2,000 patients (D1, n = 1,042; D2, n = 1,002) evaluated the safety and efficacy of extended lymphadenectomy in gastric cancer.210 A significant increase in operative morbidity and mortality was evident in patients undergoing extended D2 lymphadenectomy, with a trend in decreased disease-specific mortality in those having spleen- and pancreas-preserving gastrectomy. Longer-term survival is required to ascertain oncologically relevant outcome benefit with D2 gastrectomy.
Partial Pancreatectomy and Splenectomy Resect or Preserve? Partial (left, distal) pancreatectomy and splenectomy have been performed as part of D2 lymph node dissection to remove the lymph nodes along the splenic artery (station 11) and lymph nodes within the splenic hilum (station 12), primarily for patients with tumors located in the proximal and midstomach. Indeed, partial pancreatectomy and splenectomy were required for patients with proximal tumors in the D2 arm of the Dutch and MRC RCTs but were required only for direct tumor extension in the D1 arm. In the Dutch and MRC D1 versus D2 randomized trials, splenectomy was associated with increased risk of surgical complications and operative mortality. In addition, a multivariate analysis suggested that splenectomy is associated with inferior long-term survival. The frequent performance of splenectomy (e.g., 30% of patients in the D2 arm versus 3% in the D1 arms of the Dutch trial) in the patient undergoing extended D2 lymphadenectomy, with its associated adverse effects on both short- and long-term mortality, confounds the interpretation of the Dutch and MRC RCTs. Thus, the hypothesis that spleen- and pancreas-preserving D2 lymph node dissection improves survival remains unproven. There is an evolving consensus that splenectomy should be performed only in cases with intraoperative evidence of direct tumor extension into the spleen, or its hilar vasculature, or when the primary tumor is located in the proximal stomach along the greater curvature.211 Partial pancreatectomy should be performed only in cases of direct tumor extension to the pancreas.
Recent reports have described pancreas- and spleen-preserving forms of D2 dissection.212–218 This organ-preserving modification of classic D2 dissection allows for dissection of some station 11 and 12 lymph nodes without the potential adverse effects of pancreatectomy and/or splenectomy. In a small single-institution RCT recently reported from Chile, Csendes et al.219 randomized 187 patients with localized proximal gastric adenocarcinoma to treatment by total gastrectomy with D2 lymph node dissection plus splenectomy or total gastrectomy with D2 lymphadenectomy alone. Operative mortality was similar in both groups (splenectomy group, 3%; control group, 4%). However, septic complication rates were higher in the splenectomy arm than in the control arm (p <0.04). There was no difference in 5-year OS between study groups, although it is not clear that the trial was designed with survival as the primary end point. Other investigators confirmed these findings.218,220,221
The JCOG is conducting a multi-institutional RCT (JCOG 0110-MF) comparing D2 dissection with and without splenectomy for patients diagnosed with proximal gastric cancer.222 The hypothesis to be tested is that 5-year OS of patients treated by extended D2 dissection without splenectomy is 5% less than that of patients treated by D2 dissection with splenectomy. With a planned accrual of 500 patients, this design will provide 70% power to reject the null hypothesis when 5-year OS is 3% greater following splenic preservation compared with splenectomy.222 The results of this trial will better define the short- and long-term effects of splenectomy for patients with proximal gastric cancers undergoing extended lymphadenectomy.
In an effort to reduce operative morbidity and mortality without compromising oncologic outcome in patients undergoing extended lymphadenectomy for gastric adenocarcinoma, eight clinical trials have compared laparoscopic to open gastrectomy with D2 lymph node dissection. A meta-analysis was conducted of these eight trials, which enrolled nearly 1,500 patients with gastric cancer to assess the feasibility and safety of laparoscopic total gastrectomy with D2 lymphadenectomy compared to the same operation done in the standard open manner.223 The laparoscopic technique was associated with significantly longer operative time, less operative blood loss, fewer analgesic requirements, earlier return of bowel function, shorter hospital stay, and reduced operative morbidity (relative risk = 0.70; 95% CI = 0.50 to 0.98; p = 0.035). The total number of lymph nodes removed surgically and analyzed pathologically as well as operative mortality was not significantly different between groups. Further well-designed clinical RCTs are warranted to define the role of laparoscopic gastrectomy and extended lymphadenectomy for gastric adenocarcinoma. (A more detailed discussion follows in the section titled “Minimally Invasive Surgery for Gastric Resection.”)
Laparoscopy clearly has a role in the complete staging of disease in patients with gastric adenocarcinoma and the detection of radiologically occult macroscopic, or microscopic peritoneal cytology positive-only metastasis. Laparoscopy and peritoneal cytology are important for accurate staging and the detection of occult metastatic disease. This methodology adds value to modern imaging techniques, for positive microscopic peritoneal cytology-only disease is tantamount to macroscopic M1 disease in terms of oncologic outcome.
A recent international multidisciplinary expert panel created statements to define processes of care relevant to the perioperative management of patient with gastric cancer.224 Ten processes were deemed essential to maintaining quality of care:
1. CT of the abdomen and pelvis is part of preoperative staging.
2. PET scans are not routinely indicated.
3. Adjuvant therapy should be considered.
4. Clinical trials should be conducted and patients considered for participation.
5. Treatment decision making should involve a multidisciplinary team.
6. Hospitals must have sufficient systems in place to support the care of patients with gastric cancer.
7. Sixteen or more lymph nodes should be resected and staged pathologically.
8. Surgery should only be performed to palliate major symptoms in the setting of metastatic disease.
9. Surgeons experienced in the treatment of gastric cancer should be performing the operations.
10. These surgeons should also have advanced laparoscopic surgery experience for laparoscopic gastric resection.
These processes were deemed to be of indeterminate necessity for maintaining quality of care:
1. Diagnostic laparoscopy before treatment
2. Multidisciplinary approach to patients with linitis plastica
3. Genetic testing for diffuse gastric cancer, family history, or age <45 years at time of diagnosis
4. Endoscopic removal of select T1aN0 lesions
5. D2 lymphadenectomy in curative intent cases
6. D1 lymphadenectomy for EGC or patients with comorbidities
7. Frozen section analysis of gastric resection margins
8. Nonemergent cases performed in a hospital with a volume of >15 gastric cancer resections per year
9. By a surgeon who performs more than six gastric resections per year
Individualized Assessments of Lymph Node Involvement. Recent attention has focused on methods of individual assessment of risk of lymphatic spread. These techniques offer the possibility of tailoring surgical therapy for an individual patient based on clinicopathologic risk assessment of the primary tumor and/or preoperative or intraoperative identification of SLNs, or primary draining lymph nodes. At present, at least three approaches to individual nodal risk assessment have been evaluated: computer modeling, preoperative endoscopic peritumoral injection, and SLN biopsy.
Preoperative Computer Modeling of Individual Patient Nodal Involvement. Kampschoer et al.225 have developed a computer program to estimate the probability of spread to specific nodal regions for an individual patient using his or her pretreatment clinicopathologic data. The program incorporated data on tumor size, depth of infiltration, primary tumor location, grade, type, and macroscopic appearance of primary tumors from 2,000 patients with surgically resected gastric cancers treated at the National Cancer Center of Tokyo. The data set used for matching individual patient data is continuously updated and now includes >8,000 patients. This computer model has been validated in non-Japanese patients in Germany226 and Italy.227 In the United States, Hundahl et al.228 retrospectively applied this computer model to evaluate the surgical treatment of patients entered into the intergroup trial of adjuvant 5-FU–based chemoradiation. The Kampschoer et al.225 program was used to estimate the likelihood of disease in undissected regional node stations, defining the sum of these estimates as the Maruyama index of unresected disease. Fifty-four percent of participating patients underwent D0 lymphadenectomy. The median index was 70 (range = 0 to 429). In contrast to D level, the Maruyama index proved to be an independent prognostic factor of survival, even with adjustment for the potentially linked variables of T stage and number of positive nodes. More recent and smaller studies confirmed these findings.229–231
Preoperative Endoscopic Peritumoral Injection. The hypothesis that peritumoral injection of compounds designed to optimize lymph node dissection improves lymph node clearance was addressed in a small RCT evaluating preoperative endoscopic vital dye staining with CH40 prior to D2 dissection. The frequency of positive lymph nodes in patients injected with CH40 before D2 dissection was greater than that observed in patients treated by D2 dissection alone.232 This approach optimized the yield of lymph node dissection presumably by directing surgeons to include specific lymph nodes in the dissection that might have otherwise been left in situ and/or by directing pathologists to examine specific areas of the lymphadenectomy specimens. Further prospective studies of this approach are required to confirm the feasibility of this technique and to assess its impact on intraoperative decision making regarding the extent of lymphadenectomy and accuracy of specimen dissection and nodal retrieval in anatomic pathology.
Sentinel Lymph Node Biopsy. The goal of SLN biopsy is to identify the node or nodes believed to be the first peritumoral lymph nodes in the orderly spread of gastric adenocarcinoma from the primary site to the regional lymph nodes. Sampling of this lymph node(s) may allow for prediction of the nodal status of the entire lymph node basin, possibly obviating extended nodal dissection and its attendant morbidity in patients found to have a negative SLN. Recent pilot studies have evaluated the feasibility, sensitivity, and specificity of SLN biopsy for patients with gastric cancer.233–242 These pilot studies demonstrated that SLN identification is feasible in approximately 95% of patients. However, most patients with gastric cancer have multiple “sentinel” nodes, with mean numbers of SLNs per patient ranging from 2.6 to 6.3. The aggregate experience to date suggests that among patients with pathologically involved lymph nodes, SLN results in false-negative assessment of pathologic regional nodal status in 11% to 60% of patients. Thus, the preliminary data available suggest that SLN biopsy cannot reliably replace lymph node dissection as a means of accurately staging regional nodal basins in gastric adenocarcinoma.243–247
In a large study of nearly 400 patients, the ability and accuracy of SLNs was examined in a prospective, multicenter phase 2 study.248 Patients with early T stage gastric cancer (cT1 or cT2, tumor <4 cm) were evaluated with SLN mapping, followed by gastrectomy and D2 dissection. The SLN mapping technique identified 57 patients who had nodal involvement, of which 53 had true positive SLNs, resulting in 99% accuracy. Though further validation is needed, these results are quite encouraging.
The JCOG multicenter trial (JCOG-0302) assessed the feasibility and accuracy of indocyanine green (ICG) SLN mapping at time of surgery prior to gastrectomy and lymphadenectomy for EGC (T1).249 Single sections of ICG-stained SLNs were examined intraoperatively using frozen section with hematoxylin and eosin stain. The primary study end point was the proportion of false-negative SLNs. Study accrual was halted after 440 of the planned 1,550 patients were enrolled when the proportion of false negatives was found to be unexpectedly and unacceptably high (46%). The authors appropriately concluded that SLN mapping and biopsy using ICG and intraoperative single nodal frozen section evaluation using hematoxylin and eosin staining is inappropriate for clinical use in EGC.
Volume-Outcome Relationships for Gastrectomy. Recent studies have established a clear relationship between institutional gastrectomy volume and perioperative mortality rates—the so-called volume-outcome relationship. The recent analysis of a national database by Birkmeyer et al.250–252 of 31,854 patients who underwent gastrectomy between 1994 and 1999 demonstrated an inverse relationship between institutional gastrectomy volume and operative mortality rates. The odds ratio for gastrectomy-related death was lowest among patients treated within hospitals in the highest gastrectomy volume quintile (odds ratio = 0.72; 95% CI = 0.63 to 0.83). A separate analysis evaluating surrogate end points for morbidity demonstrated that gastrectomy at high-volume centers was associated with the shortest duration of hospital stay and the lowest readmission rates.253–256 Similar findings were noted by Hannan et al.257 in an analysis of the New York State Department of Health’s administrative database. Their analysis of 3,711 patients who underwent gastrectomy between 1994 and 1997 included adjustments for covariates such as age, demographic variables, organ metastasis, socioeconomic status, and comorbidities. Patients who had a gastrectomy at hospitals in the highest-volume quartile had an absolute risk-adjusted mortality rate that was 7.1% (p <0.0001) lower than those treated at hospitals in the lowest-volume quartile, although the overall mortality rate for gastrectomy was only 6.2%.
These studies demonstrate that the risk-adjusted mortality rates for gastrectomy are significantly lower when gastrectomy is performed by high-volume providers.258–261 It is likely that the variations in gastrectomy-related mortality rates relate in part to surgeon training and their patient age-volume262–264 and experience with the procedure. Data on gastrectomy volume obtained from general surgeons undergoing recertification after a minimum of 7 years in practice demonstrate that the mean number of gastric resections performed by recertifying general surgeons in the United States is only 1.4 per year.265 Thus, given the data supporting a relationship between hospital and provider volumes and the morbidity and mortality rate of gastric resection, there are reasons to consider regionalization of the surgical treatment of gastric cancers.
Outcome in Japan Versus Western Countries. Stage-stratified survival rates for gastric adenocarcinoma are higher in Japan than in most Western countries. The reasons for this are complex, are incompletely understood, and cannot be fully addressed within the context of a chapter covering all aspects of gastric cancers.
Important differences in the epidemiology of gastric cancer may contribute to observed differences in outcome in Japan versus Western countries. First, the better-prognosis intestinal-type (Laurén classification)266 tumors are seen more commonly in Japan, whereas the diffuse-type cancers (poorer prognosis) are more frequent in Western series. These regional differences in the frequencies of intestinal and diffuse cancers are believed to be related to the higher incidence of H. pylori infection and atrophic gastritis in Japan. Second, poorer-prognosis proximal gastric cancers are less frequent in Japan.267–269 Indeed, the progressive increase in proximal gastric cancers observed in the West has not been observed in Japanese populations.
Regional differences in the diagnostic criteria for EGC also may contribute to regional differences in observed outcome. In Japan, gastric carcinoma is diagnosed based on its structural and cytologic features without consideration of invasion of the lamina propria. In contrast, Western pathologists consider invasion of the lamina propria to be an essential element of the diagnosis of carcinoma.270–274 As a consequence, unequivocally neoplastic noninvasive lesions are classified as carcinoma in Japan, but as dysplasia by Western pathologists.270 To overcome these differences, the Padvova,275 Vienna,276 and Revised Vienna276 classifications have recently been proposed. However, until there is worldwide consensus and implementation of uniform diagnostic criteria for EGC, comparative assessments of the outcome of patients with EGC treated in Japan and Western countries should acknowledge the selection bias associated with different diagnostic criteria.
Stage migration is a well-documented factor contributing to the stage-specific differences in outcome between Japanese and Western patients.277 Stage migration arises because there is widespread use of extensive D2 or D3 lymphadenectomy combined with rigorous pathologic assessment of the lymphadenectomy specimen in Japan. More accurate stage assignment of Japanese patients leads to secondary stage migration—improvement in stage-specific survival without improvement in OS. The frequency and impact of stage migration were quantified by the Dutch Gastric Cancer Group in their RCT comparing D1 and D2 lymph node dissection.190,278 Stage migration occurred in 30% of patients in the D2 group, and the stage-specific decreases in survival rates attributable to stage migration were 3% for AJCC/UICC stage I disease, 8% for stage II, 6% for stage III, and 12% for stage IIIB, with the more accurately staged D2 group having higher survival rates.278
In addition to regional differences in epidemiology, diagnostic criteria for EGC, and stage migration, other factors may contribute to the observed differences in stage-stratified survival. Such factors may include genetic, environmental, and biologic differences between Japanese and Western patients and tumors. These factors have been less well studied, but were addressed in a comprehensive review by Davis and Sano.279
Outcome in Korea Versus Western Countries. A separate evaluation was performed comparing gastric cancer survival following curative intent resection in Korea versus the United States.126 This study compared two independent, single-institution prospectively maintained databases from 1995 to 2005: one from MSKCC (n = 711 curatively resected patients who did not receive neoadjuvant therapy) and another from St. Mary’s Hospital in Seoul, South Korea (n = 1,646 patients, also curatively resected without receiving preoperative therapy). All patients had a D2 dissection and adequate nodal staging. There were notable differences in the two cohorts: patients from the United States were more likely to have proximal tumors and more advanced stage compared with patients resected in Korea. However, when controlling for all known risk factors, stage for stage, patients from Korea still had better OS (HR = 1.3; 95% CI = 1.0 to 1.7; p = 0.05). These data cannot exclude differences in underlying cancer biology as a potential explanation for the observed differences in survival in gastric cancer between patients treated in Korea versus those treated in the United States.
Minimally Invasive Surgery for Gastric Resection
The utilization of laparoscopy in gastric surgery has been increasing over the past decade. There are a plethora of non-RCTs reporting on laparoscopic distal gastrectomy, total gastrectomy, and D2 dissection, and practically every open procedure has been tested using laparoscopy. We will review here the available data from published RCTs. There are no RCTs reporting on total gastrectomies.280 None of the current RCTs reporting on laparoscopy in gastric cancer are of sufficient quality and magnitude to be considered “practice changing.” Huscher et al.281,282 reported on the largest RCT testing laparoscopic (n = 29) versus open (n = 30) subtotal gastrectomy for distal gastric cancer. After 5 years of follow-up, overall morbidity and mortality were equivalent. Laparoscopic resection resulted in less blood loss, shorter hospital stay, longer operative time, and earlier resumption of oral intake; these were reported by other authors as well.283,284 There was no difference in the number of lymph nodes harvested. Overall 5-year and DFS did not differ significantly. The authors concluded that laparoscopic subtotal gastrectomy is feasible and has similar short- and long-term outcomes as open surgery.
Kim et al.285 reported on laparoscopy-assisted distal gastrectomy (LADG) versus open distal gastrectomy (ODG) for early gastric cancer. This trial was designed to test the 5-year survival utilizing noninferiority design. The interim analysis showed less blood loss (p <0.001), reduced amount of analgesic used (p <0.019), shorter hospital stay (p <0.0001), fewer number of lymph nodes harvested in the LADG arm (p <0.05), and improved short-term quality of life (QOL) parameters on global health (p <0.0001). The authors concluded that comparison of LADG with ODG resulted in improved QOL outcomes in patients followed up to 3 months. The authors have now reported long-term follow-up data.286 In this study, the authors evaluated 2,976 patients who were treated with either laparoscopic gastrectomy (1,477 patients) or open gastrectomy (1,499 patients) between April 1998 and December 2005. With a median follow-up of 70.8 months, there was no difference in survival between the two resection methodologies, with the exception that for stage IA gastric cancer, laparoscopic gastrectomy had a improved survival (95.3% 5-year survival) versus open gastrectomy (90.3% 5-year survival) (p <0.001).286 Recently, Ohtani et al.287 performed meta-analysis of RCT that compared LADG versus ODG for EGC. Their report confirmed the results from Kim et al.286 and Lee and Han.288
A recent update and meta-analysis analyzed data on 784 patients enrolled in eight RCTs289 comparing laparoscopy-assisted to open gastrectomy for resectable gastric cancer. Despite the fact that operative time was significantly prolonged in the laparoscopy group and that analgesic use, operative blood loss, nodal yield, morbidity (overall and anastomotic), and mortality were no different when compared with open gastrectomy, there were a number of clinically important benefits observed in those undergoing minimally invasive surgery. Pulmonary morbidity (odds ratio = 0.43; 95% CI = 0.20 to 0.93; p = 0.03), duration of postoperative ileus (missing data = −0.23; 95% CI = −0.41 to −0.05), and hospital stay (missing data = −1.72; 95% CI = −3.40 to 0.04) were significantly reduced in the laparoscopy-assisted gastrectomy group. The survival benefit of minimally invasive surgery in resectable gastric cancer has yet to be definitively demonstrated.289
Laparoscopy is another tool in the armamentarium of the surgical oncologist. The question is not whether it can be done laparoscopically, certainly it can; the question is whether it should be done in patients with gastric adenocarcinoma. Recently, a meta-analysis of six clinical trials (658 patients) compared laparoscopic versus open gastrectomy for distal EGC.290 In this analysis, the authors noted significant reductions in postoperative complications and length of hospital stay in the laparoscopic group, whereas the open gastrectomy group had less operating time and a greater number of lymph nodes harvested. There were no notable differences in the time to return of bowel function, incidence of wound infection, postoperative abscess formation, or anastomotic leak or pulmonary complications, suggesting that there may be some advantages of the laparoscopic approach. The oncologic equivalence, however, has not been adequately tested. Whether the energy, time, and capital invested in developing laparoscopic surgery for gastric cancer is worth it is the relevant question particularly in an era of skyrocketing medical costs. Certainly, beyond the short term there seems to be no advantage of laparoscopic surgery in gastric cancer. In view of the reported morbidity and mortality in specialty centers, the advantage gained by laparoscopic gastric resection over the first few days underlines the question of whether we should invest more in innovative therapies to eradicate gastric cancer than the simple technical aspects of noninvasive extirpative surgery.
A large prospective random assignment study from Korea is underway in which 1,415 patients with EGC (pT1-2 N0) are randomly assigned to receive either open or laparoscopic distal gastrectomy.291 Final results of this expected “definitive” phase 3 study are expected to be available in September 2015. A similar large phase 3 study for stage IA and stage IB gastric cancer was initiated in Japan in March 2010. This study randomizes 920 patients from 33 institutions, with the primary end point of OS.292 Together, it is anticipated that these two pivotal studies will definitively define the role of laparoscopic-assisted gastrectomy in EGC.
Adjuvant Therapy
Patients with EGCs (e.g., patients with AJCC stage I and some stage II) have a good chance of cure with surgery alone. However, the Japanese S-1 adjuvant study indicates that the prognosis for even stage II tumors can be improved with systemic chemotherapy.
Adjuvant therapy indicates administration of a treatment following a potential curative resection of the primary tumor and regional lymph nodes. Therapy after resections that leave microscopic or gross disease are not adjuvant treatment, but rather therapy for known disease, which is palliative in nature. Neoadjuvant chemotherapy involves the use of systemic treatment before potentially curative surgery.
There are several theoretical reasons for beginning adjuvant therapy soon after operation (perioperative chemotherapy). Studies have shown a rapid increase in cell growth of metastases after a primary tumor has been removed related to a decline in certain circulating factors, which serve to inhibit angiogenesis or other cell-cycle promotors, once the primary tumor is removed. Perioperative or neoadjuvant chemotherapy has been studied because the ability to perform a R0 resection in gastric cancer is difficult. In addition, a substantial number of patients undergoing gastrectomy have prolonged recovery. Neoadjuvant chemotherapy has a dual goal: allowing a higher rate of R0 resections and treatment of micrometastatic disease early in the course of treatment.
Adjuvant Systemic Therapy. The results of selected recent RCTs comparing adjuvant chemotherapy with surgery alone are summarized in Table 46.5. Japanese investigators studied S-1, an oral fluoropyrimidine, in a group of 1,059 patients (stages II to IIIB). S-1 was given for 12 months (4 weeks on/2 weeks off). A total of 529 patients received S-1 plus operation and 530 patients underwent operation only. The 3-year OS was 80.1% and 70.1%, respectively (HR = 0.68).293
The 5-year survival update was recently reported and demonstrated a sustained benefit in 5-year OS of 71.7% with adjuvant S-1 versus 61.1% with surgery alone (HR = 0.669; 95% CI = 0.540 to 0.828).294
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








