Punch Biopsy
A punch biopsy is performed under local anesthesia, using a trephine or biopsy punch (Fig. 92.1B). The operator makes a circular incision to the level of the superficial fat, using a rotating or twisting motion of the trephine. Traction applied perpendicularly to the relaxed skin tension lines minimizes redundancy at closure. Hemostasis is achieved by placement of simple, nonabsorbable sutures that can be removed in 7 to 14 days depending on anatomic site. If the punch biopsy is small and not in a cosmetically crucial area, the resulting biopsy wound can often heal very well by second intention.
Excisional Biopsy
After local anesthesia has been achieved under sterile conditions, a scalpel is used to incise an ellipse to the level of the subcutis. Hemostasis is obtained with cautery as needed, and the wound is closed in a layered fashion using absorbable and, if needed removable epidermal sutures.
GENERAL APPROACH TO MANAGEMENT OF SKIN CANCER
The management of skin cancer is guided by the histologic and biologic nature of the tumor, the anatomic site, the underlying medical status of the patient, and whether the tumor is primary or recurrent. Accurate interpretation of the diagnostic biopsy is essential for appropriate clinical management. Depending on the biologic aggressiveness of the tumor, cancers of the skin may be excised or, in some cases of superficial tumors or precancerous lesions, eliminated in a less invasive fashion. Surgical options include conventional excision and Mohs micrographic surgery (MMS). Destructive modalities include curettage and cautery/electrodessication (C&D), cryosurgery, photodynamic therapy (PDT), and laser surgery. Other techniques are topical therapy (e.g., imiquimod, 5-fluorouracil [5-FU]), intralesional interferon, chemotherapy, and radiation therapy (RT). Other than conventional and Mohs surgery, none of these latter techniques provide information about the histologic completeness of the cancer ablation.
Excision
Excisional surgery involves the removal of the cancer and a margin of clinically uninvolved tissue, followed by layered closure or second intention healing. Frozen or permanent sections interpreted by the pathologist determine adequacy of margins. Margins are assessed from representative sections of the specimen in “bread-loaf” fashion, allowing for sampling of the surgical margin. This sampling may occasionally result in a false-negative assessment of clear margins, especially in cases of infiltrating or aggressive-growth cancers. A similar misdiagnosis may occur when one relies on vertically prepared frozen specimens for intraoperative margin control. Excision, especially when performed in a physician’s office rather than in a hospital operating room, is effective and cost-efficient for primary, small (<1 cm) NMSCs, without infiltrative or other high-risk features.
Mohs Micrographic Surgery
MMS facilitates optimal margin control and conservation of normal tissue, and it has become the standard of care in a variety of skin cancer subtypes. Individuals trained in the technique perform MMS in the office setting under local anesthesia. After gentle curettage to define the clinical gross margin of the cancer, a 45-degree tangential specimen of tumor with a minimal margin of clinically normal-appearing tissue is excised, precisely mapped in a horizontal fashion, and processed immediately by frozen section for microscopic examination (Fig. 92.2). Optimal margin control is obtained by examination of the entire lateral perimeter of the specimen and contiguous deep margin. Meticulous mapping allows for directed extirpation of any remaining tumor, resulting in a cure rate of >97% to 99% for primary BCC and SCC.4
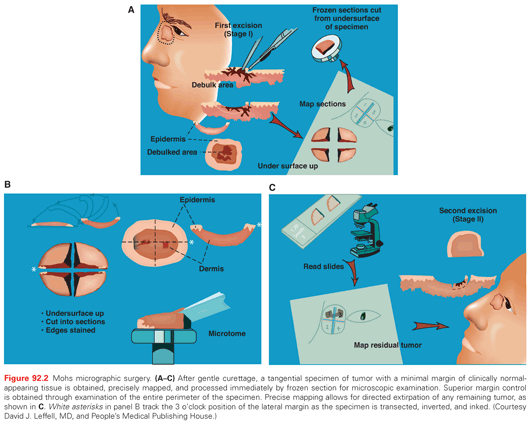
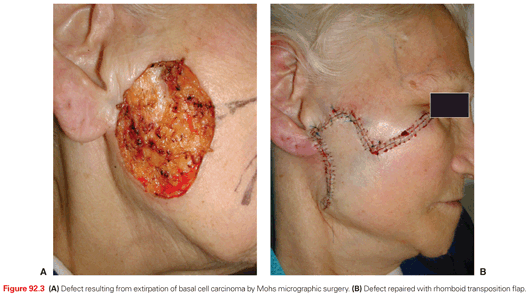
A key defining feature of MMS is that the surgeon excises, maps, and reviews the specimen personally, minimizing the chance of error in tissue interpretation and orientation. MMS has gained acceptance as the treatment of choice for recurrent skin cancers, as well as for primary skin cancers located at anatomic sites that require maximal tissue conservation for preservation of function and cosmesis (Fig. 92.3). Guidelines for the appropriate use of MMS have been published that reinforce the advantages of this technique for selected high-risk tumors.5 For those tumors with a significant risk of recurrence, MMS is a cost-effective treatment compared with surgical excision when considering associated ambulatory surgery center facility fee and a subsequent re-excision procedure. It is also significantly less expensive than radiotherapy and frozen-section–guided excisional surgery.6
Curettage and Cautery/Electrodesiccation
Common methods of treatment of uncomplicated skin cancers on the trunk and extremities and certain facial lesions include C&D and cryotherapy using liquid nitrogen. C&D is performed under clean conditions with local anesthesia. The visible tumor is first removed by curettage, which is extended for a margin of 2 to 4 mm beyond the clinical borders of the cancer. Cautery or electrodesiccation is then performed to destroy another 1 mm of tissue at the lateral and deep margins. C&D can yield satisfactory results after a single cycle of C&D for NMSC tumors <1 cm, especially if the tumor is of the superficial subtype. Salasche7 recommended that C&D be performed for three cycles to avoid recurrence. The authors believe, based on extensive clinical experience, that if the tumor requires three cycles of C&D, careful consideration should be given to more definitive approaches such as excision or MMS. Detailed reviews of primary BCC treated by C&D revealed 5-year recurrence rates of 8.6% for lesions located on the neck, trunk, and extremities, and between 17.5% and 22% for lesions located on the face.8
One potential drawback of C&D is that recurrent tumors following this treatment may be multifocal and develop a more aggressive biologic behavior. C&D is thus reserved for small (<1 cm) superficial or nodular BCCs, AKs, and SCCIS without follicular involvement located on the trunk or extremities.
Cryosurgery
Cryosurgery exposes precancers and NMSCs to destructive subzero temperatures. Tissue damage is caused initially by direct effects and subsequently by vascular stasis, ice crystal formation, cell membrane disruption, pH changes, hypertonic damage, and finally thermal shock. Successful cryosurgery requires temperatures reaching −50°C to −60°C at the deep and lateral margins of the tumor. The open-spray technique is used most often and requires pressurized liquid nitrogen spray delivery from a distance of 1 to 3 cm. With the confined-spray technique, liquid nitrogen is delivered through a cone that allows more precise tissue destruction. With the cryoprobe technique, a prechilled metal probe is applied to the tumor. Immediately following cryosurgery, local erythema and edema are apparent. An exudative phase ensues in 24 to 72 hours and is followed by sloughing at approximately day 7. Complete healing is usually seen at 2 to 3 weeks for facial lesions and up to 6 weeks for lesions on the trunk and extremities.
Temporary complications may include extensive drainage, edema, bulla formation, and hypertrophic scarring. Paresthesia may occur with thermal injury to superficial nerves. Other less common side effects may include headache, syncope, febrile reaction, cold urticaria, pyogenic granuloma, delayed hemorrhage, milia formation, or dyschromia (hypo- and hyperpigmentation). Permanent complications may include tissue contraction, hypopigmentation, and scarring.
The clinical usefulness of cryosurgery and C&D is limited by the inability to evaluate treatment margins and therefore thoroughness of tumor eradication. The absence of margin control and a dense postcryosurgery scar, which might obscure recurrence, makes these methods valuable primarily in the care of histologically superficial NMSC.
TOPICAL THERAPY FOR SKIN CANCER
Imiquimod
Imiquimod, an imidazolaquinoline, binds intracellular toll-like receptors 7 and 8 acting as a topical immune-response modifier. Imiquimod promotes a cell-mediated immune response through induction of several cytokines particularly interferon (IFN)-α and IFN-γ and interleukins (IL) 6, 8, and 12 by keratinocytes and peripheral blood mononuclear cells including monocytes, macrophages, and toll-like receptor-7 bearing plasmacytoid dendritic cells.9–12 The net effect is a T-helper type 1–dominant inflammatory response driven by CD4 T cells. In addition, IFN-γ stimulates cytotoxic T cells to kill virus-infected and tumor cells, facilitating tumor regression. The use of imiquimod is approved by the US Food and Drug Administration (FDA) for treatment of AKs and superficial BCCs on the trunk, neck, or extremities. Studies with topical imiquimod for nodular BCC, SCCIS, and malignant melanoma in situ have shown variable results.13–15 Imiquimod appears to be effective as monotherapy in carefully selected cases and may have a role postoperatively to decrease the rate of recurrence of certain skin cancers. However, long-term data on cure and recurrence remain to be determined. Close monitoring of the treatment site is essential.
Imiquimod-related adverse events include application site reactions (i.e., erythema, pain, edema, ulceration, bleeding), fatigue, myalgias, fever and chills or flu-like symptoms, headache, diarrhea, nausea, and tender lymphadenopathy.
5-Fluorouracil
5-FU, a chemotherapeutic agent that interferes with DNA synthesis by inhibiting thymidylate synthetase, has been used topically since the 1960s for the treatment of AKs. 5-FU is currently approved by the FDA for the treatment of AKs and superficial BCCs. This drug carries a black box warning urging close supervision by a physician experienced with the administration of antimetabolites. Treatment site reactions following topical 5-FU administration include erythema, edema, and pain. In severe cases, ulcerations and bleeding have been reported. Side effects of 5-FU treatment are exacerbated by prolonged UV radiation (UVR). Poor treatment compliance, due to adverse side effects, is associated with significant failure rates.11,16
Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory agent that inhibits cyclooxygenase, the rate-limited enzyme in the synthesis of prostaglandins. Diclofenac has high affinity for cyclooxygenase-2 that is frequently elevated in AKs, melanoma, and NMSC. In addition, diclofenac inhibits prostaglandin-mediated, UV-induced NMSC by decreasing proinflammatory cytokines such as IL-1, tumor necrosis factor–α, and transforming growth factor–β.17,18 Diclofenac has a modest effect on treatment of AK but has not been shown to be effective in the treatment of NMSC.
Retinoids
Both topically applied and systemic retinoids have consistently been shown to be effective in the prevention and management of NMSCs. The effect of topical retinoids is at best mild, whereas oral retinoids have a different efficacy profile and side effects that frequently limit its usefulness for cancer treatment and prevention. Retinoids downregulate the expression of AP-1 responsive genes, activate transrepression of AP-1, arrest growth, and induce apoptosis and differentiation.19,20 Retinoids also downregulate the UV-induced overexpression of cyclooxygenase-2, reducing prostaglandins that are normally elevated in AKs and NMSC.20,21 Isotretinoin at 0.25 to 0.5 mg/kg per day and acitretin at 10 to 20 mg/d are the most common systemic retinoids used for skin cancer chemoprevention, especially in immunocompromised patients.22 Randomized trials of acitretin for secondary prevention of NMSC have shown a significant benefit in patients of renal transplant and modest benefit that did not reach statistical significance in immunocompetent patients.23,24 Routine clinical monitoring for signs of retinoid toxicity and laboratory examinations (fasting lipid profile, liver function tests) during systemic therapy are mandatory.
Ingenol Mebutate
Ingenol mebutate is a macrocyclic diterpene ester found in the sap of the Euphorbia peplus plant. Ingenol mebutate gel formulations (0.015%, 0.05%) are available for the treatment of AKs. The proposed mechanism of action is induction of apoptosis in proliferating keratinocytes and activation of innate immune effector responses, including rapid release of neutrophil oxidative mediators. Treatment regimens shown to be effective for reduction of AKs include once daily application on three consecutive days to the face or scalp (0.015% gel) or on two consecutive days to the trunk or extremities (0.05% gel).25
Photodynamic Therapy
Topical PDT with 5-aminolevulinic acid (ALA) or methyl-aminolevulinic acid (MAL) is effective in the treatment of certain NMSCs. Following topical application, ALA or MAL accumulates preferentially in malignant and premalignant cells and is metabolized in the intrinsic intracellular heme biosynthesis pathway to protoporphyrin IX. Preferential accumulation of ALA in malignant and premalignant tissues results from the differences in cellular uptake, differential activities of the heme and porphyrin biosynthetic pathways, iron bioavailability, differential properties of stratum corneum, and variable tissue ALA distribution. Protoporphyrin IX is a photoactive intermediate that facilitates the transfer of singlet oxygen species and the generation of free radicals. The reactive oxygen species induce lipid peroxidation, protein cross-linking, and increased membrane permeability. All these processes contribute to irreversible damage and ultimately cell death of malignant and premalignant cells.26,27
Both lasers and noncoherent light sources (blue and red light) with wavelengths in a range corresponding to absorption peaks of protoporphyrin IX (from 400 nm to infrared) have been used to activate topically applied ALA and/or MAL.
The use of PDT is currently approved by the FDA for the treatment of AKs. The off-label uses of PDT to treat NMSCs, acne, photodamaged skin, human papillomavirus (HPV)-associated pathologies, lymphocytoma cutis, hidradenitis suppurativa, and other dermatologic conditions are currently under investigation.28 Scarring and postprocedural dyspigmentation are minimal to nonexisting following PDT.29 An apparent additional benefit of PDT is photorejuvenation with softening of the appearance of fine lines, rhytids, and acne scars. The mechanism of photorejuvenation is not fully understood, but may relate to increased type I collagen production.30
RADIATION THERAPY FOR SKIN CANCER
RT is a treatment option for certain NMSCs, angiosarcoma, Merkel cell carcinoma (MCC), cutaneous lymphomas, some adnexal carcinomas, and other primary and metastatic cutaneous neoplasms. RT, in properly fractionated doses, is indicated when the patient’s overall health status (such as elderly patients who are unwilling or unable to undergo surgery) or size of the tumor precludes surgical extirpation. The procedure is also used as an adjuvant treatment of patients with positive surgical margins, perineural invasion (PNI), or local regional nodal metastasis. The effectiveness of RT, however, is limited by the inability to definitively assess and control the tumor margins. In addition, treatment of an excessively large area of normal skin surrounding the tumor may enhance risk of both postradiation dermatitis and future skin cancers. Two modes of RT delivery are electrons and superficially penetrating photons (X-rays).31 Appropriate radiation margins for clinically visible tumors and/or surgical scars should generally be <3 cm. A protracted fractionation scheme using 2 to 2.5 Gy fractions to a total of 50 to 66 Gy for NMSCs is commonly used to achieve the best chance of durable local control and acceptable late effects.32 Nonetheless, treatment may be accelerated to a single large fraction (10 to 20 Gy) or three to five fractions of 6 to 7 Gy for patients with significant comorbidities or for smaller lesions in locations with good perfusion where cosmesis is less critical. Accelerated treatment protocols provide excellent local control but have an increased risk of fibrosis, atrophy, telangiectasias, and poor cosmesis. Although a course of RT may be protracted over several weeks, daily treatments last several minutes.
Local control rates for small (<2 cm) BCCs are 90% to 95% with adequate (>90% rated as excellent or good) cosmesis. For larger tumors with bone or cartilage involvement, local control rates decrease to 50% to 75%. These deeply invasive/destructive lesions are best approached with a combined excision and adjuvant RT. In a randomized trial of patients with incompletely excised recurrent BCCs, adjuvant RT improved the 5-year local control rates from 61% to 91%.33 The 10-year local control rates were similar for both adjuvant RT and repeat surgery (92% versus 90%). The local control for SCC is lower by 10% to 15% compared with equivalent-sized BCCs with RT. For selected cases of small, superficial BCC and SCC, office-based treatment with superficial X-ray therapy was shown in one series to have a favorable recurrence rate of 5.0% at 5 years.34
The consideration of acute and permanent tissue effects of RT, such as acute and chronic radiation dermatitis, epidermal atrophy, telangiectasias, altered pigmentation, delayed radiation necrosis, alopecia, and secondary cutaneous malignancies, must be anticipated and managed.35 Recurrent SCC after RT may be more resistant to treatment than recurrence after surgery alone.36 The late cosmetic effects are more pronounced with a large dose per fraction (over 3 to 4 Gy), if the total dose is >55 Gy, following treatment to large fields and/or deeply invasive lesions and with continued unprotected sun exposure. RT is most commonly used in the head and neck region, and treatment of distal extremities should be considered cautiously as poor vascularization and edema increase the risk of posttreatment complications.33
AKs are common cutaneous lesions that occur on sun-exposed areas, particularly in blond or red-haired, fair-skinned individuals with green or blue eyes. They, of course, can occur in patients who do not possess these phenotypic features, and relate most directly to cumulative sun exposure. AKs represent the initial intraepidermal manifestation of abnormal proliferation of keratinocytes and harbor the canonical mutations in tumor suppressor genes (such as p53) seen in SCC. As such, AK are precursor lesions with the possibility of progression to SCCIS and invasive SCC.37
Clinically, AKs demonstrate one of three behavior patterns: spontaneous regression, persistence, or progression to invasive SCC.38 The risk of progression of AK to SCC has been calculated between 0.025% and 16% per year, and the calculated total risk of malignant transformation for a patient with AKs followed for a period of 10 years ranges from 6.1% to 10.2%.39 Approximately 60% to 65% of SCCs arise from prior AKs. Spontaneous regression has been reported in as high as 25.9% of AKs over a 12-month period, although a 15% recurrence rate was noted at follow-up.37
PATHOGENESIS OF ACTINIC KERATOSIS
Factors linked to pathogenesis of AKs include (1) exposure to UVB light—UVB causes mutations of the tumor suppressor gene (TSG) p53; (2) genetic DNA instability (i.e., xeroderma pigmentosum) or melanin deficiency (albinism); (3) older age; (4) male gender; (5) anatomic location—>80% of AKs are located on the head and neck, dorsal forearms, and hands; and (6) history of immunosuppression—solid organ transplant patients are at significantly increased risk.
Studies of the molecular pathogenesis of AK and SCC have revealed a stepwise progression from normal skin to actinically damaged skin to AK to SCC. Global gene profiling has shown similar patterns of abnormal gene expression in AK and SCC, and the identification of expanded clones of p53-mutated keratinocytes in precursor AKs confirms that AKs indeed represent an early stage in the molecular carcinogenesis of NMSC.40,41 A study of asymptomatic AKs, inflamed AKs, and SCCs similarly showed a stepwise loss of differentiation manifesting as diminishing 27-kD heat-shock protein, an initial increase in lymphocytes suggesting the occurrence of an active inflammatory and immune response, a stepwise increase in the number of cells expressing detectable levels of p53 suggesting an increase in DNA damage, decreasing levels of Bcl-2, an apoptosis inhibitor, and loss of Fas antigen, suggesting these cells become less sensitive to FasL-mediated apoptosis as they progress.42
CLINICAL FEATURES OF ACTINIC KERATOSIS
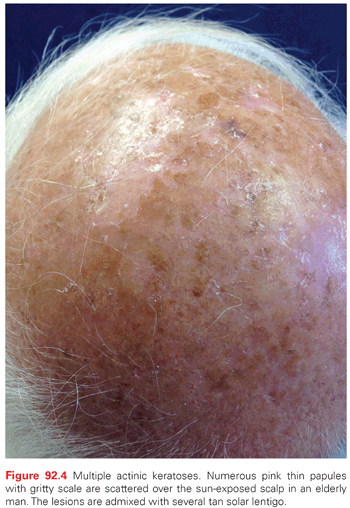
AKs are red, pink, or brown papules with a scaly (hyperkeratotic) surface. They occur on sun-exposed areas and are especially common on the balding scalp, forehead, face, dorsal forearms, and hands (Fig. 92.4). Subclinical (nonvisible) AKs are estimated to occur up to 10 times more often than clinically visible AKs, particularly on sun-exposed skin.37 Actinic cheilitis is a clinical subtype of AK on the lower lip marked by diffuse scaling and erythema or hypopigmentation along the vermilion border. More severe cases may have recurrent erosions or fissures. Although the presentation can be subtle, the elevated risk of mucosal SCC development demands clinical vigilance and warrants treatment of actinic cheilitis, most often with field-directed therapy. Actinic cheilitis is particularly amenable to treatment with PDT, although shorter incubation times must be used on the thin and sensitive skin of the vermilion lip.
The histologic spectrum of AKs includes hyperplastic, atrophic, Bowenoid, acantholytic, and pigmented subtypes.43 Each subtype is characterized by disordered, atypical keratinocytes with nuclear atypia. In the hyperplastic variant, pronounced hyperkeratosis coexists with parakeratosis. Epidermal hyperplasia and downward displacement without dermal invasion can be noted. A thin epidermis devoid of rete ridges is characteristic of the atrophic variant. Atypical cells predominate in the basal layer. The Bowenoid AK is virtually indistinguishable from BD SCCIS. In the Bowenoid variant, considerable epidermal cell disarray and clumping of nuclei gives a windblown appearance. The presence of suprabasal lacunae is characteristic of acantholytic AK. Excessive melanin is present within the basal layer in the pigmented variant of AK.
TREATMENT OF ACTINIC KERATOSIS
Prevention of disease is always superior and preferable to the need for treatment. Effective preventative measures include avoidance of excessive sun exposure (use of broad-brimmed hats, sun-protective clothing, and sunscreen), patient education, and regular self-examinations to detect the earliest signs of malignant transformation. The effectiveness of topical chemoprevention was demonstrated in a double-blind, randomized clinical trial in which 25 patients applied topical all-trans-retinoic acid (tretinoin) 0.05% cream twice daily for 16 weeks, resulting in a 30.3% reduction in the number of AKs compared with baseline.44
Because of their low but real potential to develop into invasive SCC, AK therapy is generally recommended. The management of AK should be based on whether a lesion-directed or field-directed therapy is preferred. Lesion-directed therapy, including ablative and surgical procedures, is reserved for selected cases when only a few clinically visible AKs are present. Field-directed therapy, including ablative, nonablative, and topical treatments, offers the advantage of treating both clinically evident and subclinical lesions that may progress to visible AKs and, potentially, SCC.
Lesion-Directed Therapy
Cryotherapy is the most commonly used lesion-directed treatment modality. Clearance rates range from 39% to 98.8%.37,45 In a large, multicenter Australian study evaluating the efficacy of cryotherapy for the treatment of AKs on the face and scalp, of the 89 patients and 421 lesions in the intended-to-treat population, there was an average of 67.2% lesion response rate per patient.46 As mentioned previously, cryotherapy results in the local side effects of pain, erythema, and potentially blistering or crusting, which are generally transient and well-tolerated. Cryotherapy treatment was associated with “good” and “excellent” cosmetic outcomes in 94% of the lesions.
Other lesion-directed treatment options include C&D and surgical excision. Treatment with C&D may require more than one cycle. High cure rates and good cosmetic outcome have been reported, although this treatment is not commonly used for AK. C&D should be generally avoided in recurrent lesions, lesions that have undergone punch biopsy, and lesions in hair-bearing areas.37
Field-Directed Therapy
Field-directed treatment modalities include topical pharmacologic therapies including imiquimod, 5-FU, and diclofenac, ablative and nonablative laser resurfacing, PDT, dermabrasion, and deep and medium-depth chemical peels.
5-Fluorouracil
Manufacturer-recommended 5-FU treatment protocol for AKs is twice daily for 2 to 4 weeks. Other protocols proposed in the literature include 5% 5-FU once to twice daily for 2 to 7 weeks; 5% 5-FU once daily, 1 day per week for 12 weeks; and 0.5% 5-FU once or twice daily for 1 to 4 weeks.37 The literature reports rates of AK resolution with 5-FU that reach 89% with twice-weekly application for 16 weeks, albeit recurrence rates of up to 55% have been reported.47 In addition, low compliance, because of adverse effects of medication, is associated with significant treatment failure rates.
In a phase 3, double-blind randomized study of 117 patients with at least five AKs treated with 0.5% 5-FU cream once daily, complete clearance rates at 1, 2, and 4 weeks were 26%, 20%, and 48%, respectively.48 In a large systematic review of 5-FU randomized clinical trials, treatment with 5% 5-FU resulted in an average reduction of 79.5% in the mean number of lesions with clearance rates of 94% and 98% at 2 and 4 weeks, respectively. In comparison, treatment with 0.5% 5-FU resulted in an average reduction in the mean number of lesions of 86.1%. Higher clearance rates with 0.5% 5-FU may represent increased patient medication compliance from lower side effects profile. In the study comparing 0.5% and 5% 5-FU, 85% of patients preferred 0.5% 5-FU.37
Imiquimod (Aldara, Medicis Pharmaceutical, Scottsdale, AZ)
The FDA-approved imiquimod treatment protocol for AKs is twice weekly for 16 weeks. Other treatment protocols including more frequent dosing for a shorter time period or pulsed treatment with a 2- to 4-week rest period have been reported with similar efficacy.37,49 Clearance rates with imiquimod range from 45% to 85%.50 Reported recurrence rates are 10% and 16% within 1 year and 18 months of treatment, respectively.
Imiquimod is effective and well tolerated for the treatment of AKs in posttransplant, immunosuppressed patients. The safety and efficacy of 5% imiquimod cream for the treatment of AKs in posttransplant patients receiving immunosuppressive therapy within the prior 6 months was evaluated in a multicenter, randomized, placebo-controlled study with reported imiquimod clearance rate of 62.1%.51 Common reported side effects of imiquimod therapy included site reactions (edema, erythema, vesicles, and erosions/ulcerations), fatigue, headache, diarrhea, nausea, and leucopenia. No serious adverse events were reported.51 Few randomized trials have directly compared the efficacy of the leading topical treatments for AK, 5-FU, and imiquimod. One study compared 5-FU 5% cream applied twice daily for 2 to 4 weeks with imiquimod 5% cream applied twice daily for 16 weeks in 36 patients with AKs on the face and scalp.52 At week 24, the total AK count was reduced by 94% and 66% with 5-FU and imiquimod, respectively. Another study randomized patients to either cryotherapy, 5-FU (twice daily for 4 weeks), or imiquimod (three times per week for 4 weeks, repeated if necessary) therapy for multiple AK.53 Cryotherapy resulted in a 68% initial clinical clearance, compared with 96% for 5-FU and 85% for imiquimod. Sustained clearance at 1 year was greatest in the imiquimod group (73%), compared to 54% for 5-FU and 28% for cryotherapy. Overall, the two topical treatments appear to have similar efficacy, although additional studies are needed to assess whether imiquimod has superior long-term clearance rates.
Photodynamic Therapy with 5-Aminolevulinic Acid. PDT with ALA activated by blue light showed reported overall clearance values for AKs between 50% to 80%.37 Response rates vary with duration of ALA incubation and thickness of AK lesions. In a randomized, blinded, placebo-controlled study, 243 patients with a total of 1,403 AKs on the scalp and face were treated with ALA (incubation time 14 to 18 hours) and exposure to blue light.54 At 8 weeks, 30% of patients with partial response were retreated. At 12 weeks, 91% lesion clearance rate was reported. Discomfort was the most commonly reported adverse event.
A randomized study of 36 patients with at least four AKs compared efficacy of two sessions of ALA-PDT or ALA-pulsed dye laser (ALA incubation time, 1 hour) with 0.5% 5-FU cream applied once or twice daily for 4 weeks.55 At 1 month posttreatment, the overall individual lesion clearance rates for 5-FU, ALA-PDT, and ALA-pulsed dye laser were 70%, 80%, and 50%, respectively. ALA-PDT and ALA-pulsed dye laser were better tolerated than 5-FU. The efficacy of PDT was compared with that of cryotherapy in an open, randomized, controlled study of 202 patients with 732 AKs.56 Clearance rates for PDT and cryotherapy were 69% and 75%, respectively. Response rates correlated with the lesion thickness, with thinner lesions having higher response rates. Satisfaction with the cosmetic outcomes for PDT and cryotherapy were 96% and 81%, respectively.
Other Field Treatments. Efficacy of topically applied 3% diclofenac gel in 2.5% hyaluronic acid vehicle gel versus vehicle was addressed in a multicenter, randomized, double-blind, placebo-controlled study of 195 patients. Treatment with 3% diclofenac gel twice daily for 30 to 90 days resulted in an improvement of 59% compared with 31% with placebo.57 As described previously, ingenol mebutate is a recently approved therapy for AK with a simplified dosing regimen that was shown to reduce AK counts by 75% to 83% compared to 0% in patients treated with placebo.25 Other treatment modalities primarily used for cosmetic procedures, such as chemical peels and ablative and nonablative laser resurfacing, have been reported to be effective in the treatment of AKs, but high-quality, well-controlled studies of efficacy are lacking.58
BCC is a slow-growing neoplasm of nonkeratinizing cells originating from the basal cell layer of the epidermis. BCC is the most common human cancer, accounting for 25% of all human cancers and 75% of skin malignancies diagnosed in the United States.59 Although BCC rarely metastasizes, it is locally invasive and can result in extensive morbidity through local recurrence and tissue destruction. Typically, BCC develops on sun-exposed areas of lighter-skinned individuals. Approximately 30% of lesions of BCC occur on the nose. Nonetheless, BCC can occur anywhere, including non–sun-exposed areas, and has been reported to occur on the vulva, penis, scrotum, and perianal area. Men are affected slightly more often than are women, and although once rare, before the age of 50 years, BCCs are becoming more common in younger individuals.1,4
Intermittent recreational sun exposure, exposure to UVR (UVB confers greater risk than UVA) and sun overexposure (i.e., sunburns), especially in childhood and adolescence, is a significant risk factor for development of BCC. Other factors involved in the pathogenesis include mutations in regulatory TSG, exposure to ionizing radiation, chemicals (e.g., arsenic, polyaromatic hydrocarbons), psoralen plus UVA (PUVA) therapy, and alterations in immune surveillance (i.e., organ transplantation, underlying hematologic malignancy, immunosuppressive medications, or HIV infection).1
BCC is substantially more common among survivors of childhood cancers, principally due to previous treatment with ionizing radiation therapy alone or in combination with chemotherapy. In affected individuals with a history of childhood cancers, BCC developed between 20 and 39 years of age, with an increased risk of approximately 40-fold in cancer survivors that had received ≥35 Gy versus those who did not receive radiation therapy.60
BCC can be a feature of inherited conditions. Included among these are the nevoid BCC syndrome (NBCCS), Bazex syndrome (X-linked dominant; characterized clinically by follicular atrophoderma, hypotrichosis, hypohidrosis, milia, epidermoid cysts, and facial BCCs), Rombo syndrome (features similar to those of Bazex syndrome with peripheral vasodilation with cyanosis), xeroderma pigmentosum (autosomal recessive disorder in unscheduled DNA repair, clinically characterized by numerous NMSCs and melanomas), and unilateral basal cell nevus syndrome.
NBCCS is a rare autosomal dominant genetic disorder characterized by a mutation in the human patched (PTCH1) gene and predisposition to multiple BCC and other tumors, as well as a wide range of developmental defects. Patients with this syndrome may exhibit a broad nasal root, borderline intelligence, odontogenic keratocysts of the jaw, palmar and plantar pits, calcification of the falx cerebri, medulloblastomas, and multiple skeletal abnormalities in addition to a few to thousands of BCCs.61 Tumor development in patients with NBCCS is related to sun exposure, as BCCs develop most frequently in sun-exposed areas. The clinical course is commonly benign prior to puberty; nonetheless, after puberty individual lesions may progressively enlarge and ulcerate. Individuals with NBCCS are exceedingly sensitive to ionizing radiation. Hundreds of BCCs were reported in children treated with RT for medulloblastoma.
Genomic analysis of patients with NBCCS has elucidated the molecular pathogenesis of BCC. The behavior of neoplasms occurring in NBCCS confirms a classic two-hit model of carcinogenesis—tumors develop in cells sustaining two genetic alterations.62 The first alteration or hit is inheritance of a mutation in a TSG, and the second is inactivation of the normal homologue by environmental mutagenesis or random genetic rearrangement. Sporadic BCCs would arise in cells that underwent two somatic events, resulting in the inactivation of the NBCCS TSG PTCH1. Studies of BCC have indicated an association with mutations in the PTCH1 regulatory gene, which maps to chromosome 9q22.3.63 Loss of heterozygosity at this site is seen in both sporadic and hereditary BCC. Inactivation of the PTCH1 gene is probably a necessary step for BCC formation. The PTCH1 protein is part of a receptor complex that regulates the hedgehog signaling pathway, a key regulator of embryonic development and cellular proliferation. The PTCH1 protein binds to and inhibits Smoothened, a transmembrane receptor for the secreted molecule hedgehog. On hedgehog binding, Smoothened is released from the inhibitory effects of PTCH1 and transduces an activating signal via GLI transcription factors.64,65 Loss of function mutations in PTCH1 thus permit unopposed Smoothened activity and cellular proliferation. This understanding of the molecular pathogenesis of BCC has led to the development of targeted medical therapy for BCC with small molecule inhibitors of Smoothened, as discussed subsequently. UV-induced mutations in the p53 gene, such as cyclobutane pyrimidine dimers (CC → TT) have also been reported in up to 60% of BCCs.66
CLINICAL BEHAVIOR OF BASAL CELL CARCINOMA
Although the overall prognosis of the vast majority of BCC is excellent, some tumors exhibit infiltrative growth patterns and extensive subclinical spread. Batra and Kelley67 looked at risk factors for extensive subclinical spread of more than 1,000 NMSCs treated by MMS. The most significant predictors were anatomic location on the nose of any type of BCC; morpheaform BCC on the cheek; recurrent BCC in men; any tumor located on the neck in men; any tumor located on the ear helix, eyelid, or temple; and increasing preoperative size. Invasive BCC can migrate along the perichondrium, periosteum, fascia, or tarsal plate.68,69 This type of spread accounts for higher recurrence rates in tumors involving the eyelid, nose, and scalp. Embryonic fusion planes likely offer little resistance and can lead to deep invasion and tumor spread, with very high rates of recurrence. The most susceptible areas include the inner canthus, philtrum, middle to lower chin, nasolabial groove, preauricular area, and the retroauricular sulcus.
PNI is infrequent in BCC and occurs most often in recurrent, clinically aggressive lesions. In a case series, Niazi and Lamberty70 noted PNI in 0.178% of BCC. In all cases, PNI was associated with recurrent tumors that were most often located in the periauricular and malar areas. Clinically, PNI may present with paresthesia, pain, and weakness or, in some cases, paralysis.
Metastatic BCC is rare, with incidence rates varying from 0.0028% to 0.1%.71 Interestingly, BCC appears to be uniquely dependent upon surrounding stroma, as experimental transplants of tumors devoid of associated stroma are usually unsuccessful.72 This concept of stromal dependence may explain the low incidence of metastatic BCC. Metastases, when reported, have involved the lung, lymph nodes, esophagus, oral cavity, and skin. Although long-term survival has been reported, the prognosis for metastatic BCC is generally poor, with an average survival of 8 to 10 months following diagnosis. Platinum-based chemotherapy or molecularly targeted Smoothened inhibitors may provide benefit as salvage therapy for metastatic BCC.72–74
Clinical variants of BCC include nodular, micronodular, superficial, infiltrative, morpheaform (also termed aggressive-growth BCC, fibrosing BCC, sclerosing BCC), pigmented, cystic BCC, and fibroepithelioma of Pinkus (FEP).75,76
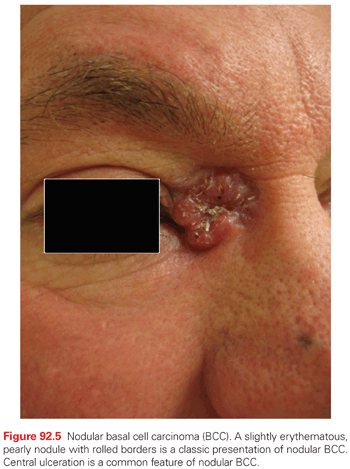
Nodular BCC is the most common BCC subtype, accounting for >60% of all tumors. Clinically, it presents as a raised, translucent pearly, skin-toned to pink papule or nodule with prominent telangiectasias (Fig. 92.5). Occasionally, the center of the tumor appears depressed or sunken, leaving a rolled, raised border with the classic pearly appearance so-called rodent ulcer. Not infrequently, history of easy bleeding and/or crusting is obtained. Nodular BCC has a propensity for involving sun-exposed areas of the face in individuals over the age of 60. In patients with severe actinic damage, nodular BCCs may appear in the third decade.
Superficial BCC is the second most common subtype of BCC, comprising of up to 15% of all BCCs. It presents in younger patients at a mean age of 57 years. Superficial BCC presents as well-defined, pink to erythematous scaly or eroded macule or plaque commonly with a thin pearly border. It appears predominately on the trunk and may be difficult to differentiate clinically from AK, SCCIS, or an inflammatory lesion.
Infiltrative BCC comprises approximately 5% of all BCCs and develops predominately in the head and neck region of older adults. Mean age at presentation is 66 years. Clinically, infiltrative BCC most often resembles the nodular form of BCC.
Morpheaform BCC accounts for 3% of all BCCs and commonly develops in the head and neck region of older individuals. It presents as a flat or indurated, slightly firm lesion, without well-demarcated borders, with a white to yellowish hue, and may be difficult to differentiate from a scar. Traction on the skin often highlights the clinical extent of the lesion. Symptoms of bleeding, crusting, and ulceration are often not present in this tumor subtype. The actual size of the cancer is usually much greater than the clinical extent of the tumor. Pigmented BCC is a variant of nodular BCC with clinically visible pigment and may be difficult to differentiate from nodular melanoma. The presence of pigment may be of value in determining adequate excision margins.
FEP, a rare variant of BCC, typically presents on the torso and extremities, but also has been noted on the genitalia, groin, and sole of the foot. Clinically, FEP usually presents as a pink, smooth, dome-shaped, or pedunculated papule, plaque, or nodule.77 It may be difficult to distinguish clinically from amelanotic melanoma.
Histologic features defining BCC are aggregates of neoplastic basaloid cells stemming from the epidermis or the epithelium of adnexal structures.75 Aggregates are organized as lobules, islands, nests, or cords of cells that display an orderly arrangement of the basaloid cell nuclei at the periphery, a palisading array. Occasionally, central necrosis or cystic changes are seen within the tumor lobules. Individual tumor cells have uniform, hyperchromatic, round to oval nuclei. Mitotic figures are uncommon, but the presence of apoptotic cells and necrosis is frequently observed. Pleomorphic variant of BCC shows tremendous variability of nuclear size and chromatism, as well as multiple mitoses and multinucleated giant cells. A stromal retraction or clefting around the neoplastic aggregates is commonly seen in the dermis. An accumulation of mucin within and around the tumor lobules may be evident.
Histologic subtypes of BCC include nodular and micronodular, superficial, and infiltrative BCC (Fig. 92.6A,B). Nodular BCC accounts for approximately 50% of all histologic variants of BCCs and exhibits characteristic features as previously described. Superficial multifocal BCC accounts for approximately 15% of BCCs and is a broad lesion characterized by basophilic buds extending from an atrophic epidermis into the papillary dermis. In a two-dimensional view, tumor buds appear to be multifocal, but upon three-dimensional imaging analysis they form a netlike pattern. Retraction artifact is present, as is peripheral palisading within the buds. FEP, which accounts for 1% of BCCs, is characterized by a polypoid lesion in which basaloid cells grow downward from the surface in a network of anastomoses of cords of cells in loose connective tissue.
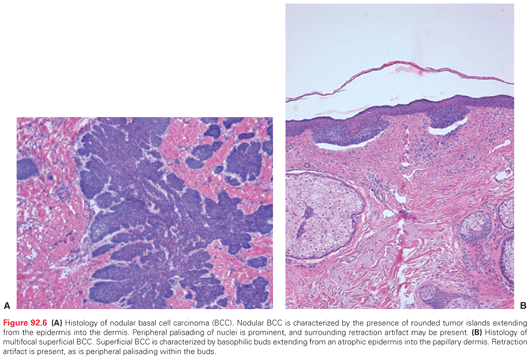
The biologic behavior of the micronodular, infiltrating, and morpheaform (sclerosing) subtypes of BCC is more aggressive than that of the nodular and superficial forms. Infiltrative histology is seen in 15% to 20% of BCCs. Individual tumor islands manifest small, irregular outlines with a spiky appearance that invade into and throughout the dermis. Palisading is characteristically absent. The stroma is less myxoid than in the nodular form. In the morpheaform variant, small groups or cords of tumor cells often only one to two cells in thickness infiltrate and dissect a dense, collagenous stroma often parallel to the skin surface. Mixed histology is frequently apparent in BCCs. Areas of follicular, sebaceous, eccrine, or apocrine differentiation may also be seen in some BCCs. BCCs may contain focal areas of squamous differentiation, ranging from individual dyskeratotic cells to keratin pearls. The term basosquamous (metatypical) carcinoma denotes BCC with a predominance of mature, atypical keratinizing squamous component. Biologic characteristics of a basosquamous carcinoma are more similar to those of SCC with a possibility for metastasis.
The significance of histologic subtype lies in the correlation with a tumor’s biologic aggressiveness. The infiltrative and micronodular types are the most likely to be incompletely removed by conventional wide local excision (WLE). Rates of incomplete excision vary from 5% to 17%.78 Incompletely excised infiltrative and micronodular BCCs recur at rates of 33% to 39%. Recurrences after RT show a tendency toward infiltrative histology and squamous transformation, and even recurrent BCC after excision or C&D may become metatypical. Although historical reports in the literature suggested that 60% of incompletely excised BCCs will not recur, none of these studies provided an appraisal of recurrence rates as a function of histologic subtype.68,69 In general, incompletely excised BCCs should be removed completely, preferably by MMS, especially if they occur in anatomically critical areas such as the central zone of the face, retroauricular sulcus, or periocular area.
Excisional surgery, MMS, and C&D have all been used to treat circumscribed, noninfiltrative BCCs. MMS is the treatment method of choice for all recurrent and infiltrative BCCs, particularly if a tumor is located on the face.6,79 RT is best suited for poor surgical candidates and patients with extensive lesions not amenable to surgery.80,81 RT is not indicated for recurrent or morpheaform lesions and in patients with NBCCS.
C&D is frequently used by dermatologists in the treatment of primary BCC. Knox82 noted cure rates as high as 98.3%. Kopf et al.,83 in an earlier study, cited a significant difference in the cure rates obtained between patients treated by private practitioners (94.3%) and those treated by trainees in the New York University Skin and Cancer Unit (81.2%). This supports the premise that C&D, although simple and cost-effective, is highly dependent on operator skill. Traditionally, it was recommended that the procedure be repeated for three cycles, but histology, location, and behavior of the tumor should dictate the number of cycles. C&D should be reserved for small or superficial BCCs, not located on the midface, in patients who may not tolerate more extensive surgery.
Surgical excision offers a unique advantage of histologic evaluation of the excised specimen. It has been demonstrated that 4-mm margins are adequate for removal of BCC in 98% of cases of nonmorpheaform BCC of <2 cm in diameter.78 Extending the excision into subcutaneous fat generally is adequate for a small primary BCC.
MMS permits superior histologic verification of complete removal, allows maximum conservation of tissue, and remains cost-effective as compared with traditional excisional surgery for NMSCs including BCCs.4,6 In a large study of treatment of primary BCC by Rowe et al.,84 MMS demonstrated a recurrence rate (RR) of 1% over 5 years. This was superior to all other modalities, including excision (RR = 10%), C&D (RR = 7.7%), RT (RR = 8.7%), and cryotherapy (RR = 7.5%). In a similar study of recurrent BCC, treatment with MMS demonstrated a long-term RR of 5.6%.85 In that treatment group, MMS was superior to all other modalities, including excision (RR = 17.4%), RT (RR = 9.8%), and C&D (RR = 40%). MMS is the preferred treatment for morpheaform, recurrent, poorly delineated, high-risk, and incompletely removed BCC, and for those sites in which tissue conservation for function and cosmesis is imperative. When the surgical approach is contraindicated, RT is a valid option for management of primary BCC. RT may be indicated postoperatively if margins are ambiguous or involved. Disadvantages of RT include lack of margin control, possible poor cosmesis over time, a drawn-out course of therapy, and increased risk of future skin cancers. The RRs for primary BCC treated by RT range from 5% to 10% over 5 years. Wilder et al.86 compared local control rates for RT among 85 patients with 115 primary or recurrent biopsy-proven BCCs. A 95% control rate was achieved for primary BCC and a 56% control rate obtained for recurrent BCC at 5 years. Considering cosmesis, RT scars tend to worsen over time, in contrast to surgical scars that tend to improve over time.
Imiquimod is approved by the FDA for the treatment of superficial BCC <2 cm in diameter on the neck, trunk, or extremities. The FDA-recommended regimen is once-daily application 5 days per week for 6 to 12 weeks. Numerous studies evaluated safety and efficacy of imiquimod for superficial BCC.11,87 Application schedules varied from 2 days per week to twice daily, and the treatment duration ranged from 5 to 15 weeks. Clinical follow-up ranged from 6 months to 5 years. Reported histologic clearance rates ranged from 52% to 81%, albeit high-risk tumors (within 1 cm of the hairline, eyes, nose, mouth, anogenital region, hands, and feet) or tumors >2 cm in diameter regardless of their location were excluded. Imiquimod has been used off-label for the treatment of nodular and infiltrative BCC.88,89 Although some studies have shown favorable cure rates, imiquimod treatment of these tumors is generally not recommended as subclinical disease may persist and lead to late recurrence.
The FDA-approved protocol for treating superficial BCC with topical 5-FU is twice-daily application for 3 to 6 weeks irrespective of tumor size or location. Longer treatment protocols with an average 11 weeks are reported in the peer-reviewed literature. In a study of 31 tumors treated twice daily for an average of 11 weeks, a 90% clearance rate was observed histologically 3 weeks posttreatment.90 Topical PDT has also demonstrated efficacy in the treatment of BCC. Clearance rates for BCC using ALA or MAL PDT range from 76% to 97% for superficial to 64% to 92% for nodular BCC after one to three treatments.26,28,91 Many studies of PDT for nodular BCC involve curettage of the lesion prior to treatment, however, and it is unclear if the response rate would be as successful without initial curettage. In a well-designed comparative trial, 601 patients with superficial BCC were randomized to treatment with MAL PDT (two treatments given 1 week apart), imiquimod, or 5-FU according to FDA-approved protocols. Complete clinical remission at 1 year was found to be 72.8% for PDT, 83.4% for imiquimod (superior to PDT), and 80.1% for 5-FU (not statistically different from the other treatments). Patients treated with imiquimod or 5-FU were more likely to report bothersome local side effects of the treatment, however.92
Historically, systemic therapy for BCC was limited to cytotoxic chemotherapy that was marginally effective as salvage therapy for metastatic disease. Elucidation of the critical role of abnormal hedgehog signaling in BCC, however, has led to development of a novel class of molecularly targeted small molecule inhibitors of Smoothened, most notably vismodegib.74 Based on a seminal trial of 33 patients with metastatic BCC and 63 patients with locally advanced BCC not amenable to surgical therapy, vismodegib was approved (2012) for treatment of advanced BCC (either metastatic or not amenable to surgery or radiation).73 With once daily oral dosing of 150 mg vismodegib, 30% of patients with metastatic BCC and 43% of patients with locally advanced BCC had an objective response (at least 30% decrease in size of tumors), and 21% of patients with locally advanced BCC had complete clinical resolution of tumors. None of the patients with metastatic BCC had a complete response. Adverse events in the trial were common, including serious adverse events in 25% of patients and fatal adverse events in 7% of patients. Most common adverse events in this and other trials were muscle spasms, alopecia, dysgeusia leading to weight loss, fatigue, diarrhea, and hyponatremia, and between 12% and 54% of patients discontinued therapy because of adverse effects.73,74,93 Due to the relatively low response rate, the high incidence of adverse events, the potential for resistance with a single mutation,94 and reports of rapid recurrence of BCC upon discontinuation of the medication,95 Smoothened inhibitors remain a limited, albeit important, addition to our treatment options for BCC. Vismodegib may also be an effective suppressive therapy for select patients with NBCCS, where it was shown to decrease the incidence of new tumors by 93% during an 8-month period.93
It is imperative that patients with a history of BCC receive annual full-body skin examinations. Although most recurrences appear within 1 to 5 years, recurrences decades after initial treatment are reported in the literature. Rowe et al.96,97 found that 30% of recurrences developed within the first year after therapy, 50% within 2 years, and 66% within 3 years. A separate new primary BCC can present at rates of approximately 40% within 3 years, with 20% to 30% within 1 year of treatment of the original lesion.
SCC is a neoplasm of keratinizing cells that shows malignant characteristics, including anaplasia, rapid growth, local invasion, and metastatic potential. More than 200,000 cases of SCC are diagnosed in the United States each year, making it the second most common human cancer after BCC.98 People of Celtic descent, individuals with fair complexions, and those with poor tanning ability and predisposition to sunburn are at increased risk for developing SCC. SCC in blacks arises most often on sites of preexisting inflammatory conditions such as burn injuries, scars, or trauma.99 Patients treated with PUVA or undergoing immunosuppressive therapy following solid-organ transplantation are at increased risk of SCC (see the following).
PATHOGENESIS OF SQUAMOUS CELL CARCINOMA
Major factors involved in the pathogenesis of SCC include cumulative exposure to UVR, genetic mutations, immunosuppression, and viral infections. UVR acts as both a tumor-initiating and a tumor-promoting factor. Both UVA and UVB (UVB more than UVA) contribute to mutagenesis of DNA by inducing UV landmark mutations (two tandem CC:GG to TT:AA and two C:G to T:A transitions at dipyrimidic sites). UV-induced mutations in TSG lead to uncontrolled cell-cycle progression and subsequent transformation of keratinocytes.100 In addition to direct mutagenesis, exposure to UVB leads to decreased density and antigen-processing capability of Langerhans cells and may suppress production of the T-helper cell type 1 cytokines IL-2 and INF-γ.101
Alterations in the TSG p53 are the most common genetic abnormality found in AK, SCCIS, and invasive SCC. Under normal conditions, UVR induces p53 gene activity. The amount of p53 protein rapidly increases in keratinocytes after UVR, and drives the expression of downstream genes including Mdm2, GADD45, and p21 CIP/WAF1, leading to cellular arrest in the G1 phase. In cases of squamous dysplasia or SCC, one allele of p53 contains a missense point mutation with UV signature, while the remaining p53 allele is often deleted. Based on whole exome sequencing and copy number variation data obtained from cutaneous SCC, it appears that loss of both copies of p53 is an early event in carcinogenesis, facilitating subsequent clonal expansion and accumulation of many additional point mutations.102 In this study and in similar studies of SCC of the oropharyngeal mucosa, loss of p53 was the most common mutation, but inactivating mutations in other TSG were also noted, including CDKN2A and NOTCH1, which encodes a membrane receptor critical for directing cell fate determination in development.102–104 Activating mutations or gene amplifications of oncogenes have also been reported in SCC, most notably involving the epidermal growth factor receptor (EGFR) and its downstream signaling components such as ras.40,62,102–105 Although these genome-wide studies highlight the mutational complexity and heterogeneity of SCC, two underlying features emerge. First, that both inactivating mutations in TSGs and activating mutations in oncogenes (often multiple) are required for malignant progression, and second, that loss of functional p53 is a central feature of SCC pathogenesis observed in a majority of tumors.
Other agents associated with development of SCC include (1) chemical agents (e.g., petroleum, coal tar, soot, arsenic); (2) physical agents (e.g., ionizing radiation); (3) exposure to PUVA: calculated adjusted relative risk for a cumulative exposure of between 100 and 337 treatments is 8.6; (4) HPV, especially important for SCC in the anogenital and periungual regions, in the setting of immunosuppression with HIV and solid-organ transplantation, and in patients with epidermodysplasia verruciformis; and (5) smoking. Development of SCC has also been associated with chronic nonhealing wounds, burn scars, and chronic inflammatory dermatoses (discoid lupus, ulcers, osteomyelitis).106 Certain cervicofacial regions such as the ear and the lower lip are more prone to developing SCC than BCC. Heritable conditions associated with higher incidence of SCC include xeroderma pigmentosum, dystrophic epidermolysis bullosa, and oculocutaneous albinism. HPV-16 and -18 are frequently implicated in the pathogenesis of subungual and periungual SCC and SCCIS of the digits.
Immunosuppression, including endogenous (underlying lymphoproliferative disorder) and iatrogenic immunosuppression plays a role in pathogenesis of SCC (see the following). In addition to immuosuppressive agents, other medications may also enhance the risk of SCC. Chronic use of photosensitizing drugs, such as the antifungal agent voriconazole, can facilitate actinic damage and have been implicated in accelerated SCC development, particularly in immunosuppressed patients.107 Vemurafenib, a tyrosine kinase inhibitor recently approved by the FDA for the treatment of metastatic and unresectable melanomas harboring V600E mutations in the BRAF gene, appears to increase the risk of keratoacanthoma and SCC development by directly altering signaling through the Ras-Raf mitogen-activated protein kinase pathway known to be involved in SCC pathogenesis.108
CLINICAL FEATURES OF SQUAMOUS CELL CARCINOMA AND ITS VARIANTS
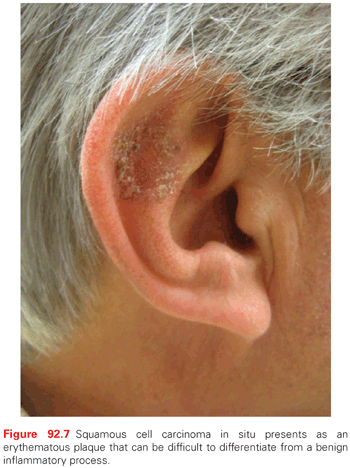
Clinically, SCCIS appears as a discrete solitary, sharply demarcated, scaly pink to red papule or thin plaque (Fig. 92.7). Erythroplasia of Queyrat (SCCIS on the glans of penis of uncircumcised male related to HPV infection) presents as a verrucous or polypoid papule or plaque, often eroded. Invasive SCC appears as a slightly raised papule plaque or nodule that is skin-colored, pink, or red (Fig. 92.8). The surface of the tumor may be smooth, keratotic, or ulcerated. The lesion may also be exophytic or indurated. Rarely, the tumor is symptomatic with pain or pruritus. Bleeding with minimal trauma is common. It can be clinically difficult to distinguish an invasive SCC from a hypertrophic AK, a benign seborrheic keratosis, or a benign inflammatory lesion. An appropriate biopsy should be performed.
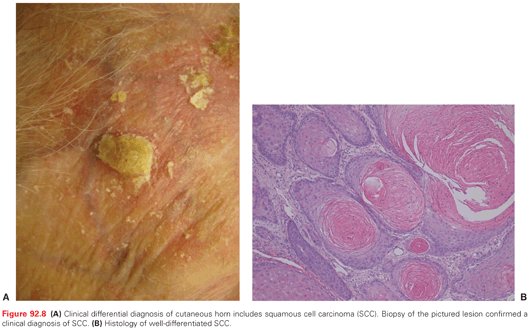
Keratoacanthoma (KA) is a variant of SCC defined by a symmetric crateriform architecture and a clinical presentation marked by rapid growth (up to several centimeters) over a period of several weeks. The tumor then typically stabilizes in size and often spontaneously regresses. Although certain KAs may thus behave in a benign fashion, it is impossible to predict which lesions will regress and which will progress. It is thus recommended to treat KAs as a subtype of SCC with appropriate surgical therapy. Verrucous carcinoma, a variant of SCC, includes oral florid papillomatosis, giant condyloma of Buschke-Lowenstein (on the genitalia), and epithelioma cuniculatum (on the plantar foot).109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree