Incidence and Mortality
In 2014, some 233,000 men in the United States are expected to be diagnosed with prostate cancer and 29,480 to die of the disease,2 accounting for 14% of all new cancers in men and women3 and 11% of male cancer deaths.4 Over the past decade, men in the United States had a 15.4% chance of being diagnosed with prostate cancer and a 2.7% chance of dying of it.2 Worldwide, there were an estimated 899,100 new cases and 258,100 deaths from prostate cancer in 2008.5 Histologic cancers, found in the prostate at autopsy in men who die of other causes, are even more common, and their age-adjusted frequency varies relatively little from country to country, about 2.4-fold.6 In contrast, the mortality rate from prostate cancer varies by 10.8-fold among different countries, suggesting different mechanisms of carcinogenesis and progression, and supporting the concept of distinct “indolent” and “aggressive” forms of the disease.6–8 There are significant age, ethnic, racial, geographic, and familial differences in incidence and mortality rates.9
Risk Factors
Age
Clinically detected prostate cancer is rare before age 40, but then the incidence increases with age faster than that of any other cancer, and continues to rise through the ninth decade of life. Histologic evidence of invasive cancer can be found in the prostates of men as early as the third decade of life, and its prevalence increases dramatically with age to reach 50% to 60% by age 90. As life expectancy increases throughout the world, morbidity and mortality from prostate cancer will impose increasing burdens in developing countries.5
Family History and Genetic Susceptibility
A family history of prostate cancer increases the risk that a man will develop the disease. The level of risk when a family member is affected is similar in breast and prostate cancers. Men with a first-degree relative with prostate cancer have a 2- to 3-fold increased risk, and those with two or more first-degree relatives affected have a 5- to 11-fold increased risk compared with the general population.10 Nevertheless, familial factors have been thought to play a role in only 11% of prostate cancers, although studies of twins suggest that inherited factors may be involved in as many as 42% of all cases.11 While over 70 risk alleles (single nucleotide polymorphisms [SNP]) have been associated with prostate cancer in genome-wide association studies, few are associated with the risk of aggressive or lethal cancer. Many such SNPs are in genes that code for PSA or related kallikreins, blood levels of which are widely used for diagnosis.12 For these SNPs, the increased risk is for a diagnosis of prostate cancer, not metastases or death from the disease. Several high-penetrance mutated genes have been identified, such as HOX13B, which are more common in patients with early-onset and familial disease, but this variant is rare (occurring in 0.1% of the population) and it is not associated with the lethal form of the disease.13 In contrast, men who carry BRCA2 mutations are more likely to develop early-onset prostate cancer, which is more likely to be aggressive and lethal.14
Race and Ethnicity
The incidence and frequency of diagnosed clinical cancers are similar in most Western countries, with the highest age-adjusted mortality rates in Scandinavia and significantly lower rates in non-Western countries. Both genetic susceptibility and exposure to causative environmental factors contribute to these variations.
Men of African ancestry in the United States and Caribbean have the highest incidence of prostate cancer in the world, with striking differences in incidence (1.8-fold) and mortality (2.4-fold) relative to American men of European descent. African American men are diagnosed at a younger age and have higher tumor burdens within each stage category,15 a two-fold higher frequency of metastatic disease at presentation,16 and lower survival rates.17 Incidence and mortality rates are significantly lower for Americans of Asian descent and somewhat lower for those of Hispanic descent.
Environmental factors also affect mortality risk.7 Asians who immigrate to the United States have a higher incidence of and mortality from the disease than in their countries of origin, which increases with each succeeding generation, but remains below the rates in men of African or European descent.18
Other Risk Factors
Diet, Supplements, and Lifestyle Factors. The increased incidence and mortality from prostate cancer evident in immigrants moving from low- to high-risk countries supports an important role for environmental in addition to genetic risk factors. Many epidemiologic studies support an association between high fat intake and breast, colon, and prostate cancer incidence and mortality.19,20 Adult obesity has been associated with aggressive prostate cancer, adverse outcomes after therapy, and increased mortality.11,21,22 The risk of death from prostate cancer has been reported to increase 15% to 20% for each 5 kg/m2 increase in body mass index (BMI).23 Among men diagnosed with prostate cancer, the risk of death from the disease is significantly associated with increased BMI (1.5-fold for overweight men and 2.7-fold for obese men).24 Physical activity may reduce the risk of mortality from prostate cancer; the data are inconsistent for development of the disease, but convincing once the diagnosis has been established.25 Smoking has not been shown to alter incidence rates, but it may be associated with the risk of prostate cancer death, especially when assessed in men after diagnosis.25
Despite many indications that certain micronutrients, minerals, and vitamins have a protective effect on the development of prostate cancer or mortality from the disease, firm evidence is sparse. In the large Selenium and Vitamin E Cancer Prevention Trial (SELECT), vitamin E and selenium, alone or in combination, failed to reduce the incidence of prostate cancer. In fact, men who took vitamin E alone may have had a greater risk of the disease,26 although there is some suggestion that aggressive, potentially lethal cancer may be reduced among smokers taking vitamin E supplements.27 There is no evidence that ingestion of calcium or administration of vitamin D affects incidence or mortality from prostate cancer. Diets rich in tomato-based products, which contain high amounts of carotenoids and lycopene, may reduce the risk of advanced prostate cancer.28,29
Alcohol use, blood group, body hair distribution, sexual activity, urban versus rural residence, and vasectomy do not affect risk.30 There are no data supporting a viral origin of prostate cancer.31
Prevention
While the evidence is incomplete and there are no large intervention trials addressing the role of diet and exercise in preventing prostate cancer, it is reasonable to recommend a low-fat diet, regular exercise, and maintenance of a normal BMI as likely having a modest effect in reducing the risk of developing prostate cancer. More evidence suggests there may be some benefit for such lifestyle changes after the diagnosis of prostate cancer is established.11,21,22
Finasteride, a competitive inhibitor of type II 5α-reductase (5αRI) that blocks the conversion of testosterone to dihydrotestosterone (DHT) within prostatic cells, is a safe and effective drug that reduces the size of the prostate and relieves voiding symptoms in men with benign prostatic hyperplasia (BPH). Hence, it was logical to test the hypothesis that finasteride, or other 5αRIs, could prevent prostate cancer. The Prostate Cancer Prevention Trial (PCPT) randomly assigned 18,882 men, age 55 years or older, who had a normal digital rectal examination (DRE) and PSA, to receive finasteride or placebo over a 7-year period.32 Finasteride reduced by 25% the risk of detecting prostate cancer on biopsy (either end-of-study biopsy or one ordered during study “for cause”). Toxicity was low, but there were more high-grade cancers (Gleason score ≥7) in the finasteride group.33 Many subsequent analyses strongly suggested that the small increase in high-grade cancers was probably a detection artifact resulting from the 20% shrinkage of the prostate by the drug,34 and there were no differences in long-term survival in either arm.35
In a separate randomized trial (REDUCE), the dual 5αRI dutasteride also reduced the detection rate of cancer by 23%.36 However, the US Food and Drug Administration (FDA) conducted an extensive review of the data from the PCPT trial and a reanalysis of the biopsy specimens from the REDUCE trial after Gleason grades were reassigned using contemporary criteria.37 The FDA reviewers agreed that while both 5αRIs reduced the risk of a prostate cancer diagnosis, the effect was seen only among low-grade cancers (Gleason score ≤6), and there was a small (0.5%) but significant absolute increase in the risk of the highest-grade cancers (Gleason score 8 to 10). The FDA concluded that the tradeoff for using a 5αRI in healthy, asymptomatic men would be the occurrence of one additional high-grade cancer for every three or four low-grade cancers (of uncertain clinical potential) prevented, and recommended against the approval of these drugs for chemoprevention of prostate cancer.34
Other agents, such as statins, metformin, and resveratrol, may have protective effects,38 but to demonstrate their benefit will require studies focusing on populations at high risk of developing clinically significant cancers.38 PSA levels at midlife hold great promise for identifying men most likely to benefit from aggressive prevention strategies (see “Prostate-Specific Antigen: A Powerful Tool For Risk Stratification”).
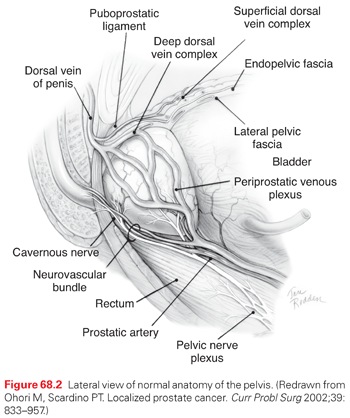
The prostate is an exocrine organ weighing 20 g to 25 g, which consists of lobular tubuloalveolar glands that secrete fluid through ducts that empty into the prostatic urethra. The fluid comprises the bulk of seminal emissions and is rich in PSA. The prostate is located deep in the pelvis between the bladder and the external urinary sphincter, anterior to the rectum and below the pubis (Fig. 68.2).39 The cavernous nerves, which control blood flow to the penis and hence erectile function, run from the pelvic plexus lateral to the rectum along the posterolateral prostate and external urinary sphincter to enter the corpora cavernosa. Because the prostate is located at this critical anatomic juncture, cancers of the prostate and the treatment of these cancers place urinary, sexual, and bowel function at risk.
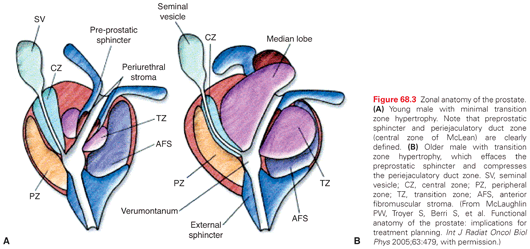
The prostate has three anatomic zones and an anterior fibromuscular stroma (Fig. 68.3). The central zone surrounds the ejaculatory ducts, the transition zone surrounds the urethra, and the peripheral zone makes up the bulk of the normal gland. The posterior peripheral zone lies against the rectum and is the area that is palpable by DRE. These zonal boundaries are indistinct in the prostate of a normal postpubescent male, but as men age the transition zone enlarges from nonmalignant growth (BPH). The frequency of malignancy in the different zones is disproportionate to the glandular tissue present. Very few cancers originate in the central zone, and only 15% originate in the transition zone; most originate in the peripheral zone.
Patterns of Spread
Localized prostate cancer is typically multifocal, in 85% of patients. Most cancers arise near the capsule in the peripheral zone; the surrounding capsule is invaded early and frequently, in up to 80% of cancers detected clinically. Local extension occurs through the capsule (termed “focal” or “established” extracapsular extension [ECE], depending on extent, when observed in a radical prostatectomy [RP] specimen), but may also extend through defects in the capsule where the neurovascular structures and ejaculatory ducts enter the gland, or in the region of the bladder neck. Local invasion can progress to involve the seminal vesicles or the bladder, or to invade the levator muscles. Rarely does tumor invade through Denonvilliers’ fascia to reach the rectal wall. Lymphatic dissemination can involve the hypogastric, obturator, external iliac, presacral, common iliac, or retroperitoneal nodes, with no consistent sentinel landing zone. Hematogenous spread most commonly involves the bones of the axial skeleton and, less commonly, the lung, liver, and other soft tissue organs. The predilection for bone seems to result from a unique bidirectional interaction between tumor cells and the marrow stroma.
Histopathology
Two main growth-related diseases develop in the prostate: BPH, which affects both the epithelial and mesenchymal components, and cancer.40 There is no direct etiologic relationship between BPH and cancer; they are related only by their close anatomic site of origin and high incidence in men over 40 years of age. More than 95% of malignant tumors of the prostate are adenocarcinomas that arise in acinar and proximal ductal epithelium. Grossly, carcinoma appears as pale yellow or gray flecks of tissue coalesced into a firm, poorly defined mass that is difficult to distinguish from surrounding normal tissue. Adenocarcinomas are often multifocal, heterogeneous, and follow a papillary, cribriform, comedo, or acinar pattern. Immunohistochemistry may assist the diagnosis when atypical areas, suspicious for carcinoma, are present in a biopsy sample, particularly in the differentiation of high-grade prostatic intraepithelial neoplasia (PIN) and atypical adenomatous hyperplasias from low-grade carcinoma. A hallmark of prostate cancer is the loss of basal cells, highlighted by negative staining for basal cell markers (high molecular weight/basal-specific cytokeratin) and p63, and positive staining for alpha-methyl-CoA racemase, which is upregulated in cancer.41
Pathogenesis
Prostate cancers develop from the accumulation of genetic alterations that result in an increase in cell proliferation relative to cell death, arrest differentiation, and confer the ability to invade, metastasize, and proliferate in a distant site. Histologic changes can be found in the prostates of men in their 20s, yet the diagnosis is typically made three to four decades later, which suggests that the development of the disease is a multistep process resulting from a variety of genetic and epigenetic alterations.42 The accumulation of changes acting synergistically seems to be more critical than the order in which the alterations occur. Identifying and understanding the events has implications for control of the disease at the earliest stages of transformation, for progression to an invasive tumor, for prognostication, and for points of therapeutic attack. Men who are castrated or who become hypopituitary before the age of 40 rarely develop prostate cancer.43 The evolution of the tumor is heavily influenced by hormonal factors; it is also influenced by environmental, infectious/inflammatory factors, and given the long history once the diagnosis is established, the response to specific treatments.
Premalignant Lesions
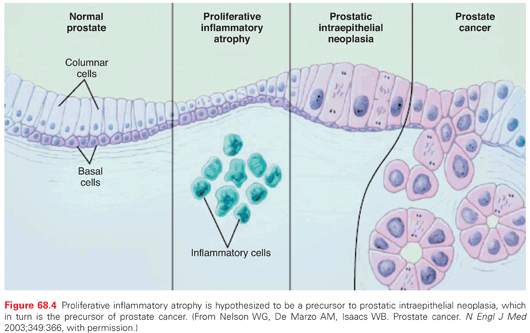
The phenotypic alterations that occur during prostate carcinogenesis and progression are shown in Figure 68.4. The earliest precursor lesion is the subject of debate, as is the cell type that is actually transformed. Recognizable changes begin with proliferation of cells within glands, termed PIN, often found adjacent to areas of proliferative inflammatory atrophy.44 PIN is defined by the presence of cytologically atypical or dysplastic epithelial cells within architecturally benign-appearing acini, and is subdivided into low- and high-grade. Only high-grade PIN is considered a precursor for some invasive carcinomas.45,46 Because high-grade PIN develops preferentially in the peripheral zone where most cancers originate, it precedes the development of cancer by 10 years or more,47 and prostates with extensive high-grade PIN tend to have multifocal tumors. With subsequent loss of the basal cell layer surrounding prostatic glands and the development of anaplastic cellular morphology with nuclear pleomorphism and prominent nuclei, the tumor invades the basement membrane, spreads locally, and begins to metastasize. Not all lesions progress to invasive prostatic cancer during the lifetime of the host. Foci of small atypical acini that display some but not all features diagnostic of adenocarcinoma are referred to as atypical small acinar proliferation, a significant predictor of invasive cancer on subsequent prostate biopsy.48,49 Atypical adenomatous hyperplasia, on the other hand, is not considered a malignant precursor lesion.
Gleason Grade
For adenocarcinomas, the degree of differentiation has prognostic significance and pathologists judge biopsy specimens using the Gleason grading system, which assesses the architectural details of malignant glands under low to medium magnification.50–52 Cytologic features under high magnification are not considered.53,54 Five distinct patterns of growth from well- to poorly differentiated were originally described by Gleason using a scale from 1 to 5 (Fig. 68.5). Pattern 1 tumors were considered the most differentiated with discrete glandular formation, while pattern 5 lesions were the most undifferentiated with strands of disorganized, free-floating cells and complete loss of the glandular architecture. Prostate cancers tend to be heterogeneous, with two or three patterns occurring within a typical prostate. So the final Gleason score is the sum of the grades of the primary (largest) and secondary patterns, ranging from 2 (1 + 1) to 10 (5 + 5).
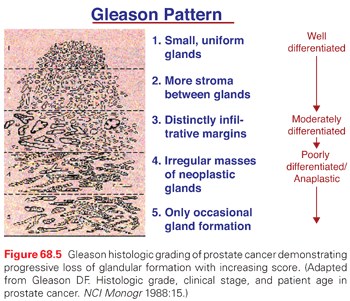
The prognostic importance of Gleason’s scoring system has been difficult to improve on, but the system has been modified several times, most recently by a consensus of expert pathologists, to reflect current data and best practices.55,56 In biopsy specimens, patterns 1 and 2 are almost never recognized, so Gleason 3 + 3 = 6 cancers are the earliest, most well-differentiated tumors currently reported by pathologists. Careful reassessment of the histologic criteria for assigning Gleason pattern 3 has resulted in reclassification of many grade 3 cancers as grade 4, and some grade 4 variants are now considered grade 3. As a result, there has been “grade inflation” over the last decade, and the prognosis for both Gleason 3 + 3/well-differentiated cancers and for 3 + 4/moderately differentiated cancers is better than in historical series.
If three Gleason patterns are seen within a single biopsy, the accepted approach is to designate the largest area as the primary grade and the highest grade as the secondary grade to arrive at a score. So a biopsy with a large area of pattern 3, a smaller area of pattern 4, and an even smaller area of pattern 5 would be designated 3 + 5 = 8. Multiple cores are typically taken during each biopsy session, and the Gleason score assigned to the patient is the score of the highest single core. In contemporary biopsy series, 25% to 50% of tumors are low-grade (Gleason 3 + 3 = 6 or less), 40% to 70% are intermediate grade (Gleason 3 + 4 or 4 + 3 = 7), and 5% to 10% are high grade (Gleason 8 to 10).55
The Gleason grading system is also used to assign grade in RP specimens, with some modifications. When the pathologist inspects all areas of cancer within the prostate, it is not unusual to identify more than two Gleason patterns.57 The original system ignored patterns that represented <5% of the cancer, but the presence of a small amount of high-grade tumor has subsequently been shown to worsen prognosis. The current recommendation is to report a tertiary grade (i.e., 3 + 4 = 7 with tertiary 5).40 Transition zone cancers tend to have lower Gleason grades than peripheral zone cancers of comparable size, and they are less likely to extend to the seminal vesicles or lymph nodes (LN).58 Despite its apparent complexity, the Gleason grading system has proven reliable and reproducible, it is strongly associated with prognosis, and it is accepted worldwide.
Other Histologic Types
Although other tumors and histologic variants of adenocarcinoma rarely develop within the prostate, the most notable include ductal carcinomas (now considered a variant of poorly differentiated adenocarcinoma), small cell or neuroendocrine tumors, and transitional cell carcinomas. Pure ductal carcinomas comprise <1% of prostate cancers, but ductal elements are present in ~5%. These tumors are biologically similar to high-grade prostate adenocarcinomas, are clinically aggressive, and are associated with lower PSA levels than comparable adenocarcinomas.59 Small cell or neuroendocrine tumors of the prostate typically comprise small, round, undifferentiated cells.60 Distinguishing these tumors from lymphomas or round cell sarcomas can be difficult without immunohistochemical analysis.40 Neuroendocrine cells can be found in almost all adenocarcinomas, but they do not affect the biology of the tumor unless they are a large component, in which case the tumors tend to metastasize early and have a poor prognosis. The presence of neuroendocrine cells may raise serum levels of neuroendocrine markers such as chromogranin-A, and the tumors should be treated with immediate chemotherapy as well as androgen ablation (androgen deprivation therapy [ADT]). Transitional cell carcinoma of the prostate is most frequently associated with and may be an extension of bladder cancer. When found in isolation, as a primary tumor on prostate biopsy without an associated bladder cancer, transitional cell carcinoma may be confined to periurethral ducts, but often invades stroma. Treatment may require cystoprostatectomy. Malignant mesenchymal tumors make up <0.3% of prostatic neoplasms, of which rhabdomyosarcomas are most common in younger patients and leiomyosarcomas in older patients. Carcinosarcomas are defined by the coexistence of adenocarcinomas of the epithelial cells, along with malignant mesenchymal elements that have differentiated into identifiable chondrosarcoma, osteosarcoma, myosarcoma, liposarcoma, or angiosarcoma.61 These tumors may be found in previously irradiated patients and are highly resistant to therapy. Metastatic tumors to the prostate include lymphomas, leukemias, adenocarcinomas of the lung, melanoma, seminoma, and malignant rhabdoid tumors, whereas tumors of the bladder and colon may sometimes involve the gland by direct extension.
Screening and Early Detection
The clinical states model (see Fig. 68.1) can also be applied to men without a cancer diagnosis by considering an individual’s need for screening or other diagnostic tests designed to detect cancer on the likelihood that he already has or will develop a clinically significant prostate cancer. Operationally, a “clinically significant” cancer can be defined as one that, left untreated, would lead to symptoms, metastases, or a premature death from cancer—but for each individual, these risks must be balanced against his competing risks of noncancer-related morbidity and mortality, and the risk of suffering harm from overtreatment or unnecessary treatment.
PSA level and DRE are commonly used for screening and early detection, although both are limited by low specificity: only one-quarter of men with an abnormal DRE or a PSA level >3 ng/ml are found to have cancer on biopsy.62,63
Prostate-Specific Antigen: A Powerful Tool for Risk Stratification
PSA is a 28 kDa protein of the kallikrein family, a group of serine proteases whose genes are found on chromosome 19q13. PSA is abundant in seminal fluid, at concentrations up to 3 mg/ml, a million times higher than in serum.64 The enzymatic activity of PSA induces liquefaction of seminal fluid and the release of mobile spermatozoa. PSA is synthesized in the ductal and acinar epithelium and is secreted into the lumina of the prostate gland. PSA is organ-specific but not cancer-specific; normal prostatic tissue (and BPH) produces more PSA per gram than cancer, and well-differentiated cancer produces more PSA than poorly differentiated cancer.65 Under pathologic conditions, PSA is thought to reach the circulation through the disrupted epithelial basement membranes. Circulating levels of PSA are inherently variable, fluctuating spontaneously by 15% from year to year.66 When cancer is present, each gram of tumor raises the serum PSA level above background by approximately 3 ng/ml, whereas each gram of BPH contributes an average of only 0.3 ng/ml. Thus, there is considerable overlap in values between patients with cancer and those with benign conditions such as BPH and prostatitis. Acute urinary retention, urethral catheterization, urinary tract infection, and prostatic manipulation by needle biopsy, or transurethral resection of the prostate (TURP) may raise serum PSA levels dramatically. Performance of DRE does not.
A commonly used threshold for a normal PSA level in adult men is 4.0 ng/ml. But there is no “normal” level; the risk of cancer rises directly with PSA levels as a continuum.67 PSA levels in healthy men vary with age. The population median PSA at age 45 to 50 is 0.6 ng/ml (interquartile range, 0.4 to 1.0), at age 60 the median is 1.1 (interquartile range, 0.6 to 2.0), and at age 70, 1.6 (interquartile range, 0.9 to 2.6).68–70
PSA levels at midlife predict with remarkable accuracy the risk that a man will develop advanced prostate cancer or die of the disease.68,70,71 For example, in the Malmö Preventive Medicine cohort of 60-year-old men followed to age 85, stored blood samples from 1981 were retrieved and analyzed for PSA. Ninety percent of deaths from prostate cancer were in men in the top quartile of PSA levels (>2 ng/ml). In contrast, the risk of death from prostate cancer was only 0.2% by age 85 for those with a PSA below median (<1.1 ng/ml) at age 60.70 PSA levels in men as young as 44 to 50 years were also prognostic, with 81% of advanced cancers diagnosed within 30 years occurring in men with PSA levels above the median (0.65 ng/ml).68
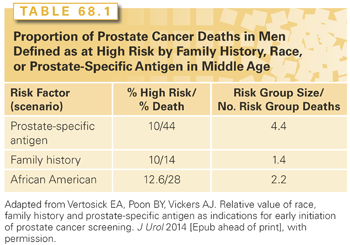
In fact, PSA levels at midlife are more informative than family history or ethnicity (Table 68.1), and can be used to stratify the intensity of screening over the next two to three decades of life, an approach that could substantially reduce false-positive test results without delaying detection of potentially lethal cancers.17,72
Prostate-Specific Antigen for Screening
Although PSA has proved to be a valuable test for early detection, prognosis, and monitoring the response to therapy, its use for population-based screening for prostate cancer remains controversial. The widespread adoption of PSA testing in the United States shifted the stage at diagnosis away from metastases in 20% of patients in the 1980s to 5% in the 1990s, with a corresponding increase in frequency of early-state cancers that are potentially curable with surgery or radiation. Over the last two decades, the age-adjusted mortality rate for prostate cancer in the United States has declined by 42% from its peak in 1992, a more rapid decline than in any other country.2 In the largest randomized trials, PSA screening reduced the risk of dying from prostate cancer by 21% to 44% (29% to 56% among men actually screened).71,73 With long-term follow-up, the number needed to screen to prevent one prostate cancer death declined from 1,410 at 9 years to 293 at 14 years, and is estimated in models to be 98 over the lifetimes of men screened at ages 55 to 69.63,71,74 The number of men who need to be diagnosed or treated (40% were managed expectantly on AS) was estimated to be 48 at 9 years, but falls to 12 at 14 years and 5 over the lifetime of men screened.63,71,74 These numbers compare favorably with other screening programs. With mammography screening from age 50 to 70, 111 to 235 women need to be screened and 10 to 14 diagnosed to avert one death from breast cancer.75–77 For colorectal cancer screening, 850 need to be screened with flexible sigmoidoscopy to prevent one colorectal cancer death.78
Nevertheless, PSA has low specificity: three of four men who have a biopsy for a PSA >3 ng/ml are not found to have cancer,63 and 10% to 56% of those in whom cancer is found would probably have lived out their lives with no symptoms from the disease, and are therefore considered “overdetected.”79 An additional consideration is that the prostate biopsy itself carries a risk of bleeding and infection in 3% to 4% of those undergoing the procedure, which increases with the number of cores obtained. In cases where “saturation” biopsies are performed, in which upwards of 24 to 30 cores are sampled in the same session, the risk is higher and mortalities have resulted. Most cancers detected have been treated immediately with radical surgery or radiation,80 with substantial risk of adverse effects on bowel, urinary, and sexual function.
In screening large populations, the lack of specificity of PSA leads to overdiagnosis, the discovery of incidental, clinically insignificant cancers that pose little or no immediate threat to life or health,81 which often leads to overtreatment with accompanying morbidities that may be permanent and compromise QOL. With rare exceptions, low-risk cancers managed expectantly, as well as intermediate-risk cancers in older men, have a good prognosis when carefully observed on an “AS” protocol, rather than proceeding to immediate treatment.82–86 AS is treatment that is designed to detect changes in the cancer that indicate it has become more aggressive and therefore requires more definitive intervention(s).
But most men with low-risk prostate cancer are treated, especially in the United States,80 with all the attendant risks of bothersome side effects and altered QOL. These findings led the US Preventive Services Task Force (USPSTF) to conclude that “there is moderate or high certainty that this service has no net benefit or that the harms outweigh the benefits” (grade D recommendation).87 Rather than screening all men or no men, a risk-adapted approach is clearly preferable (see “Clinical States Model”).
Screening Trials
Two large, prospective randomized trials of screening for prostate cancer have been published, with conflicting results.79,88 Both studies were recently updated.89,90 The European Randomized Study of Screening for Prostate Cancer (ERSPC) compared screening with PSA every 2 to 4 years to no screening in a core group of 162,243 men age 55 to 69 years in seven European countries. At a median of 9 years, prostate cancer was diagnosed more often in the screened (8.2%) than in the control (4.8%) group (relative risk [RR] = 1.63), while the risk of dying of prostate cancer was reduced by 20% (RR = 0.80; p = 0.04). The number of men needed to be screened to prevent one death from prostate cancer was 1,410 (1,068 among those actually screened), comparable to the data for breast cancer and colorectal cancer screening.75–78 However, the number needed to diagnose to prevent one death was high, 48, probably because the full impact of prostate cancer on mortality was not manifest within 9 years, and because some of the cancers detected were indolent. With further follow-up, the reduction in prostate cancer mortality at a median of 11 years was 21% in the screening arm (p <0.0001), and 29% among those actually screened. The number needed to be screened fell to 1,055 and the number needed to diagnose to 37.
The Göteborg randomized population-based prostate cancer screening trial was planned and initiated independently of the ERSPC in 1995, although the investigators subsequently agreed to include a subset of participants in the ERSPC. In Göteborg, 20,000 men ages 50 to 64 were randomly assigned to be screened with PSA every 2 years up to age 69, or to a control group with no screening. After a median of 14 years, cancer was detected in 12.7% of the screened group and 8.2% of the controls (RR = 1.64), and the risk of death from prostate cancer was reduced by 44% (RR = 0.56; p <0.002) in the screening group (56% among the 76% who were actually screened at least once, RR = 0.44) (Fig. 68.6). At 14 years, the number needed to be screened fell to 293, while the number needed to diagnose to prevent one death from prostate cancer was only 12. (Forty percent of the men diagnosed with cancer were monitored expectantly and not treated.)74
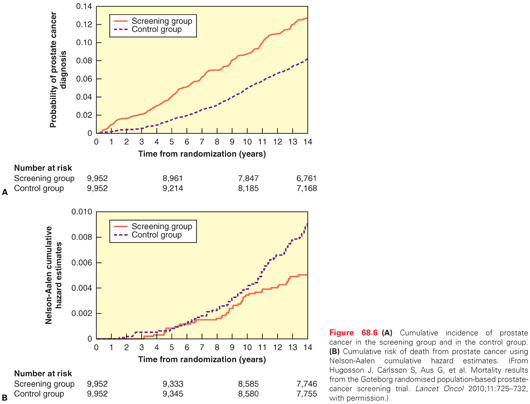
In contrast, screening with PSA and DRE in a US cohort did not reduce mortality from prostate cancer.88,89 The Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial enrolled 76,685 men ages 55 to 74 in a prospective randomized trial from 1993–2001, comparing annual PSA for 6 years and DRE for 4 years with opportunistic screening. At the most recent (13-year) follow-up, the cancer detection rate was slightly higher (RR = 1.12) in the screened arm, but there was no difference in the risk of dying of prostate cancer.
The difference in outcomes of the US and European trials largely stems from the very different contexts in which the trials were conducted. The American trial was initiated in the 1990s, when PSA screening had already become widespread in the United States. In fact, 44% of the PLCO study subjects had had at least one PSA test before randomization, which would have excluded many men with potentially lethal cancers. The mortality rate from prostate cancer in both arms of the PLCO trial (1.7 and 2 per 10,000 person-years in the control and screened arms) was much lower than in the ERSPC (3.9 and 3.2, respectively), suggesting a heavily prescreened population. Many men (45% to 85%) in the PLCO control arm had at least one PSA test after randomization, compared with <20% in the ERSPC control arm, further diluting the potential for a difference between the arms.
Screening Recommendations
The USPSTF’s recent recommendation against screening 87,91 has been understandably criticized as based on a one-time analysis of a rapidly changing field.92 The USPSTF justifiably raised concerns about the high level of overdetection and overtreatment inherent in PSA screening, which can lead to the immediate risks of harm from invasive prostate biopsies and subsequent radical therapy when cancer is found. But the potential harms from PSA screening can be greatly reduced by risk-adjusting screening so that it focuses on men at high risk of otherwise dying of prostate cancer; incorporating into screening newer, more specific biomarkers; and avoiding radical treatment of low-risk cancers.93 In a recently published computer simulation model of PSA screening, prostate cancer mortality was reduced by 28% over the lifetime of men screened annually from ages 55 to 69. Over the lifetime of this population of 1,000 men, only 98 men would need to be screened and five cancers detected (three treated and two managed expectantly) to prevent one death from prostate cancer.94
In response to the changes in the USPSTF recommendations, a number of professional organizations have revised their guidelines for prostate cancer screening. The American Society of Clinical Oncology recommends that men with a life expectancy >10 years have a discussion with their physician about whether screening for prostate cancer is appropriate, including a clear statement that screening may save lives but is associated with harms, including complications from unnecessary biopsies, surgery, or radiation treatments. Men with a shorter life expectancy should not be screened.95 The American Urological Association recommends that men ages 55 to 69 years engage in shared decision making with their physicians and proceed based on each man’s values and preferences. For men younger than 55 years at higher risk (e.g., African American men, or those with a positive family history), the decision about screening should be individualized.96
The American Cancer Society recommends that men have an opportunity to make an informed decision with their health-care providers about whether to be screened. This decision should be made after the patients have received information about the uncertainties, risks, and potential benefits of screening; men should not be screened unless they have received this information. The American Cancer Society further recommends that discussion about screening should take place at age 50 for men who are at average risk of prostate cancer and are expected to live at least 10 more years, and at age 45 for men at high risk of developing prostate cancer (e.g., African American men and those with a first-degree relative diagnosed with prostate cancer at an early age [<65 years]). This discussion should occur at age 40 for men at even higher risk, that is, those with more than one first-degree relative who had prostate cancer at an early age.97
DIAGNOSIS, RISK ASSESSMENT, AND STATE ASSIGNMENT
Signs and Symptoms
The most common symptoms arising from the prostate in men over 40 years are bladder outlet obstruction, including hesitancy; nocturia; incomplete emptying; and a diminished urinary stream. The occurrence of these symptoms, although more commonly related to BPH, should prompt a careful DRE and a PSA determination. The acute development of pelvic or perineal pain, erectile dysfunction, or hematuria should prompt further evaluation of the prostate. Today, men rarely present with symptoms of metastatic disease such as bone pain, pathologic fracture, anemia, or pancytopenia from bone marrow replacement, or disseminated intravascular coagulation.
Digital Rectal Examination
The physical examination should focus on a thorough DRE of the prostate, although palpable nodes can sometimes be detected in the inguinal or supraclavicular areas. Special attention should be paid to detect areas of induration within the prostate, extension through the capsule, or involvement of the seminal vesicles. If there is bladder outlet obstruction, the bladder may be palpable. Although not uniformly accurate or reproducible, DRE results are associated with pathologic stage and prognosis, and are the principal basis for assigning the clinical T stage of the cancer.98
Prostate-Specific Antigen and Related Biomarkers
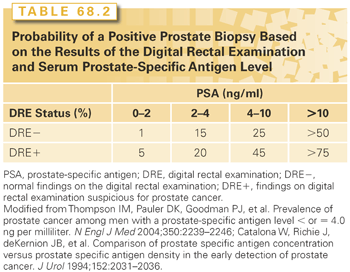
PSA levels are best considered as a continuum: the higher the level, the greater the likelihood that any cancer, or high-grade cancer, will be found on biopsy. The most commonly used threshold for recommending a biopsy is 3 ng/ml.72 A level this high is found in only 5% to 10% of men age 45 to 69 years. As PSA levels vary,66 a rise in PSA to a newly “elevated” level should be verified 2 to 3 months later, after the patient has been evaluated by a medical history, DRE, and appropriate laboratory tests to exclude causes other than cancer for the “rise.” The likelihood of finding cancer is about 15% to 20% in men with a normal DRE and a PSA level between 2.5 to 4 ng/ml. Among men with a PSA level of 4 to 10 ng/ml, 20% to 30% will have cancer. If the PSA level is >10 ng/ml, 60% of men will have cancer on biopsy. Many of these cancers will be low risk and can be managed conservatively without radical therapy.
Higher circulating PSA levels prior to treatment are associated with larger, more extensive cancers, although there may be a wide range of levels within any clinical T, N, or M category.99,100 Although poorly differentiated cancers produce less PSA per gram than well-differentiated cancers, higher PSA levels indicate a more extensive cancer and a poorer prognosis across all Gleason scores. Rare, highly aggressive, poorly differentiated cancers are found in men with low PSA levels (<2 ng/ml), but patients with these cancers usually present with rapidly progressive voiding symptoms and palpably abnormal DRE.
PSA levels rise with age because of age-related increases in prostate volume due to BPH. Adjusting the upper limit of normal for age, PSA should be <2.5 ng/ml for men age 40 to 49 years, <3.5 for men aged 50 to 59, <4.5 for men aged 60 to 69, and <6.5 for men aged 70 to 79.101 The utility of age-specific PSA levels has been challenged in screening trials because sensitivity is lost for a small increase in specificity in older men.102
PSA levels can also be adjusted for the volume of the prostate gland. PSA density (PSAD) is the ratio of PSA to gland volume, measured in ng/ml per cm3.103 As more PSA is released into the serum by cancer (3 ng/g) than by BPH (0.3 ng/g),104 PSAD can help to discriminate cancer from BPH. Because DRE correlates poorly with gland volume, an imaging study (transrectal ultrasound [TRUS] or magnetic resonance imaging [MRI]) is required to measure PSAD accurately, so PSAD is generally useful only in men who have had an ultrasound during a biopsy. PSAD has proved to be more valuable in prognosis than in detection, where it has been largely replaced by the free/total PSA ratio. The percent-free PSA in serum is higher in men with BPH than in men with cancer and can be used to discriminate cancer from BPH. Percent-free PSA values <10% are more indicative of cancer in men with values in the 4 to 10 ng/ml range.62,105
PSA levels rise more rapidly over time in men with cancer than in those without cancer, even within the normal range.106 The rate of change, termed PSA velocity, may indicate the presence of cancer, but normal biologic variations in PSA levels over time create many more false positives and lessen the accuracy of the calculated results.107 Once a man’s PSA level is known, PSA velocity contributes no additional information to predict the presence of a cancer,107 except in the rare case of an unusually aggressive, high-grade cancer that produces little PSA.
Panels of Kallikrein Markers
The major limitation of PSA for screening and early detection of prostate cancer is the high proportion of false-positive tests: 70% to 80% of men with a PSA >3 ng/ml and a normal DRE do not have cancer on biopsy. The specificity of PSA testing can be increased substantially at any given level of sensitivity by incorporating additional kallikreins into a panel of markers. There are two commercially available panels: the 4Kscore test (OPKO Lab, Nashville, TN) and the phi (Prostate Health Index; Beckman Coulter, Brea, CA). To baseline measurements of PSA and free-PSA levels the 4Kscore adds “intact” PSA and hK2, and the phi adds 2(pro)PSA.108–111 All three of these kallikreins are elevated in cancer, relative to BPH. Both the 4Kscore and phi panels increase specificity, reducing the indication for biopsy among men with an elevated PSA level. In published studies, the number of biopsies was reduced by 40% to 50% while missing few high-grade cancers. The 4Kscore preserves sensitivity for high-grade (Gleason ≥7) cancer while reducing the number of negative biopsies and biopsies finding only low-grade (Gleason ≤6), small-volume cancer.110,111
Urinary Molecular Biomarkers
Prostatic fluid may contain shed cells from prostate cancer that can be recognized by measuring the level of RNA for prostate cancer antigen-3 (PCA-3) relative to that for PSA in urinary sediment using reverse transcription–polymerase chain reaction technology. Urinary PCA-3 has been approved by the FDA to determine the likelihood of cancer in men with an elevated PSA level and a previously negative biopsy,112 but is also useful in comparable men with no previous biopsy to avoid unnecessary biopsies. The test requires collection of urine after a prostatic massage by DRE, and the levels of PCA-3 do not reflect the volume, grade, and extent of cancer,113 limiting its clinical utility, especially when the goal is to avoid biopsy in men with only low-risk cancers. Other urinary assays for molecular markers are being explored, including one for the TMPRSS fusion gene, which may be more prognostic than PCA-3.113
Biopsy
Because prostate cancer is rarely curable when it causes symptoms, and rises in incidence with age, detection has focused on evaluating asymptomatic men between the ages of 50 and 70. The principal indications for biopsy are either an abnormal DRE or, more commonly, an elevated PSA level. Any palpable induration should be evaluated further, but only about a third of men with an abnormal DRE prove to have prostate cancer. Similarly, a normal DRE does not exclude the presence of cancer. The likelihood that cancer will be found on biopsy depends on the results of the DRE and PSA test (Table 68.2).67,114
The diagnosis of prostate cancer is typically established by TRUS-guided transrectal needle biopsy. TRUS is most useful for identifying the regions within the prostate for needle biopsy and for determining prostate volume; it is not used routinely for screening. When cancers are seen on TRUS, they are typically hypoechoic relative to normal prostate tissue, but the sensitivity of detection is low and MRI has proven to be more accurate and is the preferred imaging modality for identifying suspicious lesions for TRUS-guided biopsies within the prostate.
A needle biopsy of the prostate is usually performed transrectally with an 18-gauge needle mounted on a spring-loaded gun directed by ultrasound. Any palpable abnormality on DRE should be targeted for biopsy using finger guidance. In addition, abnormal areas visible on TRUS or MRI should be sampled, along with a total of at least 10 systematic biopsies of the prostate taken from the left and right apex, middle, and base of the peripheral zone. Each core or group of cores from a single region should be identified separately as to location and orientation so that the pathologist can report the extent and grade of cancer in each region and the presence of any perineural invasion or extraprostatic extension. Higher Gleason scores are strongly associated with larger tumor volume, extension outside the prostate, probability of metastases, and duration of response to therapy.115,116 Biopsy results are used not only to assign a Gleason score to the cancer, but also to assess the volume and extent of the cancer by determining the number and percent of cores involved by cancer,117,118 the amount of cancer in each core, and the total length of cancer in all cores. Each of these features adds important additional staging and prognostic information.119–122
Because patient selection for AS is critically dependent on the results of the prostate biopsy, some investigators have suggested more extensive biopsy strategies to better assess the true extent of cancer within the prostate. Transperineal “mapping” biopsies use a brachytherapy template, with needle cores taken at 5 mm to 10 mm intervals throughout the gland.123 These template biopsies more accurately reflect the grade and extent of cancer. One study collected a median of 46 individual cores and found bilateral cancer in 55% of patients and an increased Gleason score in 23%. However, the risk of acute urinary retention, hematuria, and erectile dysfunction are increased with mapping biopsy, compared with standard transrectal needle biopsy. Further experience is needed before extensive mapping biopsies can be recommended as routine. Today, more attention is being focused on targeted biopsies of suspicious lesions seen on MRI.124
Imaging for Diagnosis and Staging
The overwhelming majority of men diagnosed with prostate cancer today do not have metastases at the time of diagnosis, so imaging studies to detect metastases are usually not indicated. Neither bone scans nor computed tomography (CT) are helpful for patients with clinically localized cancer unless they have a poorly differentiated tumor (Gleason score 8 to 10) or a PSA >20 ng/ml.125 Consequently, most patients diagnosed with a clinically localized prostate cancer need no further studies to rule out metastases. Patients with very aggressive tumors (PSA >20 ng/ml and biopsy Gleason score >7), advanced local lesions (T3-4), or symptoms suggestive of metastatic disease should have imaging studies, including a bone scan and a CT of the chest, abdomen, and pelvis.
Magnetic Resonance Imaging
With current magnet strengths of 3 Tesla (3T), a multiparametric MRI, which provides T1- and T2-weighted images as well as diffusion-weighted and contrast images, permits excellent visualization of the prostate and surrounding tissues and the pelvic LN (in which case CT imaging of the pelvis is unnecessary).126 The endorectal coil is helpful for enhanced visualization of the internal anatomy of the prostate when the magnetic strength is ≤1.5T, but magnetic resonance spectroscopy is rarely used today despite early promising results. On T1-weighted images, the prostate should appear homogenous and low intensity; cancers are not visible, but high-intensity areas resulting from recent biopsy should be noted to avoid misinterpreting corresponding low-intensity areas on T2 images as malignant lesions. On T2-weighted images, cancers can be recognized by their low signal intensity relative to the normal peripheral zone.
Diffusion-weighted imaging is a promising MRI technique that takes advantage of the known variability of random movements of water molecules observed between normal tissues and tumors. The rate of diffusion of water molecules is more restricted within tumors than in normal tissues and allows for an important metric known as the apparent diffusion coefficient. In one study comparing MRI with combined MRI and diffusion-weighted MRI, the sensitivity and specificity were 86% and 84%, respectively.127 Dynamic contrast-enhancement MRI may also identify malignant lesions within the prostate.128 In one study, the combination of T2-weighted imaging and dynamic contrast-enhancement MRI findings had sensitivity and specificity rates of 77% and 91% for detecting tumor foci that measured 0.2 cm3, but these values improved to 90% and 88%, respectively, when detecting tumors >0.5 cm3.129–131
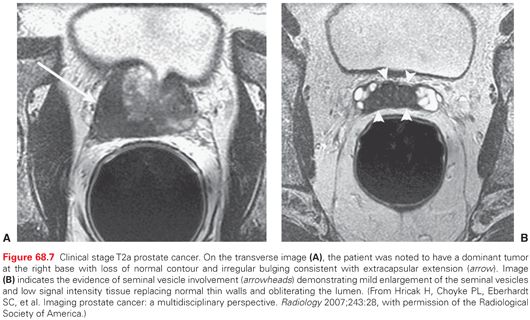
Opinions vary regarding the value of MRI in routine staging and imaging of the prostate, and a wide range in specificity and sensitivity has been reported for the detection of extraprostatic extension and seminal vesicle invasion (SVI). In general, multiparametric MRI permits excellent visualization of the prostate and is more sensitive than DRE, TRUS, and CT for identifying extraprostatic extension and SVI (Fig. 68.7). MRI also allows accurate estimates of the size and shape of the prostate, the proximity of cancer to the neurovascular bundles and the urethral sphincter, the presence of a large anterior tumor that may be invading the anterior fibromuscular stroma or bladder neck, and the length of the membranous urethral sphincter, making MRI a valuable adjunct to the preoperative evaluation of patients with apparently localized prostate cancer.132
Computed Tomography
CT scans of the abdomen and pelvis are ordered far too frequently in the initial evaluation of men with prostate cancer, as they have limited capability to detect cancer within the prostate or the presence of extraprostatic extension or SVI. CT scans can detect LN metastases within the pelvis, but these can be detected equally well with pelvic/prostatic MRI, which provides more information about the primary tumor.
Bone Scan
A radionuclide bone scan is the standard imaging study used to identify the presence of osseous metastases,130 but is not generally indicated in patients with clinically localized cancer because true positive results are much less common than false positives. In patients with a baseline PSA level <10 ng/ml, a bone scan identifies metastases in <1% of men who have no symptoms of bone pain. For patients with PSA levels between 10 ng/ml and 50 ng/ml or >50 ng/ml, the probability of a positive bone scan is 10% and 50%, respectively.131 Bone scans are frequently used to assess the response to hormonal therapy and chemotherapy in men with metastatic disease.
Risk Assessment
Characterization of the Local Tumor
A thorough evaluation of the extent of the local tumor should include a diagram of the area of induration and a recording of the clinical T stage, which reflects the size, location, and extent of the cancer (determined by DRE and imaging), histologic grade (Gleason score) in the biopsy specimen, baseline serum PSA level, and systematic biopsy results. These factors are used to predict pathologic stage, assist in treatment planning, and determine prognosis.
Tumor, Node, and Metastasis Classification
At the time of initial diagnosis, prostate cancers are staged using the TNM classification developed by the American Joint Committee on Cancer and the Union for International Cancer Control.50–52,133,134 We recommend the use of the seventh edition published in 201051 (Table 68.3). With the TNM system, designations for the primary tumor, regional nodes, and distant metastases are noted separately. A distinct category, T1c, is used to describe cancers that are neither palpable nor visible, but were detected by a biopsy performed after an abnormal PSA test or for other reasons. Cancers that are not palpable but visible on imaging such as TRUS or MRI are classified appropriately along with palpable cancers in the T2-4 categories. However, the TNM system does not fully reflect prognosis because it does not include PSA levels, Gleason grade, or the extent of cancer in the biopsy specimen.
Staging Tables and Risk Groups
While individual prognostic factors can be informative, combining multiple factors together produces more accurate estimates of pathologic stage and prognosis. Partin et al.135 developed a nomogram reported as a series of staging tables (Partin tables) that combine clinical tumor stage, biopsy Gleason grade, and PSA to predict pathologic stage. The accuracy of these tables has been widely validated.136
As pathologic stage is only a proxy for prognosis, a classification scheme has been developed to predict the risk of recurrence after treatment of the primary tumor using the same key prognostic factors (clinical stage, Gleason grade, and PSA).137 The D’Amico classification, now adopted by the American Urological Association, assigns patients to one of three logical (rather than empirical) risk groups according to their clinical T stage, Gleason grade, and PSA.137 Although it is intuitive to group patients into such risk-group categories, each “group” actually contains a heterogeneous population.138 For example, patients with a clinical stage T1c, Gleason grade 3 + 3, and PSA 9.9 ng/ml would be classified as low risk, but if the PSA were 10.1 ng/ml, the same patient would be considered intermediate risk. Using categorical values (e.g., PSA 10 to 20 ng/ml) rather than continuous values, and assigning a patient to an increased risk group if any single variable is high (e.g., tumor stage cT2c, Gleason 8 to 10, or PSA >20 ng/ml), is inherently inaccurate. Predictions are much more accurate when nomograms are used to combine individual prognostic factors into a single prognostic score assigned to an individual patient. Consequent comparisons of the results of different treatments are also more accurate when patients are more precisely matched.
Nomograms
Nomograms now widely available to predict prognosis in men with prostate cancer combine clinical and pathologic prognostic factors as continuous rather than categorical variables.139 The prognosis or probability of recurrence after definitive therapy of an apparently localized prostate cancer depends on the clinical stage and grade of the cancer, the number or percent of positive biopsy cores, as well as the PSA level before treatment. Nomograms have proved highly useful in clinical practice and have been developed for external beam radiation therapy (EBRT)140 and brachytherapy as well as surgery.141 These nomograms may provide clues about the relative efficacy of different treatment modalities in patients with comparable tumors. All these nomograms are available at http://www.mskcc.org/cancer-care/prediction-tools (accessed June 13, 2014).
Molecular Profiles
Genomic testing has recently been introduced to characterize the level of aggressiveness of prostate cancer, and, as in breast cancer,142–144 can help to guide treatment decisions.145 There are currently two commercially available genomic tests for risk-stratification of prostate cancer, the Cell Cycle Progression assay (Prolaris, Myriad Genetic Laboratories Inc., Salt Lake City, UT) and the Genomic Prostate Score assay (Oncotype DX, Genomic Health, Redwood City, CA).146–149 Both these tests use reverse transcription–polymerase chain reaction techniques to assay the expression level of a panel of genes that reflect the biologic activity of the cancer relative to the level of housekeeper genes. The Cell Cycle Progression-Prolaris assay was developed on a cohort of men with a wide spectrum of prostate cancer managed conservatively; the molecular profile added significantly to the ability of standard clinicopathologic features (stage, grade, PSA, and extent of cancer in biopsy specimens) to predict time to death from prostate cancer.147 When needle biopsy specimens from men who were candidates for AS were assayed with Genomic Prostate Score-Oncotype DX, the assay independently predicted the risk of adverse pathology (extraprostatic extension or Gleason grade 4 + 3 or greater) in RP specimens. Both tests can successfully assay expression profiles from as little as 1 mm of cancer in an 18-g needle core obtained as long as 10 to 15 years previous to the assay, and both show a wide range of expression levels, and therefore prognoses, within any clinicopathologic risk group.
The clinical utility of these molecular profiles is under active investigation; today, the assays are largely used to recommend AS in men with low- or intermediate-risk, low-volume cancer and favorable expression profiles. Assay results tend to be concordant with the clinicopathologic risk classification in approximately 45% of patients, whereas it is higher or lower in the remaining 55%. In a recent study, 14% of patients considered low risk and suitable for AS were reclassified as higher risk patients for whom active intervention was warranted, and 7% of those with clinically aggressive tumors were reclassified as low-risk and suitable for AS by the Cell Cycle Progression-Prolaris assay.150
Pathologic Stage
Several other indices have been developed that improve the biologic characterization of a given tumor. Pathologic stage, determined by examining the RP specimen, predicts recurrence much more accurately than clinical stage.151 Independent prognostic factors include the level of invasion through the capsule of the prostate, SVI, LN metastases, and positive surgical margins, as well as the Gleason score in the RP specimen and the preoperative serum PSA level. Some investigators have considered tumor volume an important prognostic factor, but others have found that it has no independent prognostic significance. Stephenson et al.152 combined these independent prognostic factors into postoperative nomograms to predict biochemical recurrence (BCR)153 10 years after RP and 15-year cancer-specific survival.139,154 These nomograms are more accurate than the preoperative nomogram, because they incorporate the final Gleason grade and pathologic stage as well as preoperative PSA.154
Stage Assignment
Clinical States Model
Given the range of prognoses among men within each of the TNM-defined stages at diagnosis, a risk-adapted approach to diagnosis and treatment is mandated. To facilitate this approach, a model was developed that divides the disease continuum from prediagnosis to advanced metastatic disease into a series of clinical states. Each state represents a distinct clinical milestone defined by the status of the tumor in the primary site, the presence or absence of metastatic disease on imaging, whether the testosterone levels in the blood are in noncastrate or castrate (<50 ng/dl) range, and prior therapy. This model differs from staging algorithms in that it applies to both the newly diagnosed, untreated patient and to the patient who has received treatment as his disease evolves. Unmet needs in diagnosis, defining treatment objectives, and assessing outcomes vary by clinical state (see Fig. 68.1). Applied clinically to men without a cancer diagnosis, this approach accommodates the need to assess an individual’s cancer based on the risk of harboring or developing clinically significant disease.
In the clinical states model, an individual is assigned to only one state and he remains in that state until his disease has progressed. He can only move forward, never back, even if his disease has been eradicated completely. Each assessment is considered a new evaluation in which the patient’s symptoms and overall tumor status are reviewed, and the decision to offer treatment, and the specific form of treatment, are based on the current state of the disease or the future risk posed by the cancer relative to comorbid conditions.
For example, the rising PSA states include patients who have a rising PSA following treatment of the primary tumor with either surgery or radiation, an indication that the disease has recurred. Issues for these patients include whether the recurrence is systemic or limited to the prostate bed (following surgery) or the prostate itself (following radiation). A more important consideration is whether any treatment is needed, based on the likelihood in the long term that the cancer will cause symptoms, local or distant, or shorten a patient’s life. Patients with rising PSA who are considered to have disease in the prostate bed (or the prostate itself) are discussed in the sections of this chapter that review the primary treatment modalities for prostate cancer. Treatment objectives and the means to assess outcomes vary by clinical state and will be considered separately. The more rapidly the disease is progressing or the more advanced the disease state, the greater the need for treatment.
Principles of Treatment and Assessing Outcomes
Treatment is offered with therapeutic intent that considers (1) why the treatment should be administered, (2) the potential benefits it can provide relative to potential morbidity, and (3) financial cost. Changing established practice paradigms requires demonstrating that a new therapy or approach provides incremental clinical benefit relative to a previous standard (if one exists) or to a suitable control in prospective randomized trials. Clinical benefit in regulatory terms represents an improvement in the way a patient functions or feels, or in how long he survives.155 Examples of clinical benefits include prolonging life, relieving pain or other symptoms, and/or reducing toxicity relative to an established standard. It is also important to demonstrate that a new approach, whether it is a therapeutic procedure or a drug, is safe and well tolerated in an elderly population.
Assessing Treatment Effects
It has long been recognized that the most common manifestations of prostate cancer, which include disease in the primary site, a rising PSA, and metastasis to bone, are not evaluable by the traditional measures of tumor regression used to monitor disease status in other solid malignancies (Response Evaluation Criteria In Solid Tumors [RECIST] 1.1).156 Changes in nodal and visceral sites that can be assessed reliably using RECIST occur less often. To address this unmet need, the Prostate Cancer Working Group (PCWG2) was formed to develop guidelines for clinical trial design in castration-resistant prostate cancer (CRPC) that also apply to earlier disease states.157
In the clinical states framework, treatment objectives are divided into early outcomes representing the control, relief, or elimination of disease manifestations present when a treatment is being considered or initiated, and later outcomes representing the delay or prevention of future manifestations. Existing manifestations may include disease limited to the prostate (localized disease), a biochemical recurrence following local treatment (rising PSA), or cancer in the extrapelvic LN, bone, or viscera with or without a rising PSA (clinical metastases). Potential manifestations that might be delayed or prevented from occurring include growth in the primary site, BCR, growth in an existing site, new sites of spread, pain, or other complications of progressive osseous disease described as skeletal-related events (SRE),158 and death from disease. SREs, first used in the regulatory filing of zoledronate, include fractures, pain requiring a change in therapy, epidural disease, and/or surgery to bone.159
“Control/relieve/eliminate” outcomes representing “response” are reported individually by the change in each disease manifestation present—PSA level, soft tissue disease, bone disease, and/or symptoms when treatment was started—rather than by grouped categorizations, such as complete and partial remission, that include all sites of disease. For example, a key PSA-based outcome for patients treated with RP is the proportion of patients who achieve an undetectable PSA level postoperatively, whereas for patients with CRPC treated with a systemic approach, a waterfall plot showing the percent change from baseline for each patient treated, or the proportion of patients achieving a defined degree of decline (e.g., ≥50%), is more informative. “Delay/prevent” outcomes focusing on time-to-event progression are also measured individually. For a patient with localized disease, outcomes from surgery or radiation include a response measure that demonstrates whether the disease was eliminated completely (e.g., a negative biopsy of the prostate at a certain time after radiotherapy) or time-to-event outcomes that include time to PSA recurrence, metastases, symptoms, or death from disease. This approach to evaluating outcomes enables the physician to focus on the specific therapeutic objective for a single patient or group of patients in a given disease state, rather than on changes in other, less-relevant measures, and at the same time enables clinical trial investigators to explore the relation between changes in individual disease manifestations and other measures of clinical benefit such as overall survival.
Clinically Localized Disease
The clinical course of newly diagnosed prostate cancer is difficult to predict. Men with similar clinical stage, serum PSA levels, and biopsy features can have markedly different outcomes. Although prostate cancer is unequivocally lethal in some patients, most men die with, rather than of, their cancer. The challenge to physicians is to identify those men with aggressive, localized prostate cancer with a natural history that can be altered by definitive local therapy, while sparing the remainder the morbidity of unnecessary treatment. Not all men with clinically localized prostate cancer require or benefit from therapy. Depending on the characteristics of their cancer, their age, and comorbidities, some men would benefit greatly by aggressive treatment, whereas others would suffer harm.
Well-established prognostic factors for clinically localized prostate cancer include age, PSA level, clinical stage based on DRE and imaging, Gleason grade, and extent of the cancer on biopsy. Prognosis can be estimated more accurately by combining these risk factors into nomograms99 that calculate the probability of a clinically important endpoint, such as freedom from BCR 10 years after surgery153 or cancer-specific survival 15 years after surgery.139
Active Surveillance
AS is a planned treatment of monitoring a patient with a potentially curable prostate cancer based on the likelihood that the cancer will progress, delaying active treatment until signs of progression to a more aggressive, potentially lethal cancer are detected. AS attempts to avoid the adverse effects of treatment in the majority of men, intervening with curative therapy for selected men only for specific indications. AS is now widely recommended for most men with low-risk cancer, based on the lack of survival benefit of immediate surgery versus observation at 12 years in the PIVOT trial160 and the low risk of prostate cancer death at 10 years in large phase 2 studies of AS.84 In this and other AS trials, the risk of progression or treatment for men with low-risk cancer is about 20% to 40% within 5 years and 35% to 60% within 10 years, depending on the initial eligibility criteria and the indications for delayed intervention.
A recommendation for AS assumes that the risk posed by the cancer at diagnosis can be assessed accurately, that progression can be identified by regular monitoring, and that deferring treatment until it is necessary will offer cancer control and survival rates similar to immediate treatment. Achieving all of these goals has not yet been realized. Standard assessment at diagnosis includes PSA, clinical stage, Gleason grade, and extent of cancer in biopsy results. This limited evaluation underestimates the grade and extent of the cancer in 15% to 25% of patients.161 A multiparametric MRI of the prostate can detect large, more aggressive cancers in some patients, and these findings can be confirmed by biopsies targeting the suspicious lesions.162 Alternatively, one can depend on annual repeat biopsies163 or PSA velocity84 to detect progression in time for effective intervention. In either case, patients under AS must accept frequent, regular, detailed evaluations of the status of their cancer for as long as they are healthy and young enough to be candidates for definitive therapy. Patients under AS are generally monitored every 6 months with DRE and measurement of free and total PSA, with repeat imaging and biopsy every 2 to 3 years after the baseline evaluation. The goal is to detect progression of the cancer while cure is still possible.
Outcomes of Active Surveillance with Selective Delayed Intervention
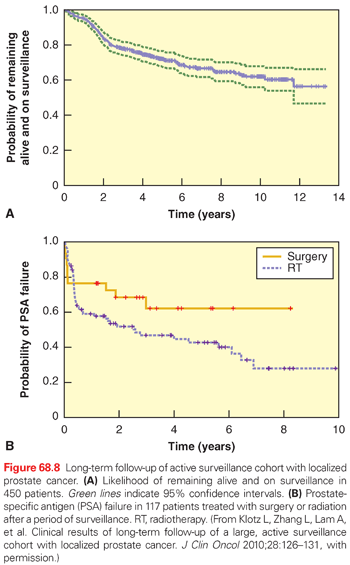
There are few reports of long-term outcomes of AS for localized prostate cancer. Klotz et al.84 conducted a prospective phase 2 study of AS in men with low-risk cancer (or those over 70 with Gleason 7 [3 + 4] and PSA <15 ng/ml). The indications for intervention were a short PSA doubling time (PSADT) or grade progression on repeat biopsy. At 8 years, overall survival was 85% and disease-specific and metastasis-free survival 99%. Some 25% of patients were treated within 5 years, and 40% by 10 years (Fig. 68.8). Delayed intervention was associated with a relatively low rate of cancer control, raising concerns that treatment for men with a short PSADT (<2 years) was too late.
Carter et al.96 observed a cohort of 769 men with very-low-risk prostate cancer and low PSA density (<0.15) from 1 to 15 years after diagnosis with DRE, measurements of total and free PSA every 6 months, and an annual surveillance biopsy. Treatment was recommended based on biopsy changes (any Gleason pattern 4 or 5, more than two cores that were positive for cancer, or >50% of any one core that was involved with cancer) or if a patient requested a change in management, but not for changes in PSA alone. A total of 41% of patients had been treated at 5 years, and 59% at 10 years, and the median time to treatment was 6.5 years. Cancer control after delayed therapy was excellent. The large number of men who required delayed intervention suggested that many tumors were underestimated at baseline, and the high cure rates at delayed intervention call into question the clinical significance of the criteria for delayed intervention. Many physicians now recommend more comprehensive initial evaluation of candidates for AS with multiparametric MRI and/or early confirmatory biopsies.164
These and other studies confirm that in appropriately selected men, AS is associated with an extremely low rate of progression to metastatic disease and/or death, and that the majority of patients do not require intervention over the first decade. Further follow-up is necessary to determine the long-term risk of progression. The National Comprehensive Cancer Network recommends AS as the appropriate initial management strategy for patients with low-risk cancer and a life expectancy <10 years, as well as for patients with very low-risk cancer and a life expectancy <20 years.125
Radical Prostatectomy
The modern anatomic technique for RP was developed nearly 35 years ago and has proven safe and effective in many large cohort studies and randomized clinical trials. The retropubic technique originally described by Walsh165 for open surgery is equally suitable for laparoscopic and robot-assisted RP. Initially focused on patients with early-stage, organ-confined cancers, RP with pelvic LN dissection (PLND) is now recommended primarily for patients with aggressive cancers (intermediate- and high-risk), whereas low-risk tumors are generally managed with AS (see previous discussion). Because of the risk inherent in major surgery, RP should be reserved for patients without serious systemic comorbidity. Although the risk of recurrence after RP rises with higher clinical stage, Gleason grade, and serum PSA level, no absolute cutoff values exclude a patient as a candidate.
Surgical Technique. The goals of modern RP are to remove the entire cancer with negative surgical margins, minimal blood loss, no serious perioperative complications, and complete recovery of continence and potency. Achieving these goals requires careful surgical planning. Because no single test provides a reliable estimate of the size, location, and extent of the cancer, we rely on the results of DRE, serum PSA levels, and a detailed analysis of the amount and grade of cancer in each individually labeled biopsy core, along with multiparametric MRI. The results are used to plan the steps necessary to remove the cancer completely and to assess the likelihood that one or both of the neurovascular bundles will have to be resected partially or fully to minimize the risk of a positive surgical margin. The retropubic procedure is performed either through a suprapubic incision (open RP) or using a minimal access (laparoscopic or robot-assisted laparoscopic) approach. The operation should be exactly the same internally, regardless of the method of access.
Selecting Patients for Pelvic Lymph Node Dissection
Cancer that has spread to the pelvic LN carries a worse prognosis than when the nodes are negative, enhancing accurate staging. However, the therapeutic benefit of PLND is uncertain. Overall rates of pelvic LN metastases found at RP vary from 2% to 35% depending on the extent of the node dissection, whether the cancer was discovered after screening, and the stage and grade of the cancer.166 Men with low-risk screen-detected cancer are rarely found to have nodal metastases (0.5% to 2%),166–168 so PLND is generally unnecessary, but it may be indicated if imaging studies or intraoperative findings suggest a more advanced cancer. The limited PLND commonly performed today, especially with robot-assisted RP, has underestimated the rate of nodal metastases.169 In men with intermediate-risk prostate cancer, LN metastases are found in 5% (screen-detected) to 20%, whereas in men with high-risk cancer, the rates are 20% to 50%, respectively, when a full or extended PLND is performed.166,170–173 The incidence of LN metastases increases with increasing PSA, clinical stage, and Gleason score.168 Our current practice is to restrict PLND at the time of RP to men with a ≥2% risk of positive nodes according to a contemporary nomogram.174 Even so, controversy persists concerning the role of PLND in patients with prostate cancer.
Limited Versus Extended Pelvic Lymph Node Dissection
No prospective studies have demonstrated the optimal anatomic limits of a PLND for prostate cancer. However, lymphatic drainage of the prostate is known to be highly variable and involves regions not sampled during an external iliac–only PLND.166 Some surgeons resect only the external iliac LN unless imaging suggests abnormal LN in other regions, whereas other surgeons routinely perform a more extensive dissection that includes the obturator, external iliac, and hypogastric areas.175 No sentinel LN has been identified in prostate cancer. The more extensive the PLND, the greater the number of LN removed, and the greater the number of positive nodes.166,168,171–173 Nevertheless, the total number of positive LN is one in 50% of patients, two in 30%, and three or more in only 20% of patients who have an extended LN dissection.166 A PLND that includes the external iliac, hypogastric, and obturator node packets is feasible in both open and minimally invasive RP, and carries no greater risk than a PLND limited to the external iliac nodes alone.
Therapeutic Benefit of Pelvic Lymph Node Dissection
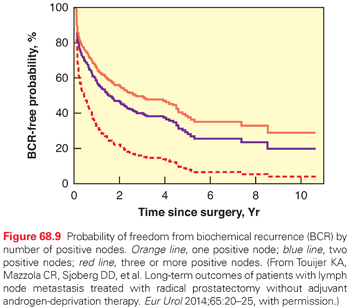
Evidence from several surgical series of patients undergoing RP demonstrates a potential therapeutic benefit of extended PLND, particularly in men with only one or two positive nodes and Gleason score ≤7 in cancer identified in the RP specimen (Fig. 68.9).170–172,176–178 In a series of patients with positive LN treated at Johns Hopkins Hospital, men who had an extended PLND were less likely to develop BCR at 5 years, compared with men who underwent a limited PLND when 15% or fewer of the removed LN were involved.170 In the Memorial Sloan Kettering Cancer Center (MSKCC) series, 25% to 30% of patients with positive nodes remained free of BCR 10 years after surgery with no additional therapy.179
Radical Prostatectomy: Surgical Approach
RP is one of the most complex operations performed by urologists. The outcomes—cancer control, urinary continence, and erectile function—are exquisitely sensitive to fine details in surgical technique. No surgeon achieves perfect results, and outcomes vary dramatically among individual surgeons.180,181 Technical refinements have resulted in lower rates of urinary incontinence and higher rates of recovery of erectile function, less blood loss and fewer transfusions, shorter hospital stays, and lower rates of positive surgical margins. Laparoscopic and robot-assisted RP promised better cancer control and functional recovery, but numerous studies have confirmed that the only consistent advantages of “minimally invasive” surgery are shorter hospital stays and fewer blood transfusions.182 A thorough understanding of periprostatic anatomy and vascular control by contemporary surgeons further increases the probability of a successful RP with reduced morbidity.
Open Radical Prostatectomy
Acute Postoperative Complications. Refinements in anesthesia, perioperative care, and surgical technique have decreased blood loss, length of hospital stay, complications, and mortality after open surgery.183 The mortality rate ranges from 0.16% to 0.66% in modern series, rising with increasing age and comorbidity. Deep venous thrombosis and pulmonary embolism occur in approximately 2% of cases, with little evidence that anticoagulants or sequential pneumatic compression are preventive. Early ambulation and shorter hospital stays are likely responsible for the lower rate of thromboembolic events. Routine perioperative anticoagulation is not used because of the increased risks of bleeding and lymphocele. Rectal injuries are uncommon. Standardized treatment pathways have been shown to decrease the cost of radical retropubic prostatectomy without compromising quality of care. Hospital stays now average 2 days for open RP and 1 day for robot-assisted RP.
Robot-Assisted Radical Prostatectomy
Surgeons have demonstrated that robot-assisted RP (RALP) can be performed with excellent results in the hands of experienced surgeons. The initial enthusiasm for RALP was based on the idea that less bleeding and a magnified surgical image would markedly improve patient outcomes, which has not been borne out in carefully performed population-based studies.182,184,185 Open RP and RALP each have a number of theoretical advantages and disadvantages (Table 68.4).186 No prospective randomized trials have yet compared the two techniques, and it is now clear that variations in outcomes among individual surgeons are much greater than variations between technologic approaches.181 Hu et al.184 have suggested that the rapid increase in utilization of minimally invasive RP despite insufficient data demonstrating superiority over the well-established open operation may be a reflection of a society and a health-care system enamored of a new technology that has increased health-care costs but has yet to uniformly realize marketed or potential benefits during early adoption. As with open surgical techniques, laparoscopic RP and RALP outcomes, including surgical margin status, continence, and potency, reflect surgical technique (the actions and expertise of the surgeon) more than surgical approach. Current data suggest that the best way to improve outcomes after RP is to have the procedure performed by a skilled surgeon, regardless of the approach he or she uses.181,187
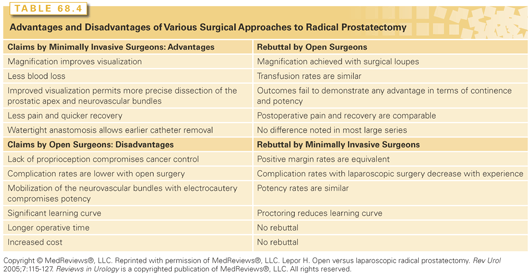
Cancer Control with Radical Prostatectomy
Benefits of Surgery Relative to Active Surveillance. The most compelling evidence that selected patients with prostate cancer benefit from active treatment compared with watchful waiting comes from the Scandinavian randomized trial (SPCG-4) of 695 unscreened men with clinically localized prostate cancer.188,189 Over 23 years of follow-up, RP (compared with watchful waiting) reduced the risk of death from any cause by 29% and risk of death from prostate cancer by 44% (an absolute difference of 11%). The need for subsequent ADT was reduced by 51%, and clinical local recurrence was reduced by 66%. At 18 years of follow-up, the number needed to treat to prevent one death from prostate cancer was eight overall and four in men under age 65. This elegant study firmly documents the overall benefit of RP in patients with clinically localized prostate cancer diagnosed in the absence of systematic screening.188,189
In a population of men subjected to widespread PSA screening, the benefit of surgery for prostate cancer was tested in the Prostate Cancer Intervention Versus Observation Trial (PIVOT).160 This trial was conducted in the United States and randomly assigned 731 men with clinically localized prostate cancer to RP or observation. The mean age was 67, the median PSA level was 7.8 ng/ml, and approximately three-quarters of the men had a biopsy as a consequence of an elevated PSA; half had no palpable tumor (cT1c) and 70% had low-grade (Gleason ≤6) cancer on biopsy. With a median of 10 years of follow-up, 48% of the patients had died, but only 7% had died from prostate cancer. There were no differences in overall or cancer-specific mortality between the two arms of the trial. But there were clear indications that RP reduced the risk of dying of cancer in the subset of men who had aggressive cancers, including those whose PSA was >10.0 ng/ml and those with high-risk cancers.
Taken together, these two trials indicate that most men with cancers detected without screening and those with screen-detected intermediate- and high-risk cancers have less risk of metastases and of death from prostate cancer when treated with early RP than with observation alone. In contrast, men with screen-detected low-risk cancer can be managed safely with AS and do not need immediate surgery or radiotherapy. Life expectancy should be considered in the choice of immediate therapy or AS, as the risks of RP increase and benefits decline progressively with age and comorbidity.190 A UK-based trial (ProtecT) is currently assessing treatment versus no treatment in a PSA-screened population in >1,500 patients.191
Progression rates after RP depend on the clinical stage, biopsy Gleason score, and serum PSA level before surgery, as well as pathologic findings in the surgical specimen. After RP, the PSA level should become undetectable. Cancer control, as measured by freedom from BCR, is excellent after RP and is reproducible among many large series (Table 68.5).179,192–196 Of 12,086 patients treated with RP between 1966 and 2003, 69% to 84% were free of progression at 5 years, and 47% to 78% at 10 years.179,192–196
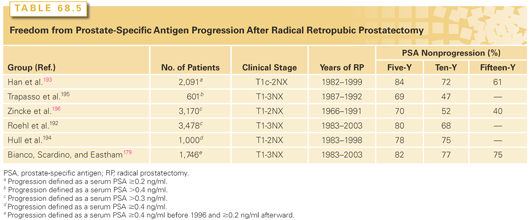
Fifteen-year outcomes have been reported after RP based on preoperative and pathologic factors (Table 68.6). Bianco, Scardino, and Eastham179 calculated the risk of recurrence in 1,743 consecutive patients with clinical stage T1-T3, N0 or X, M0 cancer treated with RP and followed with serum PSA levels for a mean of 72 months (range, 1 month to 240 months). Failure after RP was defined as a rising serum PSA >0.2 ng/ml, clinical evidence of local or distant recurrence, or the initiation of adjuvant radiotherapy or hormonal treatment. At 5 years 84% of patients, at 10 years 78%, and at 15 years 73% were free of progression (see Table 68.5).
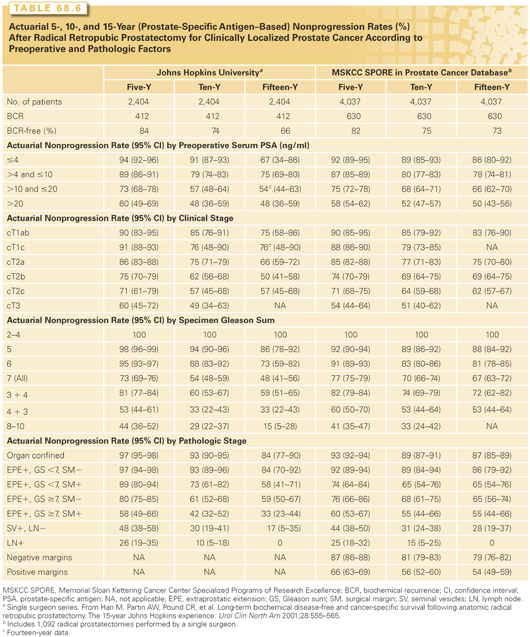
Of particular interest are patients with high-grade cancers (Gleason sum 7 to 10). Of patients with Gleason 3 + 4 = 7 cancers in the RP specimen, 68% were free of progression at 15 years. When the tumor was Gleason 4 + 3 = 7, 51% were free of progression at 15 years.179 Even patients with Gleason sum 8 to 10 cancers fared well, with 27% free of progression at 10 years. These progression rates are substantially lower than the 15-year cancer mortality rates reported for patients with Gleason sum 7 to 10 cancers managed with watchful waiting.197
Once the prostate is removed, the most powerful prognostic factor is the pathologic stage (see Table 68.6). When the cancer is confined to the prostate (defined as cancer not extending into the periprostatic soft tissue), 92% to 98% of patients remain free of progression at 5 years and 88% to 96% remain free 10 years after RP.194 Focal penetration through the capsule into the periprostatic soft tissue alone, in the absence of SVI, results in a 73% 10-year nonprogression rate. Established (extensive) penetration through the prostatic capsule into the periprostatic soft tissue, in the absence of SVI, results in a 42% 10-year nonprogression rate. Even some patients with SVI (pT3cN0) can be cured with surgery, with 30% being free of disease recurrence at 10 years (see Table 68.6).
The slow clinical progression of prostate cancer after RP has led to the widespread use of PSA recurrence as the primary end point for evaluating treatment outcome. However, doing so ignores the fact that the prognosis of men in the rising PSA state is highly variable, and that the rising PSA by itself does not necessarily mean that a patient will develop metastatic disease, develop symptoms, or die of his cancer. For many, the threat posed to a man’s duration and quality of survival is limited at best.
Reports of long-term, prostate cancer–specific survival rates after RP have clearly shown that many patients with PSA recurrence live out their lives free of cancer.179,193,194,196,198 The long-term risk of prostate cancer–specific mortality (PCSM) after RP for patients treated in the era of widespread PSA screening has recently been estimated based on a multi-institutional cohort of 12,677 patients treated with RP between 1987 and 2005.139 Fifteen-year PCSM and all-cause mortality were 12% and 38%, respectively. The estimated PCSM ranged from 5% to 38% for patients in the lowest and highest quartiles of nomogram-predicted risk of PSA-defined recurrence (Table 68.7). Only 4% of contemporary patients had a predicted 15-year PCSM of >5%.
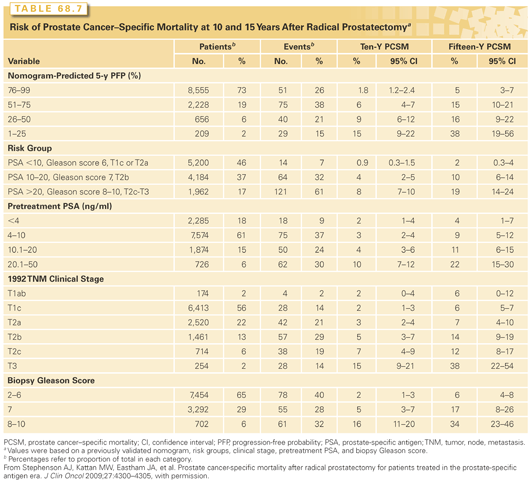
Clearly, few patients will die from prostate cancer within 15 years of RP, despite the presence of adverse clinical features. It is not known whether this favorable prognosis is related to the effectiveness of RP (with or without secondary therapy) or to the low lethality of cancers detected by early screening. Although year of surgery, biopsy Gleason grade, and PSA level are associated with PCSM risk, an individual patient’s risk cannot be predicted on the basis of clinical features alone. Further research is needed to identify novel markers specifically associated with the biology of lethal prostate cancer.
High-Risk Prostate Cancer
Monotherapy is often believed to be inadequate for high-risk cancers, and some clinicians are reluctant to consider RP in high-risk patients. However, there are no standardized criteria to define high-risk before definitive treatment. Among 4,708 patients undergoing RP, high-risk patients were identified based on eight established definitions, and their pathologic characteristics and PSA outcomes were examined (Table 68.8). Depending on the definition used, high-risk patients composed 3% to 38% of the study population. Among patients defined as high-risk, 22% to 63% of tumors proved to be confined to the prostate on pathologic examination. Although high-risk patients had a 1.8- to 4.8-fold increased hazard of PSA relapse, their 10-year relapse-free probability after RP alone was 41% to 74% (see Table 68.8).199 Disease-specific survival at 12 years was between 78% to 94% (Table 68.9).200 Of the high-risk patients who relapsed, 25% (across all definitions) relapsed more than 2 years after surgery, and in 26% to 39% the PSADT at recurrence was ≥10 months. These results show that the commonly used definitions of high risk have the potential to deny patients potentially curative treatment. New criteria are needed to identify those patients who need the integration of systemic therapy to improve outcomes beyond what can be achieved with monotherapies directed to the prostate itself.201
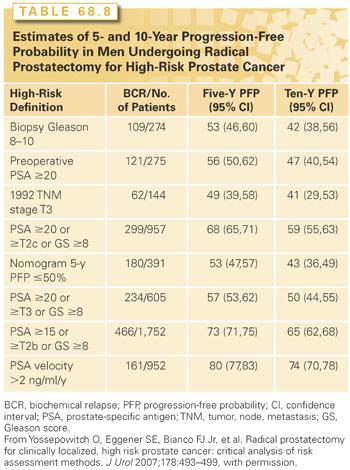
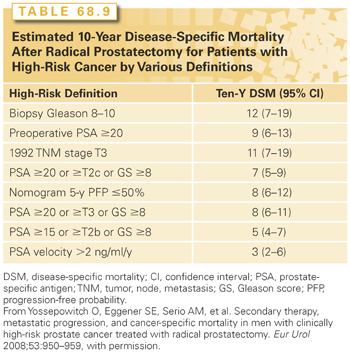
Impact of the Surgeon on Outcomes After Radical Prostatectomy
Recent research has focused on the ways in which surgical volume and the individual surgeon influence results after RP. Begg et al.202 used the Surveillance, Epidemiology, and End Results–Medicare-linked database to evaluate health-related outcomes after RP. The rates of postoperative complications, late urinary complications (strictures or fistulas 31 days to 365 days after the procedure), and long-term incontinence (>1 year after the procedure) were inferred from the Medicare claims records of 11,522 patients who underwent RP between 1992 and 1996. These rates were analyzed in relation to hospital volume and surgeon volume (the number of procedures performed at individual hospitals and by individual surgeons, respectively). Neither hospital volume nor surgeon volume was significantly associated with surgery-related death. Significant trends in the relation between volume and outcome were observed with respect to postoperative complications and late urinary complications. Postoperative morbidity was lower in very-high-volume hospitals than in low-volume hospitals (27% versus 32%; p = 0.03) and was also lower when the prostatectomy was performed by very-high-volume surgeons than when it was performed by low-volume surgeons (26% versus 32%; p <0.001). The rates of late urinary complications followed a similar pattern. Results for long-term preservation of continence were less clear-cut. In a detailed analysis of the 159 surgeons who performed a high or very high volume of procedures, wide surgeon-to-surgeon variations in clinical outcomes were observed, and these variations were much greater than would have been predicted on the basis of chance or observed variations in the case mix. These findings suggest that, in general, high-volume surgeons have superior results compared with low-volume surgeons, in terms of early postoperative morbidity and urinary complications after RP. However, the much better than anticipated outcomes among the highest-volume surgeons suggest that individual surgical technique also influences clinical outcomes.
Individual patient data from four institutions was used to study the association between a surgeon’s prior experience and BCR after RP (the principal reason that patients visit an oncologic surgeon).181 This relationship has often been termed the learning curve and probably reflects differences in surgical skill. A retrospective cohort study of consecutive patients treated from 1987 to 2003 was conducted at four academic, tertiary referral centers in the United States. In this study, 7,850 patients with localized prostate cancer received no neoadjuvant therapy and underwent open radical retropubic prostatectomy by 1 of 73 different surgeons.179 For each patient, surgeon experience was coded as the total number of RPs conducted by the surgeon prior to the incident case, and cancer control was defined as a corroborated rising PSA level >0.4 ng/ml. The study demonstrated that cancer control improved with increasing surgeon experience (Fig. 68.10), and this relationship181 remained highly significant (p <0.001) after adjustment for tumor characteristics and year of surgery. The learning curve for cancer control after radical retropubic prostatectomy was steep but did not begin to plateau until a surgeon had completed approximately 250 prior operations. Five-year probability of BCR was 17.8% for patients treated by surgeons in the early phase of their career (10 prior operations), compared with 10.9% when surgeons had performed 250 operations (absolute risk difference, 6.9%; 95% confidence interval [CI] = 4.3% to 9.5%) (see Fig. 68.10). We saw no evidence that patient risk attenuates the learning curve: there was a statistically significant association between BCR and surgeon experience in all analyses. The relative risk for a patient receiving treatment from a surgeon with 10 rather than 250 prior RP was 2.5, 1.8, and 1.3 for low-, medium-, and high-risk patients, respectively.
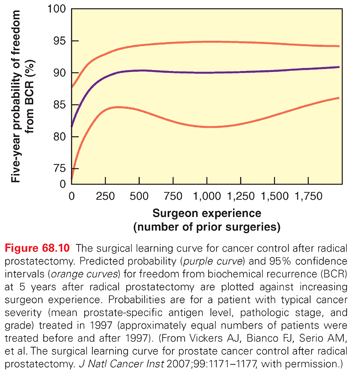
Radiotherapy for Localized Prostate Cancer
Radiotherapy given by external beam or brachytherapy techniques is a second treatment option for men with clinically localized prostate cancers. With the availability of sophisticated treatment planning systems and image-guided approaches, there have been significant advances in radiotherapy enabling higher doses to be delivered more safely, and concomitant improvements in disease-free survival outcomes. Intensity-modulated external beam radiotherapy (IMRT) has become a standard mode of treatment delivery and has facilitated the application of higher radiation dose levels of ≥80 Gy, with lower risks of late rectal and urinary toxicities. Such treatments coupled with image guidance have enabled the routine use of tighter margins, incorporating less normal tissue within the high-dose region and leading to further decrements in late toxicities. Brachytherapy using permanent or temporary radioactive implants within the prostate alone or combined with IMRT is another commonly used radiotherapeutic approach. This approach has also improved with more accurate image guidance and intraoperative real-time planning for brachytherapy, resulting in more highly conformal dose distributions, which in turn has resulted in better outcomes and lower toxicity rates, relative to what has been previously reported. In some cases, ADT is used in several contexts: to improve local eradication of locally advanced tumors by reducing tumor size, to eliminate tumor clonogens inherently resistant to radiotherapy by impairing DNA repair pathways, and/or reducing prostate volumes by 30% to 40%, which improves the ability to deliver maximal radiation dose levels without exceeding the tolerance for the surrounding normal tissue. Hormone therapy also has a favorable effect on micrometastatic disease that may be present at the time of diagnosis in men with high-risk tumors.
Although there are no randomized trials comparing modern, sophisticated high-dose EBRT to brachytherapy for the treatment of localized prostate cancer, there are several criteria used to help select the most appropriate form of therapy for the patient. For patients with low-risk disease in whom AS may not be considered—owing to PSA velocity, the presence of a dominant lesion on imaging studies, or a larger volume of disease determined by biopsy—high-dose IMRT alone (in the range of 80 Gy) or brachytherapy alone are excellent treatment options.
Larger prostate volumes, the presence of urinary obstructive symptoms, and medical comorbidities may influence the selection away from a brachytherapy-based treatment. In contrast, brachytherapy may be the preferred choice for patients without these factors because of its excellent ablative capabilities (leading to long-term PSA relapse-free survival outcomes) and its convenience (as a treatment accomplished in a single outpatient setting). For patients with intermediate- and higher-risk disease, combined modality treatments including brachytherapy and supplemental IMRT are preferred to allow for the delivery of a high and concentrated dose of radiation to the prostate. Ultrahypofractionated EBRT regimens have generated increasing interest as another therapeutic option for patients with localized disease; however, the long-term results of this approach are not available, and the results of ongoing trials are awaiting more mature follow-up.
External Beam Radiotherapy
Intensity-Modulated Radiotherapy and Image-Guided Techniques
IMRT, a type of conformal radiotherapy (CRT), has become a standard mode of treatment planning for patients with localized prostate cancer and takes advantage of inverse-planning methods to optimize dose distribution. Inverse planning is part of a mathematical optimization algorithm that creates a treatment plan based on predefined, desired dose-distribution parameters for the target and dose constraints imposed on the normal tissues. The highly conformal radiation beam is produced with the ability to vary the intensities of the X-rays from each treatment field over the entire cross-section of the beam.
More recently, image-guided approaches have enhanced IMRT delivery. Acquisition of CT images or kilovoltage images on the treatment machine immediately before the radiation treatment allows for visualization of the target and correction of its position, which can fluctuate on a daily basis, related to bladder or rectal filling. Linac-based kilovoltage image guidance systems have become commercially available; they possess capabilities for kilovoltage two-dimensional projection imaging (radiographs), fluoroscopy, and three-dimensional cone beam CT, and are thus ideally suited for monitoring inter- and intrafractional motion. The use of image-guided radiotherapy and enhanced target localization could lead to a further reduction of safety margins and a decrease in morbidity related to ultrahypofractionated regimens. A recent retrospective report demonstrated reduced urinary-related treatment toxicities and improved tumor control outcomes among patients with clinically localized prostate cancer treated with image-guided approaches, compared with patients not treated with image-guided therapy.203 Among a cohort of patients treated to dose levels of 86.4 Gy, the incidence of late grade 2 urinary toxicities was 20% for those treated with daily image guidance, compared with 10% for patients treated with daily image guidance and target positional corrections (p = 0.02)
Definition of Target Volume
The multifocal nature of prostate cancer and the well-documented risk of microscopic ECE, even for patients with early clinical stages of disease, are important considerations that influence the design of the target volume for radiation treatment planning. The clinical target volume (CTV) includes the entire prostate gland and immediate periprostatic tissues, as well as the seminal vesicles, as visualized on CT. For patients with low-risk disease with unremarkable imaging studies, the CTV may exclude the seminal vesicles, owing to the low likelihood of disease involvement. The planning target volume places an additional margin around the CTV to take into account patient setup uncertainties and organ motion. With the use of image-guided approaches, the margin can be safely reduced to 5 mm to 6 mm around the CTV, except at the prostate-rectal interface, where an even tighter margin (3 mm to 5 mm) is used.
Role of Dose Escalation in Patients with Clinically Localized Disease
Findings from several randomized phase 3 trials (Table 68.10)204–208 and the long-term results of single-institution studies209–212 demonstrate a significant improvement in treatment outcomes with higher radiation doses in patients with clinically localized disease. As shown in Table 68.10, these studies have generally demonstrated a 10% to 20% improvement in 5- to 10-year PSA survival outcomes when higher doses of 78 Gy to 80 Gy are applied, compared with dose levels of 70 Gy, and such benefits have been observed for low-, intermediate-, and high-risk cohorts. Although overall survival benefits have not been demonstrated with dose escalation, improvements in distant metastases–free survival are emerging with longer follow-up, suggesting that survival benefits will be seen as these studies mature. In one report, a reduction in death due to prostate cancer was noted at 10 years for intermediate- and high-risk patients treated with doses of 78 Gy, compared with those treated at 70 Gy.213
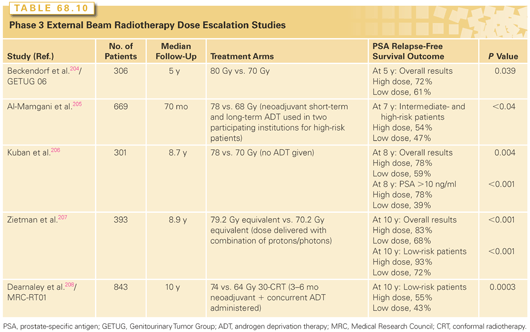
For patients with intermediate- and especially high-risk disease, doses beyond 80 Gy may be required to achieve optimal tumor control outcomes. To do so requires ultra-high-dose IMRT, which constrains the dose delivered to normal tissues, such as the bladder and the rectum. In a recent update of the long-term outcomes of ultra-high-dose IMRT at MSKCC, 1,002 patients treated with 86.4 Gy, using a five- to seven-field IMRT technique, had 7-year PSA-relapse-free survival rates of 99%, 86%, and 68% for low-, intermediate-, and high-risk patients, respectively. The incidence of distant metastases for these respective risk groups was 0.5%, 6%, and 18% at 7 years, and incidence of grade 3 rectal and urinary toxicities was 0.7% and 2.2%. The median follow-up was 5.5 years.214
Sequelae of External Beam Radiotherapy
Complication rates after EBRT vary as a function of the dose, the volume of normal tissues irradiated at particular dose levels, and the treatment field. Acute symptoms typically appear during the third week of treatment and resolve within days to weeks after its completion. Acute intestinal symptoms, especially those associated with whole pelvic irradiation, are most commonly relieved with dietary manipulations. Otherwise, medications such as loperamide hydrochloride (Imodium; McNEIL-PPC, Inc., Fort Washington, PA) or diphenoxylate hydrochloride and atropine sulphate (Lomotil; Pfizer, New York, NY) are appropriate to relieve symptoms. Internal and external hemorrhoids, which may become inflamed during the course of therapy, are often best treated with sitz baths and hydrocortisone suppositories. Patients may also experience changes in the consistency of their bowel movements or an increase in mucous discharge. Acute urinary symptoms are treated with phenazopyridine hydrochloride (Pyridium; Warner Chilcott, Rockaway, NJ), nonsteroidal anti-inflammatory agents, or alpha-blockers, such as tamsulosin hydrochloride (Flomax; Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT). Alpha-blockers have been reported to be significantly more effective than nonsteroidal anti-inflammatory agents, resulting in significant resolution of urinary symptoms in 66% of patients and moderate improvement in 22%.215
Late rectal toxicities attributed to radiotherapy typically manifest 12 months to 18 months after completion of treatment and may persist for several years thereafter; the development of rectal complications after 5 years is rare. Late rectal toxicities may include rectal bleeding, mucous discharge, and mild incontinence of stool. The more-severe toxicities, including ulcer development and fistula formation, are observed in ≤1% of patients. Grade 2 rectal bleeding can be effectively treated with steroid suppositories, sitz baths, and an increase in dietary fiber. Because of the increased risk of further trauma to the rectal mucosa and the risk of fistula formation, deep rectal biopsies and cauterization procedures should be avoided unless absolutely necessary. For radiation-induced proctitis not responsive to conservative measures, argon-beam plasma laser coagulation can decrease the frequency of rectal bleeding episodes.216 Hyperbaric oxygen treatments, at a pressure of 2.4 atmospheres for a median of 36 sessions (90 minutes per session), may be helpful; in one study, they were associated with a complete resolution of rectal bleeding in 48% of patients and a reduction in bleeding episodes in 28% of patients.217 It is assumed that hyperbaric oxygen improves the delivery of oxygen to ischemic rectal mucosa, promoting angiogenesis and advancing mucosal healing and fibroblast proliferation.
Late urinary toxicities include chronic urethritis, which occurs in approximately 10% to 15% of patients, and urethral strictures, which occur in 2% to 3%. Hemorrhagic cystitis is uncommon with conformal radiation techniques, which reduce the dose to the bladder. Current treatment approaches for hemorrhagic cystitis include intravesical formalin therapy and selective embolization of the hypogastric arteries. For patients refractory to such measures, hyperbaric oxygen therapy can be considered for radiation-induced hemorrhagic cystitis. In one report, 49 of 57 patients (86%) experienced complete resolution or marked improvement of hematuria following hyperbaric oxygen treatment.218
Whereas any form of radiotherapy can increase the risk of developing a secondary cancer, the incidence after prostate radiotherapy appears to be lower than expected. One report comparing the risks of secondary cancers among patients treated with RP, EBRT, and brachytherapy did not find the therapeutic intervention to be a significant predictor of secondary cancer at 10-year follow-up.219 Longer follow-up will be required to determine whether any significant differences eventually develop between the treatment arms. Nonetheless, given the risk of secondary cancer with any form of radiotherapy, close monitoring is important. Colonoscopy every 5 years and careful evaluation of patients who present with hematuria after radiotherapy are recommended to rule out the possibility of a secondary bladder cancer.
IMRT is also associated with a reduced risk of rectal-related toxicities after high-dose radiotherapy. In the initial cohort of patients treated with 81 Gy, late rectal and urinary complications were significantly reduced with IMRT relative to conventional three-dimensional CRT (Fig. 68.11). In 561 patients treated with IMRT (81 Gy) at MSKCC,220 the 8-year actuarial likelihood of developing grade 2 rectal bleeding was 1.6% with no grade 4 events and late grade 2 and 3 (urethral strictures) urinary toxicities were 9% and 3%, respectively. Among patients who were potent before IMRT, 49% developed erectile dysfunction.220
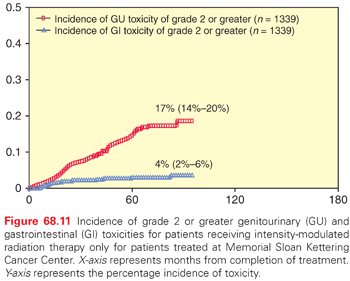
Factors predictive of late urinary toxicities after high-dose IMRT included a lower baseline urinary symptom score (International Prostate Symptoms Score) (p = 0.006) and an increased maximal dose beyond the prescribed dose to the bladder trigone region (p = 0.003).221 When high-dose radiotherapy is used, efforts should be made to restrict the high-dose region from receiving >15% to 20% of the prescription doses for the urethra and, in particular, for the bladder neck and the region of the bladder trigone. These and other data indicate that patients with significant urinary symptoms before treatment may be better served by surgery, rather than radiotherapy.
Potency Preservation with External Beam Radiotherapy
The reported rates of impotence at 3 years or longer after EBRT ranges widely from 36% to 68%,222 a reflection of the differences in methodologies used to assess this end point and the heterogeneity of the EBRT patient population. Factors such as advanced age and the presence of medical comorbidities (coronary artery disease, diabetes, and use of antihypertensive medications) can have a profound effect on erectile function. Besides erectile dysfunction, aspects of sexual dysfunction that occur after radiotherapy include decreased volume of ejaculate, absence of ejaculate, decreased intensity of orgasm, and decreased libido.
Hypofractionated External Beam Radiotherapy
The administration of larger doses for each treatment fraction has potential radiobiologic advantages (hypofractionated radiotherapy) that can result in a greater degree of cell kill.223,224 One approach is stereotactic body radiosurgery, which has been used increasingly during the last several years. Unfortunately, most reports to date are uncontrolled single-center series of selected patients which, despite recognized limitations, are showing promising results using PSA-based outcomes.225–227 These treatments are delivered with a linear accelerator mounted with an image-guided device to achieve greater precision of the dose delivery. With tight margins used, high doses per fraction allow completion of the course of therapy in 1 week. A pooled analysis of 1,100 patients treated from 2003 to 2011 at eight institutions was recently reported.228 The median dose delivered for these patients was 36.25 Gy in five fractions. The median follow-up was 3 years; the 5-year PSA-relapse-free survival rates were 95%, 84%, and 81% for low-, intermediate-, and high-risk patients, repsectively. Stereotactic radiosurgery appears promising, but this form of treatment cannot be considered an established form of radiotherapy delivery, and ongoing studies will define its role better in the years ahead.
The Role of Proton Therapy for Localized Prostate Cancer
Recently, there has been increasing interest in the use of proton therapy for clinically localized disease because of the known physical advantages of this charged particle—namely, the Bragg peak—by which the majority of the energy of the beam is deposited at the end of its track, creating a rapid falloff of dose beyond the target. The result is that exit dose beyond the target volume is eliminated, providing the potential to achieve greater sparing of normal tissues with dose escalation. Theoretically, physical advantages of the proton beam may also translate into a reduced risk of second malignancies, owing to the reduced exposure of normal tissues to the radiation beam relative to photon therapy. A series by Mendenhall et al.229 examined the 5-year clinical outcomes of 211 prospectively treated patients who received proton therapy using an image-guided approach. Low-risk patients received 78 Gy, intermediate-risk 78 Gy to 82 Gy, and high-risk 78 Gy with concomitant docetaxel followed by ADT; the 5-year PSA-relapse-free survival rates were 99%, 99%, and 76%, respectively. The incidence of grade 3 rectal and urinary toxicities was 1% and 5.4%, respectively, comparable if not superior to those with high-dose IMRT. Randomized trials to confirm these findings are ongoing, including a multi-institutional trial comparing 80 Gy versus a similar dose of protons for low- and intermediate-risk patients; the end point is 2-year QOL and late toxicities.
Reports addressing the question of whether protons are associated with reduced long-term toxicities relative to high-dose photon therapy are conflicting. One recent study of 1,242 patients treated with either proton therapy at doses of 76 Gy to 82 Gy or IMRT at doses of 75.6 Gy to 79.4 Gy showed similar QOL scores for the bowel, urinary incontinence, urinary irritative symptoms, and sexual domains at the last follow-up evaluation. Further exploration of specific function outcomes showed that patients treated with proton therapy had less frequent bowel movements and rectal urgency, compared with patients treated with IMRT.230 However, an analysis of the relative toxicities of IMRT and proton therapy in patients with localized prostate cancer based on the Surveillance, Epidemiology, and End Results–Medicare-linked database showed that IMRT was associated with fewer gastrointestinal (GI)-related toxicities and hip fractures, whereas proton therapy was associated with a lower risk of erectile dysfunction.231 It is anticipated that future improvements in intensity-modulated proton therapy will also improve the conformality of the proton beam, resulting in fewer complications with proton dose escalation. To date, however, there is no established evidence that proton therapy provides superior tumor control outcomes, compared with well-delivered high-dose IMRT or image-guided radiotherapy, for the treatment of prostate cancer.
Prostate Brachytherapy
Excellent long-term tumor control can be achieved with brachytherapy, and the approach is considered a standard treatment intervention associated with outcomes comparable to those with prostatectomy and EBRT for patients with clinically localized disease.232–237 By category, low-risk disease is managed with seed implantation alone (i.e., monotherapy), whereas intermediate- and selected high-risk disease is managed with a combination of brachytherapy (low-dose-rate [LDR] permanent interstitial implantation or high-dose-rate [HDR] brachytherapy via after-loading catheters) and EBRT. Whether the addition of EBRT is necessary in all patients with intermediate-risk prostate cancer is being studied in a phase 3 randomized trial (Radiation Therapy Oncology Group [RTOG] 0232).
Technical Aspects of Prostate Brachytherapy
Transperineal ultrasound-guided approaches have facilitated image-guided placement of the seeds and are credited with improved long-term outcomes and reduced treatment-related complications. These approaches have further improved the accuracy and consistency of the dose delivery to the target, with a concomitant reduction of dose to the urethra and rectum. The use of adjunctive intraoperative CT scanning to verify the actual deposited seed coordinates may eliminate the need for postimplantation assessments and may allow for opportunities, if necessary, to correct suboptimal implanted regions within the gland before the reversal of anesthesia. Close collaboration between the radiation oncologist and the medical physicist in the design of the pre- or intraoperative treatment plan is critical for a successful outcome.
The two most commonly used radioisotopes for permanent seed brachytherapy are iodine-125 (125I) and palladium-103 (103Pd). The half-life of 125I is 60 days, with a mean photon energy of 27 KeV and an initial dose rate of 0.07 Gy/h. In contrast, the half-life of 103Pd is 17 days, with a mean photon energy of 21 KeV and an initial dose rate of 0.19 cGy/h. The active periods for 125I and 103Pd are 10 months and 3 months, respectively. When 125I is used, the typical prescription dose is 144 Gy; 125 Gy is routinely used for 103Pd.
The quality of the implant and dose distributions are routinely evaluated using CT scans obtained on the day of the implant or 30 days later. Postimplantation CT scans are used to generate dose-volume histograms, which allow detailed analysis of the radiation dose distribution relative to the prostate and surrounding normal tissues. Dosimetric parameters include V100 for the target (volume of the prostate receiving 100% of the prescription dose), D90 of the target (dose delivered to 90% of the prostate), and the average and maximum rectal and urethral doses.238
Table 68.11 summarizes the published biochemical control outcomes after LDR interstitial seed implantation, according to prognostic risk groups. 235,237,239–243 The size of the prostate gland should preferably be <60 cm3 and optimally <50 cm3. In larger gland sizes, the pubic arch may interfere with needle placement reaching the anterolateral portions of the gland, resulting in inadequate dose coverage of the target volume. Larger glands require more seeds and activity to achieve coverage of the gland with the prescription dose, which may result in an increase in the central urethral doses and potentially increase the risk of urinary morbidity.244 The size of the prostate can be effectively addressed with combined androgen-blockade therapy. An approximately 30% reduction in volume is commonly observed after 3 months of ADT. For patients who have imaging findings consistent with gross ECE or SVI not detected on rectal examination, monotherapy is not sufficient, and supplemental EBRT should be considered.
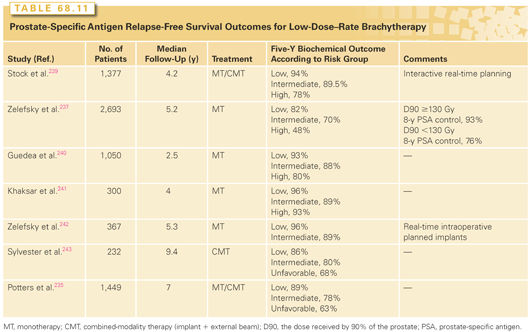
Patients with a significant degree of urinary obstructive symptoms are more prone to develop prolonged morbidity after brachytherapy and would be better suited to other treatment interventions. A previous TURP may increase the risk of urinary morbidity after seed implantation245; brachytherapy should be performed with caution in such patients. Careful attention to dose-volume considerations to the periurethral region and the area of the TURP defect should reduce the likelihood of long-term morbidities.
Seed implantation may be a more suitable intervention for patients with bilateral hip replacements, in whom CT-based treatment planning is technically difficult because of the substantial artifact created by the prosthesis, precluding adequate visualization of the target volume. Ultrasound-based seed implantation would be an appropriate alternative for such patients, as artifacts would not pose a difficulty with this imaging modality. In most cases, patients with hip prostheses are able to tolerate the extended dorsal lithotomy position for adequate perineal exposure during seed implantation. Patients with small bowel in close proximity to the prostate volume are also better suited to brachytherapy, owing to the lower doses to the bowel expected with brachytherapy. Brachytherapy may be safe for patients with a history of inflammatory bowel disease, a condition that represents a relative contraindication for EBRT. Of 24 patients with a history of inflammatory bowel disease who were treated with brachytherapy, none experienced grade 3 or higher rectal toxicities (median follow-up, 4 years), but late grade 2 rectal bleeding (19%) was significantly higher than among patients without a history of inflammatory bowel disease.246
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree