Cancer of the lung
Charles Lu, MD, SM  Daniel Morgensztern, MD
Daniel Morgensztern, MD  Anne Chiang, MD, PhD
Anne Chiang, MD, PhD  Amir Onn, MD
Amir Onn, MD  Boris Sepesi, MD
Boris Sepesi, MD  Ara A. Vaporciyan, MD
Ara A. Vaporciyan, MD  Joe Y. Chang, MD, PhD
Joe Y. Chang, MD, PhD  Ritsuko K. Komaki, MD
Ritsuko K. Komaki, MD  Ignacio I. Wistuba, MD
Ignacio I. Wistuba, MD  Roy S. Herbst, MD, PhD
Roy S. Herbst, MD, PhD
Overview
Worldwide, lung cancer is responsible for the largest number of cancer-related deaths, and reducing tobacco consumption is the most effective way to reduce lung cancer mortality. Standard therapy options continue to include surgery, radiotherapy, and/or chemotherapy. As lung cancer is a heterogeneous disease, it is hoped that translational proteomic and genomic research will advance our understanding of driver molecular pathways and allow development of more effective, less toxic, targeted therapies. Recent developments in immunotherapy have generated significant excitement and represent a novel therapeutic strategy, which has already impacted current standard of care.
Etiology and epidemiology
Worldwide, lung cancer is the most common (1.61 million of 12.7 million new cases) and the deadliest (1.38 million of 7.6 million cancer-related deaths) form of cancer.1 The US 2015 cancer statistics indicate that lung cancer is the second most common cancer for both men and women (14% and 13% of all cases, respectively), but, as in previous years for both sexes, it is the number one cause of cancer death (86,380 men, 28% of all cancer-related deaths, and 71,660 women, 26% of all cancer-related deaths). In fact, more people in the United States die of lung cancer than of the next three causes of cancer-related deaths combined, which are prostate, breast, and colorectal cancer.2 In 1920, fewer than 1000 cases of lung cancer were reported, and it was regarded as a rare malignancy. Since the 1950s, however, lung cancer has been recognized as a major public health problem. The incidence of new cases rose first in men and reached a peak in the mid 1980s; a steady decline has been noted since then. The incidence in women increased until the late 1990s and has recently stabilized. These changes occurred in parallel to the widespread adoption of cigarette smoking by both sexes. The decline in lung cancer incidence and mortality among men has been explained by reduction in smoking rates.3
Smoking and lung cancer
Tobacco is the world’s single most avoidable cause of death. Lung cancer is the most common tobacco-related cause of cancer mortality: one case occurs for every 3 million cigarettes smoked. As described by Proctor,4 cancers caused by tobacco were among the earliest discovered environmental cancers. First reports on the association between tobacco use and cancer of the oral cavity and lip were published in Europe in the eighteenth and nineteenth centuries. More than 60 years ago, Muller in Germany was the first to recognize the positive association between cigarette smoking and lung cancer. Since then, multiple epidemiological studies have confirmed these observations and elaborated on the molecular mechanisms of smoking carcinogenesis, providing sufficient evidence to establish a strong causal association between cigarette smoking and cancer of the aerodigestive tract, urinary bladder, kidney and uterine cervix, as well as myeloid leukemia. A meta-analysis of over 50 studies on never smokers showed a consistent and statistically significant association between exposure to environmental tobacco smoke and lung cancer risk.4
To date, smoking accounts for about 85–90% of lung cancer deaths in both sexes. The rate at which lung cancer develops is strongly correlated with the duration of tobacco exposure. After 45, 30, and 15 years of cigarette smoking, the annual incidence rates of lung cancer are 0.5%, 0.1%, and under 0.01%, respectively. Thus, a threefold increase in the duration of tobacco use can increase the annual incidence of lung cancer by 50-fold. As smoking ceases, the annual risk remains roughly constant thereafter. For instance, after 30 years of smoking, the risk is approximately 0.1%, and if a smoker stops after 30 years, this annual rate will persist indefinitely. Thus, 15 years later, the annual risk is 0.1% instead of 0.5%, which it would have been if smoking had continued. About 80% of the risk is, therefore, avoided by stopping smoking.5
Passive smoking
Smokers are not the only people at increased risk from exposure to tobacco smoke. “Passive smoking” from environmental tobacco smoke also increases the risk of lung cancer death. According to the Environmental Protection Agency, each year about 3000 nonsmoking adults die of lung cancer as a result of breathing the smoke of others’ cigarettes. Analysis has shown that the sidestream smoke emitted from a smoldering cigarette between puffs contains virtually all carcinogenic compounds that have been identified in the mainstream smoke inhaled by smokers.
Familial predisposition
It is interesting that the vast majority of cigarette smokers, including heavy smokers, do not develop lung cancer. This suggests that cancer formation is dependent on an inherited predisposition or cofactors such as additional carcinogens. Studies that have compared risk factors of individuals with histologically confirmed lung cancer and of individuals with other smoking-related cancers found that having relatives with lung cancer did not increase the risk of developing lung cancer, but it did increase the risk of having cancer at some site.6 This suggests a heritable variation in response to carcinogens. Respiratory diseases also predispose to development of lung cancer. Studies of families predisposed to lung cancer showed that the development of lung cancer in young individuals (aged 50 years or younger) was compatible with Mendelian codominant inheritance or a rare autosomal gene.7
Other environmental causes
Lung cancer occurs in association with occupational and environmental exposures to carcinogenic agents other than tobacco smoke. Occupational agents classified as group 1 carcinogens by the International Agency for Research on Cancer include inorganic arsenic, asbestos, bis(chloromethyl)ether, chromium (hexavalent), nickel and nickel compounds, polycyclic aromatic compounds, radon, and vinyl chloride. Group 2A of probable carcinogens includes acrylonitrile, beryllium, cadmium, formaldehyde, acetaldehyde, synthetic fibers, silica, and welding fumes. Currently, occupational exposures have been estimated to account for 5–15% of all lung cancer cases worldwide (Table 1).
Table 1 Documented occupational lung carcinogens
| Substance | Occupational exposures |
| Arsenic | Smelters, pesticide manufacturers |
| Asbestos | Miners, millers, insulators, railroad, and shipyard workers |
| Chloromethyl ethers | Ion-exchange resin manufacturers |
| Chromium | Chromate and pigment manufacturers |
| Hydrocarbons | Coal-gas workers, roofers |
| Mustard gas | Poison-gas manufacturers |
| Nickel | Refiners |
| Radiation | Miners of uranium and other ores |
Source: Adapted from Roth 1989.8
Molecular pathogenesis
The rapidly developing technology of molecular biology has allowed the identification of multiple genes responsible for lung carcinogenesis. Interestingly, these genes are altered forms of genes normally present in eukaryotic cells. The plethora of genetic abnormalities and redundancy of altered pathways induced by tobacco and other carcinogens determines lung cancer heterogeneity, which is remarkable in comparison to that of other solid tumors. In this regard, it has to be remembered that individual tumors are characterized by specific genetic alternation(s) and that there is a gradual accumulation of abnormalities in a given tumor, from normal epithelium to invasive carcinoma. Table 2 lists genes that have been implicated in lung carcinogenesis. A detailed review of cancer biology may be found in other sections of this textbook.
Table 2 Summary of molecular abnormalities associated with the NSCLC adenocarcinoma and squamous-cell carcinoma histologies
| Gene | Molecular change | Adenocarcinoma | Squamous-cell |
| carcinoma | |||
| EGFR | Mutation | 10–40% | Very rare |
| Amplification/CNGa | 15% | 40% | |
| IHCb overexpression | 15–39% | ∼58% | |
| KRAS | Mutation | 10–30% | Very rare |
| BRAF | Mutation | 2% | 3% |
| EML4-ALK | Translocation | 13% | Very rare |
| ROS1 | Translocation | 1% | Very rare |
| RET | Translocation | 1% | Very rare |
| LKB1 | Mutation | 8–30% | 0–5% |
| HER2 | Mutation | 2–4% | Very rare |
| Amplification | 8% | 2% | |
| IHCb overexpression | 35% | 1% | |
| DDR2 | Mutation | Very rare | 4% |
| TP53 | LOHc and mutations | 50–70% | 60–70% |
| FGFR1 | Amplification | 1–3% | 8–22% |
| PIK3CA | Amplification/CNGa | 2–6% | 33–35% |
| Mutation | 2% | 2% |
a CNG, copy number gain.
b IHC, immunohistochemistry.
c LOH, loss of heterozygosity.
Molecular abnormalities in premalignancy
Structural and genetic epithelial changes occur gradually, and invasive carcinoma develops 5–20 years after initial insult to the airways (Figure 1). Loss of specific chromosomal regions on a single allele [loss of heterozygosity (LOH)] has been detected frequently in lung cancers and bronchial epithelia exposed to tobacco carcinogens. The regions of the earliest and most frequent allelic loss are 3p21, 3p22–24, 3p25, and 9p21.9 It is noteworthy that many of these changes are seen in histologically normal bronchial epithelium from smokers, but not nonsmokers.10, 11 However, these changes appear to become more frequent and extensive in terms of chromosome loss with advancing abnormality of premalignancy. In some cases, these molecular changes appear to be clonally independent. Methylated sequences of tumor suppressor gene promoters can be detected in tumors, smoking-damaged normal lung (preneoplastic changes), sputum, and blood. These represent attractive surrogate biomarkers for early detection and monitoring of chemoprevention, smoking cessation, and response to therapy. Recent advances in the characterization of molecular abnormalities of lung cancer, including the reports of several novel molecular pathways involved in the pathogenesis of invasive squamous cell carcinoma (SCC) and adenocarcinoma of the lung identified using high-throughput technologies such as next-generation sequencing of tumoral DNA and RNA,12, 13 have been extended to the analysis of molecular changes involved in the development of preneoplastic lesions of the lung airway.14 It has been shown that in lung adenocarcinoma harboring epidermal growth factor receptor (EGFR) mutations, those changes can be detected in bronchial normal epithelium adjacent to tumors.15 In SCC, gene amplification and protein overexpression of the cell lineage gene SOX-2 have been detected in a subset of bronchial dysplasia in patients with invasive squamous tumors.16, 17 High-throughput microarray profiling studies of the lung airway has shown that global alterations in the gene expression of normal epithelial cells can (1) predict cancer development in smokers,18 (2) identify the activation of pathways (e.g., PI3K) that can be potentially targeted using chemoprevention strategies,19 and (3) better define the localized molecular field of cancerization that can explain the development of different histologies of lung cancer.14, 20, 21
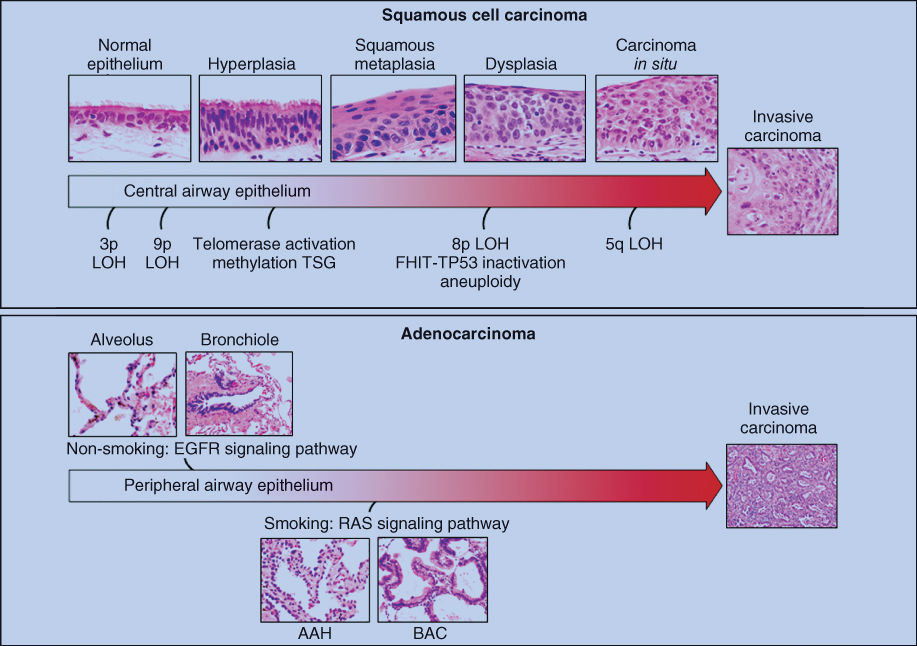
Figure 1 Summary of histopathologic and molecular changes involved in the pathogenesis of squamous-cell carcinoma and adenocarcinomas of the lung.
Pathology of lung cancer
From histopathologic and biologic perspectives, lung cancer is a complex neoplasm. The 2004 WHO (World Health Organization) histologic classification of lung cancer has been recently revised with support from the pathology panel of the International Association for the Study of Lung Cancer (IASLC).22 The new classification is based on the analysis of lung tumors by light microscopy with standard histology techniques and immunohistochemical analysis of proteins representing differentiation markers (Table 3).22, 23 The most common histologic types of lung cancer are non-small-cell lung carcinomas (NSCLC), which include SCC, adenocarcinoma [including minimally invasive adenocarcinoma (MIA) and large-cell carcinoma, and small-cell lung carcinomas (SCLC).24 Lung neoplasms are generally classified by the best-differentiated region of the tumor and graded by its most poorly differentiated portion.
Table 3 Histological classification of lung cancer
|
Precursor lesions
Lung cancers are believed to arise after the development of a series of progressive pathological changes (preneoplastic or precursor lesions) in the respiratory mucosa. The new WHO histological classification of preinvasive lesions of the lung lists three main morphologic forms: (1) squamous dysplasia and carcinoma in situ (CIS); (2) atypical adenomatous hyperplasia (AHH) and adenocarcinoma in situ (AIS); and (3) diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH), which has been associated with the development of carcinoid tumors of the lung.22, 23 While the sequential preneoplastic changes have been defined for centrally arising squamous carcinomas, they have been poorly documented for large-cell carcinomas, adenocarcinomas, and SCLCs.25, 26
Invasive tumors
Minimally invasive adenocarcinoma (MIA)
This lesion is defined as a small (≤3 cm), solitary adenocarcinoma with a predominantly lepidic pattern and with ≤5 mm invasion area in greatest dimension.22, 27 MIA is usually composed of nonmucinous cells, but infrequently, it may be mucinous. The measurement of the invasive area should include the presence of histological subtypes other than a lepidic pattern (i.e., acinar, papillary, micropapillary, and/or solid), or the identification of malignant cells clearly infiltrating stroma.22 If a tumor invades lymphatics, blood vessels, or pleura or contains tumor necrosis, it should be diagnosed as invasive adenocarcinoma.
Adenocarcinoma
This tumor type accounts for nearly 40% of all lung cancers (Figure 2a, b). Most adenocarcinomas are histologically heterogeneous, and according to the new WHO classification, adenocarcinoma can be subclassified based on the predominant histological pattern present (i.e., acinar, papillary, solid with mucin production, micropapillary, and lepidic).22 When tumor cells grow in a purely lepidic manner without evidence of invasion, they are regarded as AIS.22 The solid adenocarcinoma pattern is, by definition, poorly differentiated, and these poorly differentiated tumors usually demonstrate mucus production as shown by mucicarmine or periodic acid-Schiff staining. Lung adenocarcinomas typically immunostain for thyroid transcription factor-1, Napsin A, and cytokeratin 7. There are four variants of adenocarcinoma tumors, including mucinous, colloid, fetal (low and high grade), and enteric. While adenocarcinomas of the lung spread primarily by lymphatic and hematogenous routes, aerogeneous dissemination often occurs in mucinous tumors and is characterized by spread of tumor cells through the airways forming lesions separate from the main mass.28
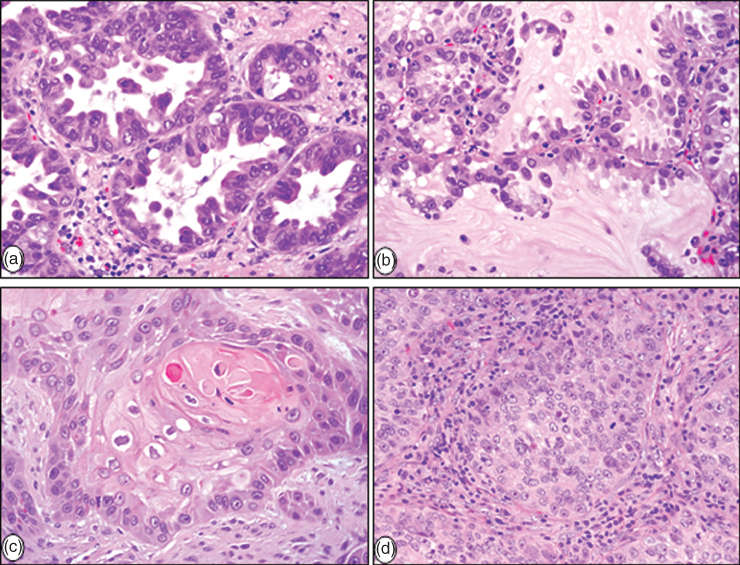
Figure 2 Histopathological characteristics of major forms of NSCLC: (a) invasive adenocarcinoma with acinar pattern, (b) adenocarcinoma with lepidic (noninvasive) pattern, (c) keratinizing squamous-cell carcinoma, and (d) large-cell carcinoma.
Squamous-cell carcinoma (SCC)
SCC accounts for approximately 30% of all lung cancers. Intercellular bridges, squamous pearl formation, and individual cell keratinization characterize squamous differentiation in this tumor type (Figure 2c). While all these features are very apparent in well-differentiated SCCs, they are difficult to find in poorly differentiated tumors. The histologic subtypes included in the new WHO classification include keratinizing, nonkeratinizing, and basaloid.29 In tumors with keratinizing features, there is no need for immunohistochemistry analysis. The nonkeratinizing tumors necessitate immunohistochemistry examination to distinguish theses tumors from large-cell carcinoma with a null immunophenotype in surgical resections or other types of poorly differentiated NSCLCs. For such tumors, diffuse positive staining with p40, p63, and/or CK5 or CK5/6 confirms their squamous phenotype and classification as a nonkeratinizing SCC. Both TTF-1 and mucin stains should be negative or only focally positive (<10% of cells with faint staining).
Approximately, 70% of SCCs of the lung present as central lung tumors.30 The tumor may grow to a large size and central cavitation secondary to necrosis is a common gross finding.31
Adenosquamous carcinoma
Adenosquamous carcinoma of the lung is characterized by the presence of SCC and adenocarcinoma with each comprising at least 10% of the tumor.32 They account for 0.4–4% of lung cancers and are usually located in the periphery of the lung and may contain a central scar. The routes of dissemination and metastasis are similar to other NSCLCs.
Sarcomatoid carcinomas
Sarcomatoid carcinomas of the lung are a group of poorly differentiated NSCLCs that contain a component of sarcoma or sarcoma-like (spindle and/or giant cell) differentiation.33 Currently, there are five variants identified: pleomorphic carcinoma, spindle-cell carcinoma, giant-cell carcinoma, carcinosarcoma, and pulmonary blastoma.33, 34 Sarcomatoid carcinomas are rare tumors (0.3–1.3% of lung tumors).33, 35
Large-cell carcinoma
Large-cell carcinoma is defined as an undifferentiated NSCLC that lacks the cytologic, architectural, and immunohistochemical features of SCC, adenocarcinoma, or SCLC (Figure 2d). Thus, it is a diagnosis of exclusion. Large-cell carcinomas account for approximately 10% of all lung cancers, and they represent a spectrum of morphology, most consisting of large cells with abundant cytoplasm and large nuclei with prominent nucleoli (Figure 2d).31 The 2004 WHO classification listed four histological variants of large-cell carcinoma: large-cell neuroendocrine carcinoma (LCNEC), basaloid carcinoma, lymphoepithelioma-like carcinoma, clear-cell carcinoma, and large-cell carcinoma with rhabdoid phenotype. However, in the revised WHO classification, LCNEC is being reclassified into a new category (neuroendocrine carcinoma), and basaloid carcinoma is included as a variant of SCC. Pure large-cell carcinoma with clear cells or rhabdoid phenotype is extremely uncommon. If these components are detected in a large-cell carcinoma, their presence should be added in the description of the tumor.
Neuroendocrine tumors
Lung neuroendocrine tumors account for approximately 15% of lung cancers. They are composed of malignant cells showing neuroendocrine differentiation and representing a wide spectrum of tumors: typical and atypical carcinoids, considered low- and intermediate-grade malignancies, respectively, and LCNEC and SCLC, considered high-grade tumors (Figure 3a–c).
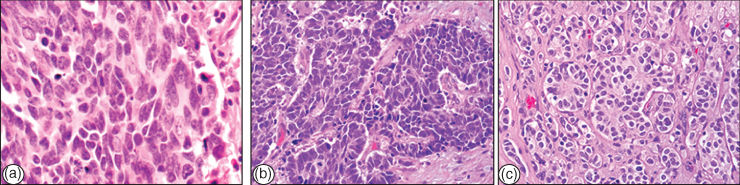
Figure 3 Neuroendocrine tumors of the lung: (a) SCLC, (b) LNEC, and (c) typical carcinoid.
Carcinoid tumors
Carcinoid tumors are characterized by organoid growth pattern, uniform cytologic features, and immunohistochemical expression of neuroendocrine markers, such as chromogranin and synaptophysin (Figure 3c).36 Carcinoid tumors have been divided into two categories, typical and atypical types, based on their clinical behavior and pathologic features, with atypical crinoids having more malignant histologic and clinical features.37 Typical and atypical crinoids are also referred to as low- and intermediate-grade neuroendocrine carcinomas, respectively. Histologically, typical carcinoids show fewer than 2 mitoses per 2 mm2 field and lack necrosis, while atypical carcinoids show 2–10 mitoses per 2 mm2 field and/or foci of necrosis.36 Typical carcinoids are uniformly distributed throughout the lungs, whereas atypical carcinoids are more commonly peripheral tumors.38 Compared to typical carcinoids, atypical carcinoids have a larger tumor size and a higher rate of metastases, and their survival is significantly reduced.38 At presentation, approximately 10–15% of typical and 40–50% of atypical carcinoids demonstrate regional lymph node metastases.36
Large-cell neuroendocrine carcinoma (LCNEC)
This tumor type is defined by the presence of large undifferentiated cells with prominent nucleoli, neuroendocrine pattern of growth, high mitotic rate, and neuroendocrine differentiation demonstrated by immunohistochemistry (Figure 3b).39 They are usually peripheral, nodular masses, with necrosis. LCNEC is considered an aggressive malignancy with a prognosis similar to SCLC.39 The term combined LCNEC is used for tumors associated with other better differentiated types of NSCLC, mostly adenocarcinomas.
Small-cell lung carcinoma (SCLC)
This tumor type accounts for approximately 15% of all lung cancers.29 They characteristically consist of small epithelial tumor cells with finely granular chromatin and absent or inconspicuous nucleoli (Figure 3a). Necrosis is frequent and extensive and the mitotic count is high. Although there is not a precise upper limit for cell size to be defined as small cell, it has been suggested that the cells should measure approximately the diameter of two or three small mature lymphocytes.40 While SCLC represents a light microscopic diagnosis, electron microscopy shows neuroendocrine granules in at least two-thirds of cases and immunohistochemistry for neuroendocrine markers (chromogranin and synaptophysin) is positive in most (∼90%) cases.40, 41 Less than 10% of SCLCs demonstrate a mixture with NSCLC histologic types, usually adenocarcinoma, SCC, or large-cell carcinoma, and they are termed combined SCLCs.
NSCLC histological classification applied to small biopsies and cytology specimens
In these tumor samples, the NSCLC diagnosis has been lumped together without attention to more specific histologic typing. One of the major recent changes in lung cancer pathology has been the development of standardized criteria and terminology for pathologic diagnosis of lung cancer in small biopsies and cytology.42 In addition to the criteria and terminology, there is a paradigm shift for pathologists in tumor classification and management of specimens, which indicates the need to perform immunohistochemistry to further classify tumors formerly diagnosed as NSCLC not otherwise specified (NOS). Currently, if a NSCLC does not show clear glandular or squamous morphology in a small biopsy or cytology specimen, it is classified as NSCLC-NOS. Tumors with this morphology should be studied with limited special immunohistochemical markers to classify them further. It is recommended to use a single adenocarcinoma maker (i.e., TTF-1), a single squamous marker (i.e., p40 or p63), and/or mucin stains.42 Tumors that are positive for an adenocarcinoma marker or mucin are classified as NSCLC, favor adenocarcinoma. Tumors that are positive for an SCC marker with negative adenocarcinoma marker are classified as NSCLC, favor SCC. Cytology is also a powerful diagnostic tool that can accurately subtype NSCLC in most cases, and immunohistochemistry is readily available if cell blocks are prepared for the cytology samples.
Clinical manifestations
Clinical presentation
Some patients present with an asymptomatic lesion were discovered incidentally on chest radiograph. The majority of lung cancers, however, are discovered because of the development of a new or worsening clinical symptom or sign. Although no set of signs or symptoms is pathognomonic for lung cancer, they may be divided into four categories: (1) those due to local tumor growth and intrathoracic spread, (2) those due to distant metastases, (3) nonspecific systemic symptoms, and (4) paraneoplastic syndromes.
Manifestations of local tumor growth and intrathoracic spread
Signs and symptoms referable to the primary tumor vary depending on location and size of the tumor. Centrally located tumors produce cough, a localized wheeze, hemoptysis, and symptoms and signs of airway obstruction and postobstructive pneumonitis such as dyspnea, fever, and productive cough. Peripheral tumors are more likely to be asymptomatic when they are small and confined within the lung; occasionally, cough and pleuritic chest pain may be evident.
Intrathoracic spread of lung cancer, either by direct extension or by lymphatic metastasis, is associated with a variety of sign and symptom complexes. Mediastinal invasion may be manifested as vague, poorly localized chest pain in association with other findings of nerve entrapment, vascular obstruction, and/or compression or invasion of the esophagus. One of the most common neurologic disorders arising from mediastinal involvement is hoarseness owing to entrapment of the recurrent laryngeal nerve. Because of its longer intrathoracic course, the left recurrent laryngeal nerve is more likely to be the source of hoarseness than the right recurrent laryngeal nerve.43 Compression of the esophagus by the tumor also may lead to dysphagia. The formation of a tracheoesophageal or bronchoesophageal fistula, which occurs with a frequency of 0.16%, can be manifested by vigorous cough, especially on swallowing, and recurrent aspiration pneumonia.44 Involvement of the phrenic nerve is associated with hiccups early, and later leads to paralysis and elevation of the hemidiaphragm with resulting dyspnea.
The principal vascular syndrome associated with the extension of lung cancer into the mediastinum is superior vena cava (SVC) syndrome, most commonly caused by invasion of the vein and extrinsic compression by the tumor, but also by intraluminal thrombosis.45 Lung cancer accounts for 65–90% of all cases of SVC syndrome, and in approximately 85% of these cases, the primary lung tumor is on the right, primarily in the right upper lobe or right mainstem bronchus. Establishment of a histologic diagnosis is important before initiating treatment, because the SVC syndrome is no longer considered a radiotherapeutic emergency.
With apical tumors, the classic Pancoast’s syndrome (lower brachial plexopathy, Horner’s syndrome, and shoulder pain) is due to local invasion of the lower brachial plexus (C8 and T1 nerve roots), satellite ganglion, and chest wall.46 The tumor may cause symptoms through involvement of the first or second rib or vertebrae and other nerve roots. The radiographic signs are those of an asymmetric apical cap or an apical mass. Most superior sulcus tumors are SCCs, although they may be adenocarcinomas, or even in 1–2% of cases, SCLC, underscoring the importance of establishing a histologic diagnosis.
Approximately 15% of patients with lung cancer have pleural involvement at initial presentation, and 50% of patients with disseminated lung cancer develop pleural effusion during the course of their illness. A pleural effusion may cause dyspnea, cough, or chest pain.47 A number of pathogenic mechanisms have been suggested, but the presence or absence of malignant cells in cytology specimens does not significantly influence survival outcome, although the presence of malignant cells in pleural washings at the time of pulmonary resection for lung cancer has been shown to have a negative impact survival.48
Pericardial involvement arises from direct extension of the tumor or as a result of retrograde spread through mediastinal and epicardial lymphatics. Lung cancer is the single most frequent source of pericardial metastases.49Clinical findings include cardiac dysrhythmias, enlargement of the cardiac silhouette, and, infrequently, cardiac tamponade (Table 4).
Table 4 Clinical manifestations of lung cancer caused by local tumor growth and intrathoracic spread at presentation
| Frequency (%) | ||
| Clinical manifestation | SCLC | NSCLC |
| Cough | 50–76 | 40 |
| Dyspnea | 34–40 | 30–40 |
| Chest pain | 35–36 | 25–40 |
| Hemoptysis | 15–23 | 15–35 |
| Pneumonitis | 21–25 | 13–24 |
| SVC syndrome | 12 | <10 |
| Pleural effusion | 10–15 | 15 |
| Pancoast syndrome | Rare | 3 |
| Pericardial effusion | Uncommon | Rare |
NSCLC, nonsmall-cell lung cancer; SCLC, small-cell lung cancer; SVC, superior vena cava.
Manifestations of distant metastases
Approximately 60% of SCLC and 30–40% of NSCLC patients present with stage IV metastatic disease. Although lung cancer can metastasize to virtually any organ site, the most common sites of hematogenous spread that are clinically apparent are the central nervous system (CNS), bones, liver, and adrenal glands. Many of these patients do not have symptoms that can be attributed to a specific distant site.
Systemic, nonspecific signs, and symptoms
As shown in Table 5, systemic, nonspecific signs and symptoms are common in both SCLC and NSCLC. The 30% rate of anorexia is probably underreported. Weight loss, which is usually but not always accompanied by anorexia, occurs in approximately one-half of the patients and generalized weakness in one-third. Fever and anemia occur in fewer than 20% of the patients. Fever is generally not considered paraneoplastic in lung cancer patients; if present, it is usually associated with a documented infection (e.g., postobstructive pneumonia) or with liver metastases.
Table 5 Clinical manifestation caused by systemic effect at presentation
| Frequency (%) | ||
| Clinical manifestation | SCLC | NSCLC |
| Anorexia | 30 | 30 |
| Weight loss (≥10 lb) | 35–52 | 45–52 |
| Fatigue | 23–42 | 35 |
| Fever | 11–15 | 7–16 |
| Anemia | 11–15 | 16–20 |
NSCLC, nonsmall-cell lung cancer; SCLC, small-cell lung cancer.
Paraneoplastic syndromes
Table 6 lists 21 paraneoplastic syndromes, which induce signs and symptoms away from the primary tumor or its metastasis. The major categories of paraneoplastic syndromes include endocrine, neurologic, cutaneous and musculoskeletal, and cardiovascular and hematological manifestations.50
Table 6 Major paraneoplastic manifestations of lung cancer
| Syndrome | Clinical | Comments |
| frequency (%) | ||
| Endocrine | ||
| Inappropriate ADH | 5–10 | Mainly SCLC |
| Atrial natriuretic factor | ? | — |
| Ectopic ACTH | 3–7 | Most commonly with SCLC |
| Hypercalcemia of malignancy | 10 | Most commonly with squamous-cell types |
| Gynecomastia | 6 | More with large-cell type |
| Other hormones | — | No significant clinical manifestations |
| Neurologic | ||
| Eaton–Lambert | 6 | Mainly SCLC |
| Subacute sensory neuropathy | Rare | Mainly SCLC |
| Subacute cerebellar degeneration | Rare | Mainly SCLC |
| Limbic encephalopathy | Rare | Mainly SCLC |
| Visual paraneoplastic syndrome | Rare | Mainly SCLC |
| Subacute necrotic myelopathy | Rare | Mainly SCLC |
| Cutaneous/musculoskeletal | ||
| Hypertrophic pulmonary osteoarthropathy | <10 | More with adenocarcinoma |
| Acanthosis nigricans | Rare | — |
| Tylosis | Rare | — |
| Tylosis | Rare | — |
| Cardiovascular/hematologic | ||
| Nonbacterial thrombotic endocarditis | Uncommon | More with adenocarcinoma |
| Migratory thrombophlebitis | Uncommon | More with adenocarcinoma |
| Hypercoagulable status | 10–15% | Renal |
| Glomerulonephritis | Rare | — |
| Nephrotic syndrome | Rare | — |
ADH, antidiuretic hormone; SCLC, small-cell lung cancer; ACTH, adrenocorticotropic hormone.
Endocrine syndromes
Syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
Excess secretion of arginine-vasopressin associated with hyponatremia is the hallmark of SIADH (syndrome of inappropriate secretion of antidiuretic hormone). The cardinal findings are hyponatremia with corresponding serum hypo-osmolality; continued renal excretion of sodium; absence of fluid volume depletion; inappropriately high urine osmolality; and normal kidney, adrenal, and thyroid function. SIADH may be caused by a variety of malignant tumors, and SCLC is the most common (up to 15%, though only one-third are symptomatic). Water restriction is usually sufficient to control symptoms until systemic anticancer treatment is initiated, which typically leads to improvement or resolution of the hyponatremia. Saline infusion, furosemide, or demeclocycline is infrequently required.
Syndrome of ectopic adrenocorticotropic hormone
Hyperadrenocorticism in association with ectopic adrenocorticotropic hormone (ACTH) production (>200 pg/mL) is a frequently observed hormonal syndrome in lung cancer, particularly in SCLC. Serum ACTH levels are elevated in 30–72% of SCLC patients, and cortisol secretion is abnormally regulated in 51%, but only 3–7% of SCLC patients become symptomatic. Patients with ectopic ACTH syndrome generally fit the demographic characteristics of lung cancer patients and rarely exhibit the classic cushingoid features of centripetal obesity or moon facies. Ectopic ACTH syndrome was found to be associated with frequent complications from chemotherapy and shortened survival in patients with SCLC.51
Other hormone production
Other hormones elevated in lung cancer, particularly in patients with SCLC, include calcitonin, growth hormone, prolactin, serotonin, insulin, gastrin, and melanocyte stimulating factors. In most cases, however, these laboratory abnormalities bear minimal clinical significance.
Hypercalcemia of malignancy
It has long been known that cancer patients may have hypercalcemia even without demonstrable bone metastases. Hypercalcemia has been reported to occur in up to 30% of patients with cancer at some time during the course of their disease. This incidence may be falling owing to the wide use of bisphosphonates in patients with multiple myeloma or breast cancer, although data are lacking. Hypercalcemia leads to progressive mental impairment, including coma, as well as renal failure. These complications are particularly common terminal events among patients with cancer. The detection of hypercalcemia in a patient with cancer signifies a very poor prognosis; approximately 50% of such patients die within 30 days. A parathyroid hormone-related protein has been shown to be responsible for the majority of cases of hypercalcemia of malignancy. SCC is the lung cancer mostly associated with hypercalcemia, and SCLC is rarely involved. Hypercalcemia may be completely reversible with effective treatment of the underlying cancer, and bisphosphonates may be used as a specific therapeutic modality.52 Denosumab, a monoclonal antibody, which binds the bone resorption mediator RANKL, has recently been approved for hypercalcemia of malignancy refractory to bisphosphonate therapy.53
Neurologic syndromes
Neurologic syndromes associated with lung cancer may occur through autoimmune mechanisms, mainly in patients with SCLC. Symptoms may precede diagnosis of the cancer by many months or may be the first sign of tumor recurrence. The severity of neurologic symptoms is unrelated to tumor bulk, and a primary malignant lesion may be undetected before death, despite disabling symptoms. Most of these conditions are not specific for malignancy.
Eaton–Lambert syndrome
A myasthenia gravis-like disorder that was originally linked to SCLC but was found in other cancers, this syndrome, characterized by proximal limb muscle weakness and fatigue, is caused by the formation of IgG antibodies directed at calcium channels present in both the tumor and the neuromuscular junction. A type-1 antineuronal nuclear autoantibody (ANNA-1, also known as “anti-Hu”) has been identified as a marker of neurological autoimmunity that is highly associated with SCLC (97%) and other paraneoplastic neurologic disorders.54
Subacute sensory neuropathy
This is the most characteristic peripheral neuropathy associated with SCLC. Clinical symptoms characterized by progressive impairment of all sensory modalities, with areflexia and marked sensory ataxia followed by stabilization after a period of weeks, may precede the diagnosis of SCLC by several months. It may be accompanied by more widespread evidence of paraneoplastic encephalitis, with cerebellar brainstem dysfunction and dementia.
Cutaneous and musculoskeletal syndromes
Digital clubbing and hypertrophic pulmonary osteoarthropathy (HPO) are the other major paraneoplastic syndromes associated with lung cancer, most commonly with NSCLC. Digital clubbing is more common than HPO, which often resembles rheumatoid arthritis. It is characterized by a symmetric polyarthritis (usually involving the ankles, wrists, and knees), proliferative periostitis of the long bones, and neurovascular changes of the hands and feet. Lung cancer accounts for more than 80% of cases of HPO in adults.55 Radionuclide bone scans typically demonstrate increased uptake at the distal ends of the affected long bones, and the results may be confirmed by evidence of new bone formation on plain films; the spine is spared. The onset of HPO is often acute and may precede the diagnosis of cancer. A variety of underlying mechanisms have been suggested, including the release of platelet-derived growth factor by megakaryocytes or platelet clumps that bypass the pulmonary capillary network. The syndrome may resolve with response of the cancer to therapy. No effective form of treatment is recognized, including aspirin and nonsteroidal anti-inflammatory agents.
Cardiovascular and hematological manifestations
Arterial and, more commonly, venous thrombosis is a frequent complication of cancer and sometimes a harbinger of occult cancer. Moreover, the use of new and aggressive therapy for cancer increases the risk of thrombosis. The two most notable manifestations in lung cancer are nonbacterial thrombotic endocarditis (NBTE) and venous thromboembolism (VTE).
Diagnostic and staging techniques
Accurate clinical staging includes a combination of noninvasive and invasive procedures. Noninvasive studies include sputum cytology and imaging studies; most commonly used are chest radiography, computed tomography (CT) scanning, and positron emission tomography (PET) (usually PET–CT) scanning. For mediastinal or spinal lesions, magnetic resonance imaging (MRI) is considered. Invasive procedures include bronchoscopy, CT or ultrasound (US)-guided fine-needle aspiration (FNA) or biopsy, lymph node biopsy, and surgical (open) biopsy using mediastinoscopy or thoracoscopy. Patients who present with clinical or radiographic evidence of extensive disease (ED) usually require the least invasive procedure to establish both the diagnosis and disease stage. Cytologic or histologic confirmation through FNA or biopsy usually is sufficient to confirm suspicion of N3 or M1 disease. Thoracentesis or pericardiocentesis should be performed on associated effusions to assess malignant cytology. Tissue may be required for molecular assessment of the tumor to better select medical therapy. Therefore, in some cases repeated biopsy is needed.
Noninvasive studies
Sputum cytology
Cytologic evaluation of sputum, bronchial washings, bronchial brushings, and FNA specimens have high diagnostic yield, but the positive and negative predictive values of each and their accuracy of diagnosis certainly are dependent on sampling error, tissue preservation, processing quality, and observer experience. Sputum cytology is a simple test with a specificity rate of 99%. However, the sensitivity rate is approximately 70% for central tumors, and <50% for peripheral lesions. To increase the yield, three specimens are usually collected. In practice, more invasive measures to obtain a diagnosis are used in most cases.
Imaging
Lung cancer is generally first imaged by chest radiography. CT scan, PET/CT scan, and occasionally MRI are used to stage a known or suspected lung cancer and monitor response to therapy.
Chest radiography
Posterior–anterior and lateral chest radiographs remain the simplest method for identifying patients with lung cancer. It is widely available, has low cost and low radiation dose, but most cases are identified at an advanced stage. A standard chest radiograph can detect a lesion as small as 3 mm in diameter; however, unsuspected nodules generally are not seen unless larger than 5 mm in diameter. Associated atelectasis, postobstructive pneumonitis, abscess, bronchiolitis, pleural reaction, rib erosion, pleural effusion, or bulky mediastinal lymphadenopathy may be identified on radiographs, raising suspicions of a primary lung malignancy.
Plain chest radiography may identify abnormal pulmonary nodules. There are no absolute criteria to confirm a benign lesion on the basis of its radiographic appearance, but stability of size for 2 years and the presence of specific patterns of calcification (multipunctate foci, a dense central nidus, a popcorn ball, or laminated “bull’s eye” appearance) are considered indicators of benignancy. However, some tumors such as AIS and typical carcinoids occasionally appear to be stable for 2 or more years.56
Computed tomography
As a single comprehensive study, CT scan remains the most effective noninvasive technique for evaluating suspected or known lung cancer and the mediastinum, which may contain associated metastatic disease. However, the accuracy of CT scanning in identifying metastatic disease in mediastinal lymph nodes is highly variable and its sensitivity ranges from 51% to 95%. Such a wide range in accuracy is secondary to variations in the criteria for nodal abnormality, which are based on size and shape of a lymph node, CT scanner differences, and nonuniformity in nodal mapping. A lymph node size ≥1 cm in shortest diameter has been generally accepted as the criterion of abnormal nodal enlargement. Approximately 8–15% of patients considered to have a negative CT scan for mediastinal nodal enlargement, with lymph nodes ≤1 cm, will ultimately be found to have mediastinal nodal involvement at the time of operation. Mediastinal lymph nodes that are ≥2 cm in diameter contain metastatic disease in over 90% of cases. Lymph nodes that are 1.5–2 cm in size contain disease in over 50% of cases. Lymph nodes that are 1–1.5 cm in size harbor metastatic disease in 15–30% of cases. The negative predictive accuracy of CT scan is 85–92% for mediastinal lymph node metastases. For these reasons, many centers are now using PET–CT imaging.
Magnetic resonance imaging
MRI is not used for the routine evaluation of patients with lung cancer, but it does have specific advantages over CT scan. Because of its heightened ability to discern neurologic and vascular structures, tumors that reside in close proximity to neurovascular structures may be more accurately assessed by MRI than by CT scan. MRI is most useful in evaluating patients with superior sulcus tumors.
Positron emission tomography
Over the past several years, PET scanning with 2-[18F]fluoro-2-deoxy-D-glucose (FDG-PET) has been increasingly used in the diagnosis, staging, and therapeutic monitoring of lung cancer. This test identifies areas of increased glucose metabolism, which is a common trait in pulmonary tumors. As commercial PET scanners provide nominal spatial resolution of 4.5–6.0 mm in the center of the axial field of view, even lesions that are ≤1 cm in diameter can be detected on the basis of an increased uptake of FDG. Although initially heralded as a reliable noninvasive method of identifying and staging pulmonary neoplasms, a number of limitations have become apparent. Many inflammatory processes such as abscesses and active granulomatous diseases, as well as hypoxic conditions such as those that exist after radiotherapy, may cause high FDG uptake and lead to false-positive results. Treatment-induced hypermetabolic inflammatory changes also may lead to difficulty differentiating between treatment effects and those of the residual tumor. False-negative results have occurred primarily in tumors with low glucose metabolism (carcinoid and AIS) and in small tumors, owing to the limited spatial resolution of current PET scanners.57
Integrated PET–CT
PET provides imprecise information on the exact location of focal abnormalities. Thus, even if the results of PET and CT scan are visually correlated, the precise location of lesions is sometimes difficult to determine. To overcome this limitation, integrated PET–CT was introduced.58 Recent studies suggested that integrated PET–CT improved the diagnostic accuracy of NSCLC. Tumor staging was significantly more accurate with integrated PET–CT than with CT scan alone (p = .001), PET alone (p < .001), or visual correlation of PET and CT scan (p = .013); node staging was also significantly more accurate with integrated PET–CT than with PET alone (p = .013).58 In patients with potentially resectable NSCLC, the accuracy of PET–CT is insufficient for mediastinal staging, and pathologic assessment of mediastinal lymph nodes remains the standard of care.59 Randomized clinical trials (RCTs) suggest that PET–CT may help in the preoperative assessment of metastatic disease, potentially leading to the avoidance of unnecessary surgery.60
Invasive studies
Tissue is collected for histopathological diagnosis, molecular studies, and genetic testing. In most cases, FNA for cytological assessment does not suffice and core biopsies are recommended. Endobronchial and centrally located tumors are usually diagnosed with bronchoscopy, whereas peripheral lesions with CT- (or US-) guided biopsies. In many cases, the biopsy is targeted to a lesion that would determine the diagnosis and the stage of the disease. For example, biopsy of a contralateral lymph node should be considered in cases where it would alter the disease stage (N stage) and a biopsy of a liver lesion would confirm the diagnosis of the primary tumor and the presence of a metastasis (M stage). In this regard, one has to consider the potential differences in the genetic expression between the primary tumor and its metastatic lesions and to avoid a biopsy of a lesion in a bone because decalcification processes could alter the genetic expression of the tumor.
FNA biopsy
Transthoracic percutaneous needle biopsy (TPNB) has significantly heightened the ability to diagnose intrathoracic pathologic processes. With CT or US guidance, tissue samples can be obtained from poorly accessible sites in the lung, mediastinum, abdomen, and retroperitoneum. The procedure is performed under local anesthesia using a small-gauge needle to either aspirate or biopsy lesions. Aspirated material is immediately processed with optimal procedural coordination. Many centers use an on-site cytopathologist for interpretation. Should the material be inadequate, a repeat aspiration can be performed. TPNB has been shown to be over 90% effective in establishing a final diagnosis. The false-positive rate is low (1%) and the false-negative rate ranges from 23% to 29%.61
Fiberoptic bronchoscopy (FOB)
Fiberoptic bronchoscopy (FOB) is an essential and standard technique for the evaluation of patients with pulmonary neoplasms; it remains the most important procedure for determining the endobronchial extent of disease. FOB permits careful survey of the supraglottic, glottic, tracheal, and bronchial regions to the level of most subsegments. Tumor (T) status can be defined by measuring tumor proximity to the carina and various bronchi and by identifying unsuspected occult lesions that indicate multiplicity of disease. For lesions that are visible by endoscopy, an accurate histologic diagnosis can be achieved in over 90% of cases. For central lesions, cytologic studies via needle aspiration, washings, and brushings, coupled with biopsy, heighten the diagnostic yield to over 95%. Peripheral lesions not visible endoscopically may be approached by cytologic studies of brushings and bronchioloalveolar lavage (BAL), which yield a diagnosis in 50–60% of patients. Cytologic studies, coupled with transbronchial fine-needle aspiration (TBNA), greatly enhance diagnostic yield.
TBNA biopsy
TBNA biopsy was introduced by Wang and Terry62 using FOB. It has been used most widely to sample endobronchial and peripheral lesions and significantly improves the diagnostic yield when coupled with standard diagnostic measures (washings, brushings, and biopsies). TBNA is best performed in a suite that is equipped with fluoroscopy to enhance localization of the lesion. One of the most important applications of TBNA is the evaluation of mediastinal lymphadenopathy. The true sensitivity and specificity of TBNA appear to range from 14% to 50% and 96% to 100%, respectively. Thus, negative results require definitive operative confirmation, but the risk of a false-positive finding appears to be quite low.63
Advances in bronchoscopy
The development of linear echo-endoscopes has opened up new diagnostic possibilities for patients with lung cancer. Transesophageal US-guided fine-needle aspiration (EUS-FNA) and transbronchial US-guided needle aspiration (EBUS-TBNA, endobronchial ultrasound transbronchial needle aspiration) are both minimally invasive diagnostic techniques that enable real-time controlled aspirations of mediastinal lymph nodes and centrally located lung tumors. Evolving reports using these technologies suggest that EBUS-FNA has higher sensitivity than TBNA and that EUS plus EBUS may allow near-complete minimally invasive mediastinal staging in patients with suspected lung cancer. These newer diagnostic technologies may serve as an alternative approach for mediastinal staging in patients with suspected lung cancer.64 Electromagnetic registration and guidance combine virtual bronchoscopy, three-dimensional (3D) CT images, and a steerable probe to aid in the biopsy of lung lesions. The yield and safety of this technology are being tested in several centers.65
Mediastinoscopy
Transcervical mediastinoscopy is the best method for invasive evaluation of the middle mediastinum to include the peritracheal and subcarinal lymph nodes. The indication remains preoperative mediastinal nodal assessment in patients with CT scan evidence of cross-sectional lymph node enlargement of ≥1 cm. In such patients who are proven to have lung cancer, the chance that these nodes contain metastasis is over 7%. If the nodes are enlarged to 1.5–2 cm or more, the risk of having metastatic involvement is over 30%. The accuracy of cervical mediastinoscopy ranges from 80% to 90%, and the false-negative rate ranges from 10% to 12%. The lymph node station most commonly missampled is the subcarinal region, which is difficult to access in some patients. The subaortic and aortopulmonary window regions are inaccessible by standard cervical mediastinoscopy.66 Extended cervical mediastinoscopy, a variation of standard mediastinoscopy, has been useful for staging lesions in the left upper lobe. The standard mediastinoscopy incision is used, with the plane of dissection extending anterior to the innominate artery and aorta, anterolaterally to the level of the aortopulmonary window. “Anterior mediastinotomy,” originally described by McNeil and Chamberlain, permits direct visual access to the anterior mediastinum through the second, third, or fourth anterior interspace, with or without removal of a short portion of the adjacent cartilage. For right-sided lesions, the procedure provides access to the proximal pulmonary artery and SVC. The procedure is used on the left side to evaluate disease in the subaortic and lateral aortic regions.
Thoracoscopy
Thoracoscopy and video-assisted thoracoscopic surgery (VATS) are used in a broader range of applications, including resectional techniques. The VATS approach is used in many thoracic conditions, and its role continues to evolve regarding the evaluation and management of lung cancer. It is currently considered for the evaluation and treatment of pleural tumors and effusions and in the diagnosis of indeterminate pulmonary nodules, and has a complementary role to standard mediastinoscopy in the staging of mediastinal lymph nodes. It has also become an accepted approach for resection of peripheral early-stage lung cancer in many centers.67
Operative staging
Operative staging provides the opportunity to verify histologically the extent of gross and microscopic diseases. The surgeon is responsible for performing a complete nodal dissection or nodal sampling as an integral part of the thoracotomy. Lymph nodes are removed and labeled according to the location of the station on the regional station map (Figure 4).
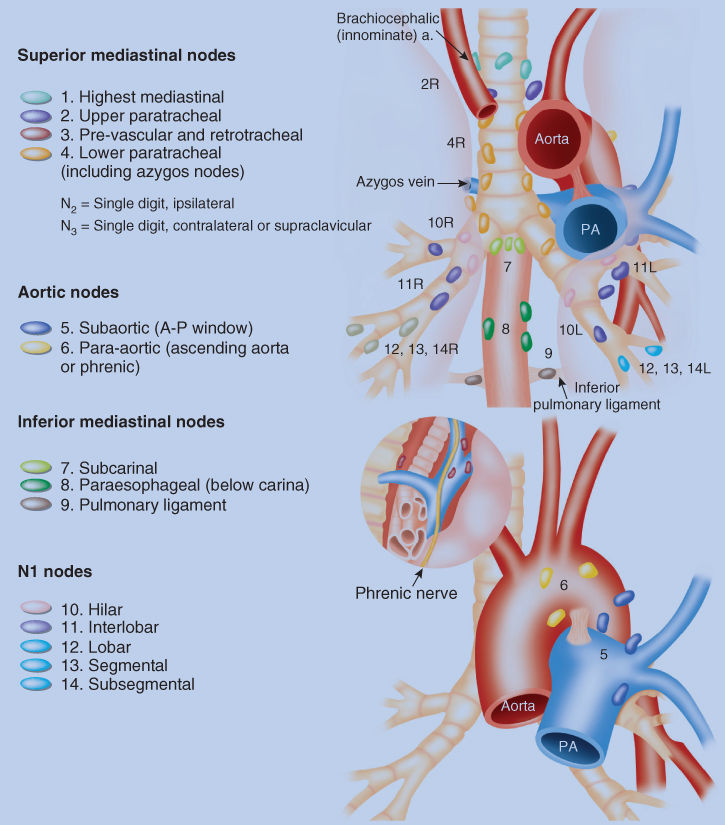
Figure 4 Regional lymph node stations for the staging of lung cancer. The location of the lymph nodes and assigned numbers are determined by the surgeon at the time of operation.
Source: Adapted from Onishi et al. 2004.68
Cancer screening and early detection
Because symptoms of early-stage localized disease are insidious and nonspecific, they are frequently attributed to the effects of smoking. By the time the patient seeks medical attention, the disease is usually advanced so that complete surgical resection is possible in fewer than 30% of cases, and the overall 5-year survival rate is <17%. Clearly, screening and early detection of cancer at a more treatable stage is a desirable goal.
In the 1970s, the National Cancer Institute (NCI) sponsored three separate RCTs to assess the efficacy of lung cancer screening in male smokers (aged 45 years or older who smoked at least one pack per day).69 By 1978, a total of 31,360 patients had been enrolled, and the final results of all three studies were unable to demonstrate a disease-specific mortality reduction.70
The introduction of low-radiation-dose spiral computed tomography (LDCT) renewed interest in screening high-risk individuals for early lung cancer.71 The NCI subsequently funded the National Lung Screening Trial (NLST), which enrolled 53,454 persons at high risk for lung cancer at 33 medical centers between 2002 and 2004. Eligible participants were between 55 and 74 years old, had a smoking history of at least 30 pack-years, and, if former smokers, had quit within the past 15 years and were randomly assigned to undergo three annual screenings with either LDCT or chest radiography. Subjects in the LDCT screening arm demonstrated a 20% reduction in lung cancer-specific mortality (95% CI, 6.8–26.7; P = 0.004).72 The current US Preventive Services Task Force (USPSTF) recommends lung cancer screening with annual LDCT for asymptomatic adults between 55 and 80 years old with at least a 30 pack-year smoking history.73
Staging systems
Staging is the determination of the extent of disease, with the intent of grouping patients with similar levels of disease for analytical, therapeutic, and prognostic purposes. The staging of lung cancer provides a scale of relative disease, which can be assigned to all patients with primary lung malignancies. Accurate staging of lung cancer is essential for defining operability, for selecting treatment regimens, for predicting survival, and for reporting comparable end results.
The accuracy of staging depends on available clinical information and relies on preoperative and subsequent evaluations at different times during the course of the disease: clinical-diagnostic staging (c), surgical-evaluative staging (s), postsurgical resection-pathologic staging (p), retreatment staging (r), and autopsy staging (a).
Staging of NSCLC
The current seventh edition of the AJCC and UICC lung cancer staging system was updated in 2009 and is based on 67,725 cases treated from 1990 to 2000, derived from 46 sources in more than 19 countries.74 This revision further improves the alignment of TNM (tumor, node, and metastasis) stage with prognosis and treatment (Tables 7–9).
Table 7 TNM descriptors
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchusa (i.e., not in the main bronchus) T1a tumor ≤2 cma T1b tumor >2 cm but ≤ 3 cm |
| T2 | Tumor >3 cm but ≤7 cm; or tumor with any of the following features:
T2a tumor >3 cm but ≤5 cm T2b tumor >5 cm but ≤7 cm |
| T3 | Tumor >7 cm or one that directly invades any of the following: chest wall (including superior sulcus tumors), diaphragm, mediastinal pleura, parietal pericardium; or tumor in the main bronchus, ≤2 cm distal to the carina but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung |
| T4 | Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, esophagus, vertebral body, carina; or tumor nodules in a different ipsilateral lobe to that of the primary |
| Regional lymph nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to ipsilateral peribronchial and/or ipsilateral hilar lymph nodes, and intrapulmonary nodes involved by direct extension of the primary tumor |
| N2 | Metastasis to ipsilateral mediastinal and/or subcarinal lymph node(s) |
| N3 | Metastasis to contralateral mediastinal, contralateral hilar, ipsilateral, or contralateral scalene, or supraclavicular lymph node(s) |
| Distant metastasis (M) | |
| MX | Presence of distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present M1a separate tumor nodule(s) in a contralateral lobe; pleural nodules, or malignant pleural or pericardial effusionb M1b distant mets |
a The uncommon superficial tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified T1a.
b Most pleural effusions associated with lung cancer are due to tumor. However, there are a few patients in whom multiple cytopathologic examinations of pleural fluid show no tumor. In these cases, the fluid is nonbloody and is not an exudate. When these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element, and the patient’s disease should be staged M0.
Source: Edge et al. 2010.75 Reproduced with permission of Springer.
| Stage | TNM subset |
| 0 | Carcinoma in situ |
| IA | T1a–T1b N0 M0 |
| IB | T2a N0 M0 |
| IIA | T1a–T2a N1 M0 |
| T2b N0 M0 | |
| IIB | T2b N1 M0 |
| T3 N0 M0 | |
| IIIA | T1a–T3 N2 M0 |
| T3 N1 M0 | |
| T4 N0–N1 M0 | |
| IIIB | T1a–T4 N3 M0 |
| T4 N2 M0 | |
| IV | Any T, Any N, M1a–M1b |
a Staging is not relevant for occult carcinoma, designated TX N0 M0.
Source: Edge et al. 2010.75 Reproduced with permission of Springer.
Table 9 Lymph node map definitions
| Nodal station | Anatomic landmarks |
| N2 nodes: all N2 nodes lie within the mediastinal pleural envelope | |
| 1. Highest mediastinal nodes | Nodes lying above a horizontal line at the upper rim of the brachiocephalic (left innominate) vein where it ascends to the left, crossing in front of the trachea at its midline |
| 2. Upper paratracheal nodes | Nodes lying above a horizontal line drawn tangential to the upper margin of the aortic arch and below the inferior boundary of No. 1 nodes |
| 3. Prevascular and retrotracheal nodes | Prevascular and retrotracheal nodes may be designated 3A and 3P; midline nodes are considered to be ipsilateral |
| 4. Lower paratracheal nodes | The lower paratracheal nodes on the right lie to the right of the midline of the trachea between a horizontal line drawn tangential to the upper margin of the aortic arch and a line extending across the right main bronchus at the upper margin of the upper lobe bronchus, and contained within the mediastinal pleural envelope; the lower paratracheal nodes on the left lie to the left of the midline of the trachea between a horizontal line drawn tangential to the upper margin of the aortic arch and a line extending across the left main bronchus at the level of the upper margin of the left upper lobe bronchus, medial to the ligamentum arteriosum and contained within the mediastinal pleural envelope. Researchers may wish to designate the lower paratracheal nodes as No. 4s (superior) and No. 4i (inferior) subsets for study purposes; the No. 4s nodes may be defined by a horizontal line extending across the trachea and drawn tangential to the cephalic border of the azygos vein; the No. 4i nodes may be defined by the lower boundary of No. 4s |
| 5. Subaortic (aortopulmonary window) | Subaortic nodes are lateral to the ligamentum arteriosum or the aorta or left pulmonary artery and proximal to the first branch of the left pulmonary artery and lie within the mediastinal pleural envelope |
| 6. Para-aortic nodes (ascending aorta or phrenic) | Nodes lying anterior and lateral to the ascending aorta and the aortic arch or the innominate artery, beneath a line tangential to the upper margin of the aortic arch |
| 7. Subcarinal nodes | Nodes lying caudal to the carina of the trachea, but not associated with the lower lobe bronchi or arteries within the lung |
| 8. Paraesophageal nodes (below carina) | Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes |
| 9. Pulmonary ligament nodes | Nodes lying within the pulmonary ligament, including those in the posterior wall and lower part of the inferior pulmonary vein |
| N1 nodes: all N1 nodes lie distal to the mediastinal pleural reflection and within the visceral pleura | |
| 10. Hilar nodes | The proximal lobar nodes, distal to the mediastinal pleural reflection and the nodes adjacent to the bronchus intermedius on the right; radiographically, the hilar shadow may be created by enlargement of both hilar and interlobar nodes |
| 11. Interlobar nodes | Nodes lying between the lobar bronchi |
| 12. Lobar nodes | Nodes adjacent to the distal lobar bronchi |
| 13. Segmental nodes | Nodes adjacent to the segmental bronchi |
| 14. Subsegmental nodes | Nodes around the subsegmental bronchi |
Staging of SCLC
When the TNM system was first developed for NSCLC in the 1960s, it was not prognostic when applied to SCLC. This was most likely explained by the very low incidence of stage I or II SCLC and the fact that without chemotherapy, all patients with SCLC had very short survival. In a placebo-controlled trial of cyclophosphamide, the Veterans Administration Lung Group (VALG) developed a two-stage system for SCLC.76 They separated patients into two groups, termed limited or extensive, based on whether or not their disease could be encompassed by a radiation port. The former group included those with malignant pleural effusion; the latter included all those with metastatic disease to distant sites. This classification was prognostic in patients on both arms of the trial, with median survival rates twice as long in limited stage patients. Through the past 20 years, the “limited” classification has been refined to identify those who are candidates for curative-intent chemoradiation.
As in NSCLC, the process of staging SCLC is key to determining therapy and prognosis. The main goal of thorough staging is to identify patients who are candidates for curative-intent chemoradiation. Patients who have clinically evident metastatic disease (extensive stage) do not require thorough staging for all potential sites of spread. Because the major intent of staging is to determine therapy, the case can be made to image the brain in all patients as positive findings are an indication for eventual brain radiation.
General guidelines for lung cancer staging
Patients who present with a new lung lesion and no evidence of metastatic disease by history, physical examination, or chest radiography should undergo CT scanning of the chest, including the liver and adrenal glands. In some circumstances, when a clinical stage I malignancy is suspected, invasive diagnostic studies can be waived, and the patient can undergo resection for diagnosis and treatment. If a resection beyond a lobectomy is required or if the patient is a high surgical risk, it is best to attempt preoperative diagnosis of the lesion. If the patient requires pneumonectomy, a cancer diagnosis should be made before proceeding with the resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree