Chemical Carcinogens
Probably the best-studied and most potent ubiquitous natural chemical carcinogen is a product of the Aspergillus fungus, called aflatoxin B1.28 Aspergillus flavus mold and aflatoxin product can be found in a variety of stored grains, particularly in hot, humid parts of the world, where grains such as rice are stored in unrefrigerated conditions. In the months following the monsoons in Southeast Asia, most village-based grains can be seen to be covered by a white layer of aflatoxin that is consumed with the grain. Data on aflatoxin contamination of foodstuffs correlate well with incidence rates of HCC in Africa and to some extent in China.
There is considerable literature on the hepatocarcinogenicity of anabolic steroids as well as the induction of benign adenomas by estrogens.29 Although estrogens are capable of causing HCC in rodents, an epidemiologic association in humans has never been clearly shown. In an industrial society, a large number of environmental pollutants, particularly pesticides and insecticides, are known rodent hepatic carcinogens. In a recent case-control study, cumulative lifetime tobacco use of more than 11,000 packs and Asian ethnicity were independent predictors of HCC development amongst a cohort of patients with chronic liver disease, including HCV.30
Multiple clinical staging systems for hepatic tumors have been described. The most widely used is the American Joint Committee on Cancer/tumor-node-metastasis (AJCC/TNM) (Table 52.2).31 Adverse prognostic features include large size, multiple tumors, vascular invasion, and lymph node spread. Macroscopic or microscopic vascular invasions, in particular, have profound effects on prognosis. Stage III disease contains a mixture of lymph node–positive and –negative tumors. Stage III patients with positive lymph node or stage IV disease have a poor prognosis, and few patients survive 1 year.
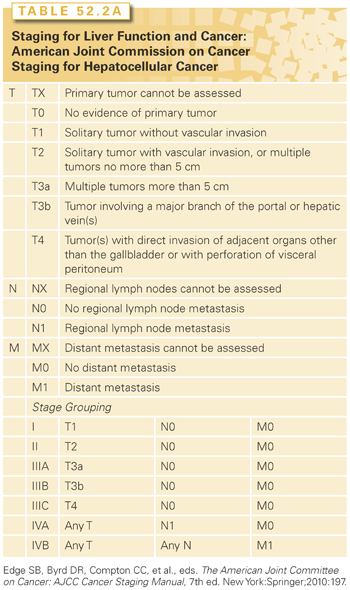

The prognosis in patients with HCC is very much influenced by the presence and severity of underlying liver disease as well. The Child-Pugh scoring system is the most commonly used tool for assessing cirrhosis (see Table 52.2).32 It encompasses five parameters—bilirubin, albumin, prothrombin time, clinical ascites, and clinical encephalopathy—each of which is scored from one to three depending on severity. The key limitation of the Child-Pugh scoring system is its lack of any parameters pertaining to the cancer itself. Despite that, it remains incorporated into many HCC clinical trials as a tool to measure the extent of liver disease in the study populations, and thus, its use may not fade away any time soon. However, this main limitation of the Child-Pugh scoring system has been overcome by other scoring systems. Among those, the first to be established is the Okuda staging system. The Cancer of the Liver Italian Program (CLIP) score was defined and studied prospectively in patients with HCC mainly caused by HCV.33,34 The CLIP score consists of the Child-Pugh score parameters combined with a subjective assessment of tumor in the liver, the presence or absence of portal vein thrombosis, and the alpha-fetoprotein (AFP) level. The addition of vascular endothelial growth factor levels to the CLIP parameters (V-CLIP) has been shown to provide a significantly more precise prognosis, but has yet to be prospectively validated.35 The Chinese University Prognostic Index (CUPI) scoring system was developed in patients with mainly HBV-related HCC.36 The CUPI parameters are bilirubin, ascites, AFP, alkaline phosphatase, the tumor extent (AJCC/TNM 5th edition), and clinical symptoms at presentation. A French system called the Groupe d’Etude et de Traitement du Carcinoma Hepatocellulaire (GETCH) staging system consists of bilirubin, Karnofsky performance score, AFP, alkaline phosphatase, and portal vein thrombosis.37 Another scoring system that is mainly used in Japan is the Japan Integrated Staging (JIS) score.38
Another commonly used scoring system is the Barcelona Clinic Liver Cancer (BCLC) classification system.39 The BCLC couples prognosis with treatment assignment and has been validated prospectively.40 However, it has been shown to be less valuable in the setting of more advanced disease, defined as BCLC category C.41,42 In retrospective analyses of patients with advanced-stage HCC seen by medical oncologists, the CLIP scoring system was noted to be the most informative regarding the outcome of this specific patient population.
The tests used to diagnose HCC include radiologic studies and pathologic diagnosis with biopsy. Core biopsies are most preferred because of the tissue architecture given by this technique. For patients suspected of having portal vein involvement, a core biopsy of the portal vein may be performed.43 Morphologic features, such as stromal invasion, help distinguish high-grade dysplastic nodules from HCC.44
The American Association for the Study of Liver Diseases (AASLD),45 and the European Association for the Study of the Liver (EASL)46 have outlined noninvasive criteria for the diagnosis of HCC. EASL recommends that lesions that are greater than 2 cm with characteristic radiologic features of arterial hyperenhancement on two different imaging modalities, or on one imaging modality alongside with a serum AFP of 400 ng/dL or more, are diagnostic of HCC, and no biopsy is needed. The AASLD added venous washout as a requisite radiologic feature. Detection of a lesion larger than 2 cm that exhibits both arterial hyperenhancement and venous washout in a single imaging modality concomitant with an AFP >200 ng/mL is sufficient to diagnose HCC.47 Bialecki et al.48 found a sensitivity and specificity of 89.1% and 100%, respectively, for liver biopsy compared to 64.9% and 62.8%, respectively, for the noninvasive EASL criteria. The fear of biopsy-related hemorrhage is dissuaded by a 0.4% rate, and tumor seeding occurs at a low rate of 1.6%. When seeding does occur, it can be treated by local resection and is seldom a cause of morbidity and mortality.49,50
TREATMENT OF HEPATOCELLULAR CARCINOMA
Many treatment options for HCC are available (Table 52.3). Resection and liver transplantation represent the potentially curative options with the longest track record. For small tumors, ablation and radiotherapy (RT) are quite effective and may be curative.

Surgical Resection for Hepatocellular Carcinoma
Patient Selection
Liver resection is the preferred treatment for the noncirrhotic patient with HCC. These patients generally have normal liver function, no portal hypertension, and can tolerate major liver resections with acceptable morbidity and low mortality. The selection of noncirrhotic patients with HCC for resection is as for other malignant lesions. Resection should be considered for patients where a complete resection of tumor is possible while preserving greater than 30% functional liver. If the potential remnant liver volume may be less than 30%, portal vein embolization is now a well-accepted preoperative preparatory method for increasing the potential remnant liver volume and safety of the resection.51
For cirrhotic patients, the primary determinant of outcome and selection of therapy is the degree of hepatic dysfunction and portal hypertension. Traditionally, only compensated cirrhotics (Child-Turcotte-Pugh class A) were candidates for hepatic resection, whereas patients with significant hepatic functional dysfunction (Child class B or C) are generally not selected for resection because of poor outcome.51 Portal hypertension can be indirectly assessed clinically by the presence of splenomegaly, esophagogastric varices, and thrombocytopenia (platelet count <100.000/mm3) or directly determined by hepatic venous wedge pressures (≥10 mmHg). With recent advances in perioperative care, there is growing evidence that liver resection for HCC in well-selected patients with mild portal hypertension is safe and can achieve a comparable outcome as in patients without portal hypertension.52,53
The future remnant liver mass is another important factor to be considered in cirrhotic patients before resection. A too small remnant liver volume is associated with an increased risk for postresectional liver failure.54 There is a general consensus that the critical remnant liver volume in cirrhotic patients is 50%,55 and portal vein embolization should be considered if the future remnant liver volume is expected to be below 50%.56–59 Some investigators have even attempted to sequential employ transarterial chemoembolization (TACE) to control the tumor or portal vein embolization (PVE) to increase residual liver volume, followed by definitive surgical resection.56,57 The sequential use of TACE and PVE results in more efficient hypertrophy of the future remnant liver compared to PVE alone.51
In other parts of the world, dynamic liver function tests are also employed for the assessment of suitability for liver resection. These include the indocyanine green (ICG) test and technetium-99m diethylenetriaminepentaacetic acid-galactosyl human serum albumin (technetium-99m galactosyl human serum albumin [99mTc-GSA] scintigraphy).51,55 An ICG retention rate of less than 14% proved to be a safe indicator for major hepatectomy in cirrhotic patients, whereas retention rates above 20% are considered a contraindication for major hepatectomy.60–62
Patient medical comorbidities considered are similar to an assessment for any major surgery. Some studies have reported age and gender to be independent risk factors for poor outcome after resection of HCC.63 Other studies indicate that advanced age is more a surrogate for medical fitness, and that, with careful patient selection, elderly patients benefit as much from resection as younger patients.64 Comorbidities as represented by American Society of Anesthesia (ASA) grade have been shown to correlate with survival.65
Outcomes of Resection and Prognostic Factors
With improving patient selection and perioperative care, the outcome of hepatic resection for HCC has been continuously improved during the past 2 decades. Many large series of the past 10 years show that resection is associated with a perioperative mortality rate of less than 7%, and patients achieve an overall survival rate of 30% to 50% (Table 52.4).65–75 Many major centers are recently reporting operative mortalities less than 2%,68,70,74 even in cirrhotic patients.

Tumor factors most important for outcome are TNM staging at presentation, macro- and microvascular invasion, and the number of tumors. Large HCC has a propensity for vascular invasion and growth of tumor intraluminally. This is associated with intrahepatic satellite metastases via the portal venous system and is frequently associated with small satellite tumors. Intraluminal spread through the hepatic veins leads to pulmonary metastases.
One surgical factor prognostic for outcome is surgical margins. There is no clear margin size that has been universally agreed upon, but there is consensus on importance of an R0 resection.76 Most surgeons prefer at least a 1-cm margin. In one 225 patient study, a 1-cm margin was associated with a 77% 3-year survival versus 21% for those with less than a 1-cm margin.72 It must be noted that in a randomized controlled trial, a 2-cm margin was associated with a decrease in recurrence as well as improved survival.77 Studies have demonstrated improved outcome for anatomic versus nonanatomic resections for HCC.78 In a series of 210 patients, 5-year survival rates were 66% for anatomic versus 35% in nonanatomic resections.72 For small solitary tumors, anatomic resections seem to be less important.79 Choice of margin and the anatomic approach for cancer clearance must be weighed against better perioperative outcome for limited parenchymal resection in cirrhotic patients.
Liver Transplantation for Hepatocellular Carcinoma
Patient Selection
Theoretically, liver transplantation is the ideal therapy for HCC in cirrhotic patients because it treats both the cancer as well as the underlying parenchymal disease. However, early experience with transplants produced dismal results. Bismuth et al.80 was one of the first groups to consider that, in advanced disease, the likelihood of systemic disease was so high that recurrence rates, and therefore long-term outcomes, were unacceptably poor. They demonstrated that patients with limited disease (uninodular or binodular <3 cm tumors) had much better outcomes with transplant than resection (83% 5-year survival versus 18%).80
The landmark works of Mazzaferro et al.81 have defined the most commonly used criteria for the selection of patients with HCC for transplantation. In their paper they defined the Milan criteria for transplantation as a single tumor less than 5 cm or three or fewer tumors all individually less than 3 cm (Fig. 52.1). Using these criteria for selection, patients transplanted had a very favorable outcome, including a 4-year actuarial survival rate of 85% and a recurrence-free survival rate of 92%. The suitability of these criteria for the selection of patients for transplant has been confirmed by numerous studies (Table 52.5).81–94

The excellent outcomes of HCC patients within the Milan criteria led many to explore more expansive and inclusive criteria.90 The most accepted of the expanded criteria is that from the University of California San Francisco (UCSF) group. They reported excellent results after transplant for solitary tumor ≤6.5 cm, three or fewer nodules with the largest ≤4.5 cm, and total tumor diameter ≤8 cm (see Fig. 52.1). The UCSF criteria was associated with a survival of 90% and 75% at 1 and 5 years, respectively.90,95

The largest experience to date using transplantation for HCC was reported from the University of California, Los Angeles (UCLA).96 In this study of 467 transplants performed for HCC, the overall 1-, 3-, and 5-year survivals were 82%, 65%, and 52%, respectively. Transplanted patients with tumors beyond the UCSF criteria had a survival below 50%.
Living Donor Liver Transplant
Because of the shortage of cadaveric livers, living donor liver transplant (LDLT) has become an increasingly utilized modality for the treatment of patients with decompensated cirrhosis. In many Asian countries, where prevalence of HCC is high, living related transplants are the most common liver transplants performed. Survival outcomes for all patients undergoing LDLT are comparable to the results with deceased donors (Table 52.6).97–102 The disadvantage is clearly the risk to the living donor, with morbidity as high as 40% and a 0.5% mortality. The greatest concern is that LDLT may encourage transplants for patients with unfavorable biology (outside of the established Milan or UCSF criteria) and pose unjustified risk to two lives, including the healthy individual.

Multimodality Management While Awaiting Transplant
To qualify for the wait list, a biopsy or one of the following criteria must be fulfilled: AFP >200 mg/mL, arteriogram confirming the tumor or arterial enhancement followed by portal venous washout on computed tomography (CT) scans or magnetic resonance imaging (MRI), or a history of local–regional treatment. Patients should be assessed radiologically for number and size of tumors, to rule out extrahepatic disease and vascular involvement. Patients with tumors less than 2 cm in size or patients who do not qualify for the Milan criteria can be listed for transplant, but they will receive no additional priority points for the tumor. The tumor should be assessed every 3 months by CT scans or MRI to rule out progression of disease beyond the established criteria.
To reduce the likelihood of tumor progression while on the wait list, many local treatments are used, including TACE, percutaneous radiofrequency ablation (RFA), or percutaneous ethanol injection (PEI). TACE involves selective embolization of the arterial feeding vessels for the hepatoma with occlusive particles with or without admixed chemotherapeutic agents. TACE limits wait list dropout, decreases posttransplant recurrence, and can downstage HCC that is beyond transplant criteria.103 A dropout rate of 14% was found in a series of patients treated with TACE as bridge to transplant, which compared very favorably to a dropout rate of 38% for an untreated group of patients reported by the Barcelona Liver Cancer study group.104,105
For small, solitary tumor, PEI106 or RFA can be effective treatment options for use as a bridge to transplant. In a series of 52 patients treated with RFA, the dropout rate was 6% at 12 months due to tumor progression with a 3-year disease-free survival of 76% for the 41 patients eventually transplanted.107 Mazzaferro et al.108 reported no dropout for their patients treated with RFA as bridge to transplant, with a 3-year survival of 83%.
ADJUVANT AND NEOADJUVANT THERAPY
Adjuvant Therapy
A recent report of a randomized trial of adjuvant IFNα-2b described an improved 5-year survival in patients with stage III/IVA tumor from 24% to 68% (p = 0.038).109 In a separate, larger study, IFNα-2b therapy was not associated with reducing postoperative recurrence in a population at risk for viral hepatitis–induced hepatocellular carcinoma. In this study of 268 patients, the median recurrence-free survival was 42.2 and 48.6 months with observation and IFNα-2b therapy, respectively (p = 0.828).110
Another approach that has been evaluated is intrahepatic 131I-lipiodol. In a randomized study of 43 patients, adjuvant 131I-lipiodol administered via the hepatic artery was compared with observation after resection of HCC. There was an improvement in median disease-free survival noted in favor of the 131I-lipiodol arm (57.2 versus 13.6 months; p = 0.037).111 However, a long-term follow-up of this trial at 10 years failed to show the same survival advantage.112
The best-studied systemic adjuvant therapy so far is acyclic retinoid, which was evaluated against placebo following surgical resection in 89 patients.113 Patients who received acyclic retinoid had a recurrence rate of 27% compared with 49% for patients who received placebo (p = 0.04) after a median follow-up period of 38 months. The prevention of second primary HCC was more marked after a median follow-up of 62 months (p = 0.002, log rank test), but the difference in survival rates did not reach significance until after 6 years, with an estimated 6-year survival of 74% versus 46%, respectively (p = 0.04).114
Sorafenib, which will be discussed at length in the Systemic Therapy section, was also studied in the adjuvant setting. In a pilot study of 31 patients with HCC who had undergone curative resection and were at high risk of recurrence, sorafenib prolonged the time to recurrence (21.5 versus 13.4 months; p = 0.006) and significantly lowered recurrence rate (29.4% versus 70.7%; p = 0.006).115 A currently fully accrued yet not reported phase III trial (STORM) randomized patients to adjuvant sorafenib versus placebo after surgery, radiofrequency ablation, or percutaneous alcohol injection with a primary end point of recurrence-free survival (Clinical Trial: NCT00692770). A phase II randomized, placebo-controlled study evaluating whether adjuvant sorafenib can prevent recurrence of HCC in high-risk orthotopic liver transplant recipients is currently enrolling (Clinical Trial: NCT01624285).
Neoadjuvant Therapy
Two randomized controlled trials and seven nonrandomized trials have evaluated preoperative transarterial chemotherapy. No clear advantage in disease-free or overall survival was found in these studies.116–118 Postoperative transarterial chemotherapy has been examined in four randomized controlled trials and three nonrandomized controlled trials. A meta-analysis of these trials revealed a significant improvement in disease-free and overall survival.117 The regimens consisted of Lipiodol and chemotherapy agents, including doxorubicin, mitomycin, and cisplatin. An analysis of postoperative adjuvant systemic chemotherapy trials demonstrated no consistent advantage in terms of disease-free or overall survival.113,119–122
A combination regimen of systemic therapy that has been studied extensively in advanced HCC provided input for its potential use in the neoadjuvant setting. PIAF (cisplatin, IFNα-2b, doxorubicin, and 5-fluorouracil) have shown in a phase II study a response rate of 26% with a median survival of approximately 9 months.123 Of the 13 patients (26%) who had a partial response, 9 underwent surgery and 4 (9%) achieved a complete pathologic response to chemotherapy. These results illustrated that chemotherapy for HCC is effective in selected patients and suggest the possible use of PIAF as neoadjuvant therapy for medically fit patients with good liver function in whom cytoreduction might permit future resectability. The potential for cure would justify the risk of the significant toxicity profile of PIAF.
Choice of Resection, Ablation, or Transplantation
Although liver resection versus liver transplantation as primary therapy for patients with small HCC and adequate hepatic reserve is hotly debated, in most cases resection and OLT are complementary and not competing therapies. A number of studies report comparable overall survival rates for primary resection and primary OLT for transplantable HCC (Table 52.7).89,124–134

Patients with limited disease have potentially curative treatment options. There is general consensus that in patients with no underlying liver dysfunction, limited disease should be resected because this gives the best chance of cure without an ongoing need of immunosuppression dictated by transplantation. The outcomes of resection are quite good, with 5-year survivals of 30% to 50% (see Table 52.4).
For patients with end-stage liver disease (ESLD) and limited HCC, OLT is currently the best treatment modality. However, OLT can only be offered only to a small proportion of patients due to donor organ shortage. Therefore, liver resection remains the most important surgical therapy in patients with HCC and well-preserved liver function (Child class A).51 Using resection as first-line therapy with salvage transplantation saved for recurrence is a common approach, especially for geographic regions with a high incidence of HCC and a low liver donation rate.135,136 Resection can, therefore, be used as bridge therapy before OLT to control the tumor burden in patients who fulfill the Milan81 or UCSF90 criteria. In a study from a large Asian transplant center, the majority of patients (79%) who developed tumor recurrence after resection of small HCC were still eligible for salvage transplantation.137 Primary resection also allows the opportunity to pathologically assess the tumor and adjacent liver tissue.136 Pathologic prognostic factors such as micro- or macrovascular invasion, satellitosis, or occult tumors can be used as selection criteria for salvage liver transplantation in the case of recurrent tumor disease.
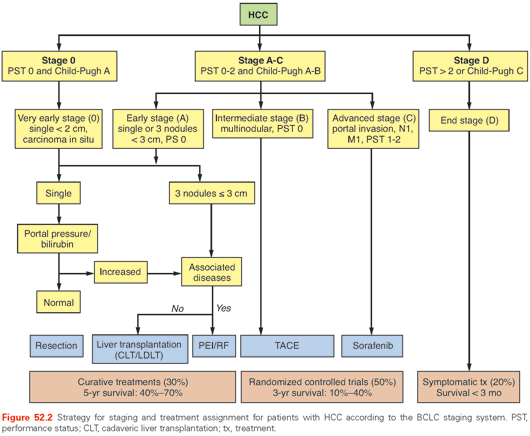
Patients with large HCC beyond the Milan or UCSF criteria have a less favorable outcome than those with small HCC.81,90 Patients in the United States outside the accepted criteria can be transplanted based on the physiologic Model for End-Stage Liver Disease (MELD) score but do not receive any exception points for HCC. In an analysis of 94 patients with HCC exceeding the Milan criteria, results of resection was compared to OLT.138 The overall survival rate was 66% for both groups even though the mean tumor size was 10 cm for the resection group and 6.4 cm for the OLT group. The results suggest that resection and OLT in patients with HCC beyond the Milan criteria have similar outcomes. A proposed algorithm of care from the Barcelona Clinic is shown in Figure 52.2.
Ablative Therapy For Localized Hepatocellular Cancer
Chemical Ablation
Chemicals destroy tumor tissue by direct dehydration of the cytoplasm, protein denaturation, and consequent coagulation necrosis as well as from indirect ischemia from vascular thrombosis from endothelial damage.139,140 The direct instillation of chemicals such as absolute ethanol or acetic acid has been long studied for the treatment of HCC.141–143 Chemical ablation is very inexpensive and, therefore, it is more widely used in developing countries with a high incidence of HCC. Intratumoral instillation of acetic acid for the treatment of HCC compares favorably with that of ethanol treatment.144,145 Moderate quality evidence supports that RFA is superior to chemical ablation for the treatment of HCC.146 Chemical ablation requires multiple applications, and thus, thermal ablation has largely replaced chemical ablation in many cases. In certain anatomic locations, such as adjacent to the major biliary tree or gallbladder, the combination of thermal ablation and ethanol ablation has shown some benefit.147,148
Radiofrequency Ablation
RFA is currently the most widely used ablative technique for the treatment of liver malignancies. The term radiofrequency applies to all electromagnetic energy sources with frequencies less than 30 MHz, although most clinically available devices function in the 375 to 500 kHz range. The technique for thermal ablation in the liver by using RFA was first described in animal liver models in 1990149,150 and was later reported in a patient in 1995.151
In this technique, the RF electrode is placed into the tumor with imaging guidance. The electrode is coupled to an RF generator and is grounded by means of a grounding pad or pads applied to the thighs. The RF generator produces a voltage between the active electrode (applicator) and the reference electrode (grounding pad), establishing electric field lines that oscillate with the alternating current. This oscillating electric field causes electron collisions with the adjacent molecules closest to the applicator, inducing frictional heating.152 Tissue heating to temperatures greater than 60°C leads to immediate cell death. Thus, for any given RFA procedure, the application of energy from the applicator is maximized to create a zone of tissue necrosis that encompasses the tumor and a margin of normal parenchyma.153 The volume of ablation achieved is based on the energy balance between heat conduction of the local RF energy applied and heat convection from the circulating blood and extracellular fluid.
In the United States, there are three commercially available percutaneous RFA systems (Fig. 52.3). Two of the systems (Boston Scientific/RadioTherapeutics and RITA Medical Systems) use a deployable RF array electrode that consists of 4 to 16 small wires (tines) deployed through a 14- to 17-G needle. Because the tines of the Boston Scientific device (LeVeen electrode) curve back toward the handle (see Fig. 52.3), the array is initially deployed in the deep portion of the tumor. In contrast, the RITA electrode tines course forward and laterally so the probe is deployed on the near surface of the tumor. The LeVeen electrode measures impedance only, and treatment time depends on repeated increases in impedance during active heating, which is a measure of tissue desiccation indicative of adequate thermocoagulation. With the RITA system, temperature readings are obtained throughout the ablation cycle from multiple peripheral thermocouples. The RITA system also has perfusion electrodes (see Fig. 52.3) that introduce small amounts of saline into the tissue to enhance the distribution of tissue heating. The third RF system (Cool-tip; Covidien) utilizes a single or triple “cluster” perfusion electrode (three single electrodes spaced 5 mm apart; see Fig. 52.3), the tip of which is positioned in the deepest part of the tumor.

Cold saline or water is pumped internally within the shaft of the electrode to keep its tip cooler than the adjacent heated tissue, thus reducing charring, which in turn helps the thermal conduction to occur at a greater distance from its source. The single or cluster RF electrode contains a thermocouple embedded in its tip, which is used to measure intratumoral temperature. A switching controller can be used with the Covidien system, allowing the placement of up to three separate single electrodes spaced 1.5 to 2.5 cm apart, thus increasing the duty cycle of the generator to enable the creation of a greater volume of tissue thermocoagulation in a single application, as compared with three separate ablations that might be required with a single electrode. Most of the thermal ablation data regarding the treatment of liver tumors has been reported with RFA.154–156
Microwave Ablation
Like RFA, microwave ablation (MWA) uses electromagnetic waves to produce heating. Unlike RFA, the MW energy is not an electrical current and is in a much higher frequency range that extends from 300 MHz to 300 GHz. The broader deposition of MW energy creates a much larger zone of active heating. MW applicators available for clinical use generally operate in the 900 to 2,450 MHz range.157 Microwave tissue heating occurs because of the induction of kinetic energy in surrounding water molecules. Because of their electron configuration, water molecules have highly polar properties and function as small electric dipoles, with the negative charges preferentially localized around the oxygen nucleus. The rapidly alternating electric field of the MW antenna causes water molecules to spin rapidly in an attempt to align with electromagnetic charges of opposite polarity. These spinning water molecules interact with neighboring tissues, transferring a portion of their kinetic energy. Because temperature is merely a proxy measurement of molecular kinetic energy, this energy transfer results in local tissue hyperthermia.
Currently, there are six MWA systems that are commercially available in the United States and Europe.158 These systems use either a 915-MHz generator (Evident, Covidien; MicrothermX, BSD Medical, Salt Lake City, UT; Avecure, MedWaves, San Diego, CA) or a 2,450-MHz generator (Certus 140, NeuWave Medical, Madison, WI; Amica, Hospital Service, Rome, Italy; Acculis MTA, Microsulis Medical, Hampshire, England) and straight antennae with varying active tips 0.6 to 4.0 cm in length. Perfusion of the antenna shaft is required for five of the six systems, with either room-temperature fluid or carbon dioxide to reduce conductive heating of the nonactive portion of the antenna, thus preventing damage to the skin and tissues proximal to the active tip. A single applicator is used with a single generator in four of the systems. Two have the ability to power up to three antennae with a single generator and treat large tumors (Fig. 52.4). Because most of the microwave systems have only recently received U.S. Food and Drug Administration approval, there are no published data at this time on the differences between systems in clinical safety or effectiveness. Similar safety and efficacy results for the treatment of metastatic colorectal liver metastases have been reported.159 Perceived advantages of MW over RF energy include a greater heating profile and less severe heat sink effects.160 Less heat sink effects may reduce local recurrences, and the larger resultant ablative volume when using the synergistic effect of multiple applicators simultaneously will allow a faster treatment time when compared to RFA.161,162 Large trials evaluating safety show a similar safety profile compared with RF.163 In Asia, MWA technology has been used for the treatment of liver tumors for a long period of time, and their extensive experience has created the development of appropriate treatment guidelines.163

Outcomes of Ablations
To date, the literature is replete with retrospective, single and multicenter cohorts with very few randomized controlled or cooperative group trials evaluating the benefits of liver ablation. Regardless, many institutions around the world perform liver ablation for primary and metastatic liver tumors given its relative safety, low cost, and low toxicity. Many factors affect the success of thermal ablation treatment for liver malignancies, some of which include: tumor size, proximity to blood vessels, operator experience, the presence of underlying liver disease, extrahepatic disease in patients with secondary liver malignancies, overall patient health, and implementation alongside synergistic therapies in a collegial, multidisciplinary treatment clinic. The ever present argument for surgery as first-line treatment in these patients would be to properly stage the patient because preoperative imaging can underestimate the extent of liver and extrahepatic disease.164 Of course, not all patients are fit for surgery, and ablation is an attractive minimally invasive option for older and frailer patients. Tumors adjacent to larger (>3 mm) blood vessels may be undertreated due to the thermal sink effect.165 Proper device selection to effectively eradicate tumors adjacent to vessels should be improved, in theory, with hotter energy sources such as MWA compared to RFA.166 A novel, largely, nonthermal electrical ablation technology called irreversible electroporation that is purported to be unaffected by thermal sink effects may play a role in treating tumors adjacent to blood vessels and critical structures, but there are no mature data at this time.167
With a myriad of thermal ablation devices available to hepatic surgeons, hepatologists, and interventional radiologists, studies have shown that it is incumbent upon these operators to gain experience with a particular device before optimal results can be expected.164,168 This can be difficult not only in low patient volume ablation practices, but also in general given the rapid technologic changes that occur in this arena whereby a newer technology is perceived to be an improvement over an existing technology prior to rigorous scientific study. The presence of underlying cirrhosis will affect treatment options and outcomes in patients presenting with HCC. In general, HCC patients with Child-Pugh class C cirrhosis who are not on a liver transplant list and do not undergo liver-directed therapies have a median survival of less than 4 months. Therefore, treatment with thermal ablation is unlikely going to affect long-term survival.169 In patients with Child-Pugh class A cirrhosis, data suggest thermal ablation can rival surgery when tumors are solitary and smaller (<5 cm) and less in number (< three tumors each under 3 cm).170 However, for patients who are healthier with a normal performance status, surgical resection may provide better long-term survival compared to RFA.171 It makes clinical sense that in patients with limited extrahepatic disease, liver-directed therapy could improve outcomes, although the outcomes themselves may be as much due to underlying tumor biology and not necessarily the treatment.154,155 Given the complexity of any given patient with hepatic malignancy, it is important to first and foremost apply any treatment or treatments under the supervision of a team of experts whose primary goal is to provide the most cost-effective, comprehensive treatment based on evidenced-based medicine when available alongside patient centered outcomes.
Liver Resection Versus Ablative Therapy for Hepatocellular Carcinoma
With recent improvements in imaging that allows for the early diagnosis of cancer and facile guidance for interventional therapies, ablative modalities such as RFA are increasingly accepted as effective treatment for small tumors. Ablative techniques are less invasive and have the promise of being better tolerated than resections or transplantation. In retrospective studies, data suggest that for small HCCs (≤2 to 3 cm), RFAs result in similar outcomes as resections.172–174
Recently, four randomized trials comparing RFA and hepatic resection have been reported (Table 52.8).175–178 Two of them, Chen et al. and Huang et al., compared tumors fulfilling the Milan tumor criteria for transplantation. Chen et al.175 compared resection to RFA for tumors less than 5 cm in size. The 1-, 3- and 4-year survivals were 93%, 73% and 64%, respectively, for resection, and 96%, 71%, and 68%, respectively, for RFA. The authors concluded that RFA was as effective as surgical resection in the treatment of solitary HCCs ≤5 cm in terms of overall and disease-free survival after 4 years with no significant difference in outcome between the two groups on follow-up. Huang et al.177 concluded that surgical resections have better outcomes than RFAs. This conclusion was based on a recurrence rate at 5 years of 63% in the RFA group and 41% in the resection group. However, it must be pointed out that more patients in the resected group had tumors less than 3 cm in size. In addition, the overall survival was not statistically different between the two treatment groups.

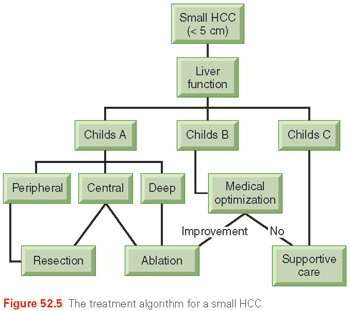
Feng et al.178 and Liang et al.176 confirmed the similar efficacy of RFA to resection in tumors <4 cm. Both groups found RFA and resection to have similar overall survival in a follow-up period after 3 years. It appears that for small HCCs, particularly in patients with cirrhosis, RFA can produce similar cancer outcomes with much lower morbidity. Figure 52.5 illustrates a recommended algorithm of care for small HCCs.
Embolic Therapies for Regional Disease
For patients with multifocal liver-predominant disease who are not candidates for resection or transplantation, transcatheter ablative methods have emerged as the most commonly used treatment worldwide. These techniques rely on the dual blood supply of the liver: arterial and portal venous. The portal vein provides over 75% of the blood flow to the hepatic parenchyma, whereas the hepatic artery is the primary nutrient supply of tumors. Selectively delivering agents transarterially targets the tumor while sparing the liver.
There are currently three main categories of percutaneously administered transcatheter intra-arterial therapies: TACE, bland hepatic artery embolization (HAE), and radioembolization (RAE). The usual chemotherapeutic agents used are mitomycin C, doxorubicin, and aclarubicin. The majority of the effects of embolic therapies derive from tumor ischemia produced by occlusion of the arterial vessels. Thus, bland embolizations (without chemotherapy), even with a nonpermanent agent such as Gelfoam, can produce a high likelihood of tumor killing. RAE involves the administration of yttrium-90 (a pure β emitter) that can be loaded in glass or resin microspheres intra-arterially.179 This is really not an embolization, in that the goal is not occlusion of the arterial inflow, but more brachytherapy. RAE will be further discussed.
Patient Selection
Performance status, underlying liver disease, and degree of portal hypertension are important patient selection criteria. Although minimally invasive, following embolization, patients commonly experience a postembolization syndrome of pain, fever, and nausea that may last for several days to a few weeks. It often takes 4 to 6 weeks to recover to baseline performance status.
Although embolization in patients with normal liver, or well-compensated cirrhosis, has a low risk of liver failure, the risk of further compromising liver function and hastening death in poorly compensated cirrhosis is significant. This is because a basis of TACE is that the portal blood flow will protect the noncancerous liver from the treatment agents and ischemia. Thus, portal vein occlusion is considered a contraindication to both TACE180 and HAE because of the risk of liver failure. Ascites, which is an indication of severe portal hypertension, or measured reversal of portal blood flow is a relative contraindication.
Results of Treatment
It has always been apparent that embolic therapies can result in a high rate of tumor response (>50%). Excellent results (level IIa evidence) following chemoembolization have also been reported from Japan in 8510 patients treated between 1994 and 2001, with 1-, 3-, and 5-year survival of 82%, 47%, and 26%, respectively.29 With well-designed trials, there is also now level I evidence of a survival benefit to conventional TACE as demonstrated in randomized trials published by Llovett et al.,181 Lo et al.,182 and Becker et al.183 In the trial by Lo et al.,182 patients were randomized to TACE (cisplatin + Lipiodol + Gelfoam) versus control (no treatment). The 2-year survival was 31% for TACE versus 11% for controls. In the trial by Llovet et al.,181 patients randomized to TACE (doxorubicin + Lipiodol + Gelfoam) had a 2-year survival of 63% versus 27% for control. In the study from Becker et al.,183 TACE (mitomycin C [MMC] + Lipiodol + Gelfoam) + PEI resulted in a 39% 2-year survival compared to 18% for TACE. What seems clear from these data is that arterial embolotherapy is an effective method of treating HCC and can prolong the patient’s survival. Comparable, or better, survival results have been demonstrated with bland embolization.119
Radiation Therapy for Hepatocellular Carcinoma
Radiation therapy for liver tumors was historically limited by hepatic toxicity but, with improved imaging, treatment planning, and treatment delivery, it now is an excellent option, particularly for patients with unresectable tumors or who are medical inoperable. Tumors not likely to be effectively treated by radiofrequency ablation due to size over 3 cm or proximity to the diaphragm, large vessel, or gallbladder are also good candidates for RT. With proper care, tumors near gastrointestinal (GI) structures can also be treated. Side effects are typically minimal, and commonly include mild fatigue, and less commonly include nausea, mild radiation dermatitis, pain associated with tumor edema, or worsening liver dysfunction. Radiation can be delivered using external beam or brachytherapy. Patients with metastases to the bone, brain, adrenals, or other locations can be effectively palliated with RT, as can patients with pain from large primary tumors.184
External Beam Radiotherapy
Fractionated Treatment
Because the liver is one of the most radiosensitive organs in the body, treatment planning and delivery must be done carefully to maximize dose to the tumor and to minimize dose to the normal liver. The primary toxicity of concern is radiation-induced liver disease (RILD), which can be categorized as classic and nonclassic. Classic RILD is a constellation of anicteric ascites, hepatomegaly, and elevated liver enzymes (particularly alkaline phosphatase), which typically occurs within 4 months of therapy and is a type of veno-occlusive disease, similar to that which can be seen after high-dose chemotherapy conditioning for bone marrow transplantation.185 Nonclassic RILD, a recently coined classification, can occur in patients with hepatitis and cirrhosis, and is characterized by jaundice and markedly elevated serum transaminases (>5 times the upper limit of normal) within 3 months of completion of therapy. This is thought to represent direct hepatocyte rather than endothelial injury.186 RILD is typically self-limited, but can be serious, even leading to mortality. It is managed symptomatically, using diuretics and paracentesis. Even low doses to the whole liver of 25 Gy in 10 fractions or 32 Gy in 1.5 Gy per fraction twice daily are associated with a more than 5% risk of radiation-induced liver disease, particularly for patients with cirrhosis and already compromised liver function. Other toxicities that can occur include gastric or duodenal bleeding,187 although both of these risks can be minimized using careful treatment planning, image guidance, and treatment delivery. It is important to consider factors including cirrhosis, prior liver-directed therapies, and hepatitis B and C, which add to the dose and volume models for the prediction of RILD. ICG is also used in Asia as a pretreatment assessment for the safety of RT, similar to its use for the safety of liver resection.188,189 In the United States, the University of Michigan has pioneered its use to measure individual tolerance to radiation, which can be quite variable. Even some patients with good pretreatment liver function can experience rapid decline, so customizing treatment is crucial.190
At the University of Michigan, investigators created the normal-tissue complication probability (NTCP) model that quantitatively described the relationship between radiation dose and liver volumes and the probability of developing RILD.191 Dose was customized per patient to give an anticipated risk of 5% to 15%. Due to the variety of tumor and liver sizes and geometries, prescribed doses ranged from 40 to 90 Gy. Patients who received 75 Gy or more had a higher median survival of 24 versus 15 months. Progression-free survival was also improved with higher doses.192–195 A prospective phase II trial from France treated patients to 66 Gy in 2 Gy fractions, with a response rate of 92%.196 The largest published experiences are from Asia, which has a very high incidence of HCC. In a multicenter retrospective patterns-of-care study among 10 institutions in Korea, 398 patients with HCC were described. Of those, 70% were Child class A, 54% had tumors >5 cm, and 40% had portal vein thrombosis. Nearly all had received prior treatment, mostly TACE. In this series, higher dose (biologic effective dose over 53 Gy) also correlated with better survival.197 Other groups have combined TACE and RT, with various schedules, although mostly using RT to treat residual disease. In a Korean study of 73 patients with incomplete response to TACE, 38 received RT, whereas the rest received additional TACE. Patients who received radiation had a higher 2-year survival of 37% versus 14% (p = 0.001).198,199
Another use for RT is to treat tumor thrombus in the portal vein, with the goals of decreasing portal pressure and allowing the safe delivery of embolic therapies. The largest series from Taiwan reports a 25% response rate. Compared with a dismal 1-year survival of 9% for nonresponders, responders had a better survival of 21% for those still not eligible for definitive therapies, and 29% for those who ultimately had additional therapy.200
Other crucial components of a liver RT program not always available for standard RT include adequate imaging with arterial and portal venous phase imaging with CT scans and MRI for tumor delineation, motion assessment, and management tools four-dimensional computed tomography (4D-CT) scan, treatment gating), and precise image guidance (cone beam CT scan) on the treatment machine. Because liver tumors move with respiration, accurate assessment and management of this will aid in proper tumor targeting and normal tissue avoidance. Some common methods include forced breath hold for tumor immobilization and 4D-CT scans to assess and help account for motion.201,202 Fiducials can also be placed within or near the tumor percutaneously prior to treatment planning, with daily alignment on the treatment machine, because external anatomy is a poor surrogate for internal anatomy and tumor location.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








