Other pathologically relevant variables in RCC include nuclear grade, sarcomatoid and rhabdoid differentiation, tumor necrosis, and vascular invasion. Nuclear grade usually follows the Fuhrman grading system18 and is most often and best used for clear cell RCC (ccRCC) as the prognostic value in non-ccRCC remains largely unproven. Sarcomatoid differentiation exists in 5% of RCC and can be seen in any subtype. As such it is not considered a distinct entity, but rather a high-grade or poorly differentiated component. The presence and extent of micro- or macronecrosis has been correlated with prognosis in ccRCC.19 Micro- or macrovascular invasion is thought to be a requisite step toward systemic disease; however, correlation between the extent of invasion and prognosis remains imprecise.
DIFFERENTIAL DIAGNOSIS AND STAGING
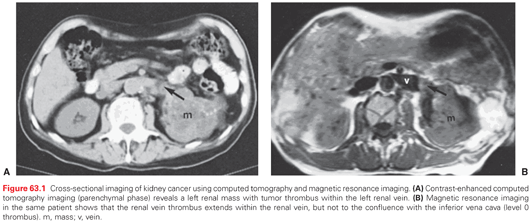
Most patients with RCC present with an incidental, radiographically detected renal mass (Fig. 63.1). While symptoms including microscopic or gross hematuria, flank pain, gastrointestinal disturbances and/or pain, bleeding or systemic disturbances related to metastases may lead to the diagnosis, the use of routine cross-sectional imaging has led to the more common scenario of an incidentally detected renal mass. While the suspicion for RCC may be high in cases such as these, RCC is a pathologic/tissue diagnosis, not a clinical one (Fig. 63.2). Proper radiographic evaluation of a renal mass requires a pre- and postcontrast computed tomography or magnetic resonance imaging (MRI) to assess enhancement.20 Duplex ultrasound, renal mass biopsy, and noncontrast diffusion-weighted MRI with antibody drug conjugates mapping may be useful adjunctive tests in various clinical settings. deoxy-2[18F]fluoro-d-glucose positron emission tomography exhibits a low sensitivity for the diagnosis of RCC and is therefore not recommended for the evaluation of RCC. ImmunoPET with G-250 using an iodine-labeled antibody against carbonic anhydrase IX (CA-IX), which is known to be overexpressed in ccRCC, exhibits near 90% sensitivity and specificity for this RCC subtype.21
The differential diagnosis of the renal mass is broad and includes a long list of benign, malignant, and inflammatory conditions. Clinical and radiographic features can assist the astute clinician in narrowing down the diagnosis of the renal mass, particularly for benign and inflammatory lesions. Cystic lesions, for example, are frequently benign,22 and fat-containing solid lesions are most commonly found to be angiomyolipomas (also benign). About 20% of enhancing renal masses and 15% of surgically removed masses are nonmalignant, with the most common diagnoses being oncocytoma and fat-poor angiomyolipoma.23–25 Young to middle-aged women, in particular, are more likely to have benign pathology, as high as 40% in some series, while the likelihood of malignancy gradually decreases with age in men.24,26 Tumor size is the most important determinant of pathology and biologic aggressiveness with larger tumors more likely to be high grade, locally invasive, and/or of adverse histologic subtype.23,24,27 Incorporation of readily available features can allow the physician to provide an individualized risk of cancer (ranging between ~50% and ~99%), but a certain diagnosis requires pathologic confirmation.24,28 The most accurate nomogram currently available gives estimates of preoperative prediction of tumor histology with an area under the curve of 0.76 and high-grade malignancy with an area under the curve of 0.73.28
Percutaneous renal mass sampling is now being performed with increased regularity at many centers.29 There is a strong rationale for biopsy when the findings will change management, such as when there is reason to suspect lymphoma/leukemia or abscess or to guide systemic therapy for metastatic disease. Even for clinically localized renal tumors, conventional renal mass biopsy can provide a definitive diagnosis in 80% to 90% of cases, and the ability to subtype and grade RCC can increase with the use of immunohistochemical and other molecular analyses.30,31 Therefore, renal mass sampling should be considered in patients with enhancing renal masses who are candidates for a wide range of management strategies.30–34 However, younger, healthier patients who are unwilling to accept the uncertainty associated with renal mass biopsy as well as elderly, frail patients who will be managed conservatively (independent of biopsy results) should still be managed without a biopsy.
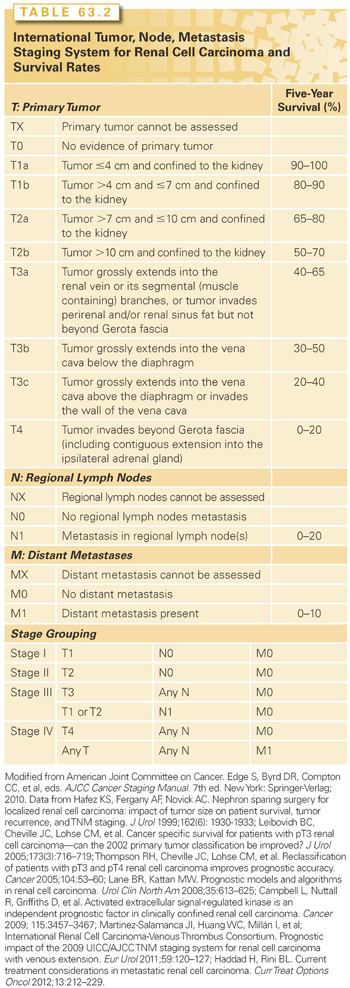
Clinical and pathologic staging systems provide a basis of standardized communication, comparison, and prognostication. They are used to communicate risk for treatment decision making and clinical trials planning. There have been several staging systems for RCC proposed. The most widely used is version 7 of the TNM (tumor, node, metastasis) staging system of the American Joint Committee on Cancer and the Union for International Cancer Control, updated in 2010 (Table 63.2). It distinguishes T1a from T1b and T2a from T2b based on tumor size.35 Additionally, adrenal involvement was changed from pathologic stage T3a to T4 and venous invasion was separated into renal vein/segmental branches (T3a) and inferior vena cava (IVC) below (T3b) or above the diaphragm (T3c). Nodal and metastatic disease are only classified as negative (N0/M0) or positive (N1/M1). Other potential prognostic features of the primary tumor not included in the TNM classification include necrosis, urothelial involvement, microvascular invasion and molecular features. Review of these and other prognostic features of RCC are available.36
HEREDITARY KIDNEY CANCER SYNDROMES, GENETICS, AND MOLECULAR BIOLOGY
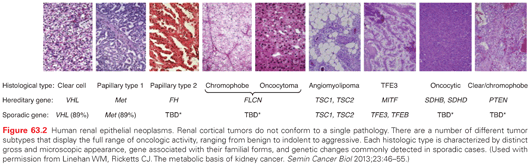
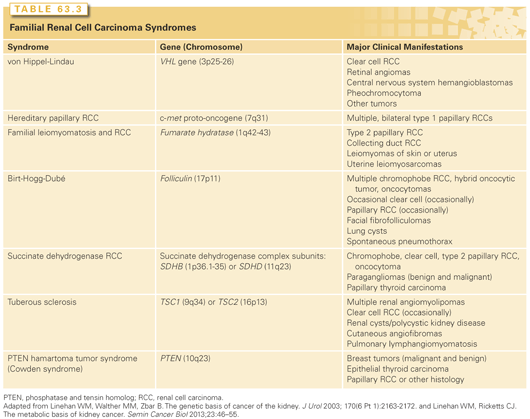
While most renal cancers are believed to occur sporadically, familial clusters have led to the discovery of at least seven RCC susceptible syndromes (Table 63.3). It is estimated that approximately 4% of RCC have a hereditary basis.37 In these cases, in addition to a provocative family history, tumors tend to be bilateral, multifocal, and arise at an early age of onset. Importantly, the study of hereditary kidney cancer has dramatically changed our understanding of the genetic and molecular basis of RCC and has led to the development of effective, approved therapeutic agents as similar cytogenetic and molecular alterations appear to be shared between sporadic and hereditary RCC (see Fig. 63.2).38
The molecular alterations in RCC appear to converge on similar pathways involved in dysregulated oxygen sensing/angiogenesis, iron metabolism, and energy/nutrient sensing.39,40 The predominant genetic and molecular defects in RCC known to date include VHL loss of function (ccRCC), neurofibromatosis type 2 loss of function (ccRCC), MET gain of function (papillary type I RCC), NRF2 gain of function and alterations in fumarate hydratase (papillary type II RCC), CCND1 gain of function and folliculin loss of function (oncocytoma/chromophobe RCC), and MiTF-TFE3 gain of function (translocation RCC). Additionally, inactivation of chromatin modifying proteins including PBRM1, BAP1, and histone methylases/demethylases, as well as inactivation of electron transporters, may represent early or common events in multiple subtypes.41 While important, the associations of these aberrant pathways with various subtypes of RCC likely represent an overly simplified model of renal tumor development.42 A variety of small nucleotide mutations, structural mutations, and large chromosomal abnormalities characterizes RCC, with as many as 5 to 70 small somatic mutations found in individual renal tumor cells.42 Moreover, variable gene expressions may reflect differences in cell types from which RCC originates, suggesting that genetic aberrations require a specific cellular context for dysregulated growth. The development of rapid sequencers continues to redefine the molecular characterization of RCC from which a genetic profile/classification is emerging with important implications for the development of the next generation of targeted therapeutic molecules.43,44
Von Hippel-Lindau Disease
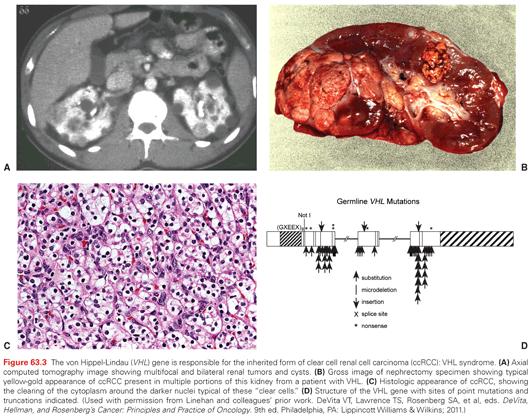
VHL is a syndrome characterized by the development of highly vascular tumors of the retina, central nervous system, pancreas, adrenal, and kidney (ccRCC). It is inherited in an autosomal dominant fashion with an incidence of 1:35,000.39 Loss of VHL function (3p25.1) by genetic or epigenetic means in a classic tumor suppressor fashion is the known cause. In an established genotype-phenotype relationship, type 1 VHL (absence of pheochromocytoma) is due to germline deletions, insertions, and nonsense mutations, whereas type II VHL (with pheochromocytoma) is associated with missense mutations.45 Between 25% to 60% of patients with VHL develop bilateral multifocal cystic and solid RCC, which represents a common cause of death (Fig. 63.3). Management of renal tumors in patients with VHL now includes surveillance of smaller tumors (<3 cm) and resection of larger ones (>3 cm) by PN with the goal of preventing metastases and optimizing renal function by “resetting the biologic clock” through appropriately timed surgeries.46 The goal of complete tumor removal with wide negative surgical margins is less appropriate for these patients where management of localized lesions supplants cure.39,46 Patients should be evaluated and followed by a team of clinicians familiar with the complexities of multisystem genetic disorders.
Hereditary Papillary Renal Cell Carcinoma
Hereditary papillary RCC is perhaps the least common familial RCC syndrome, with manifestations that appear to only affect the kidney. Affected individuals develop bilateral multifocal type I papillary RCC due to mutations in the MET gene located at 7q31. MET is a tyrosine kinase receptor with hepatocyte growth factor as its ligand.47 The syndrome is transmitted in an autosomal dominant fashion and tumors usually appear after the age of 30.39 As with VHL, management of renal tumors recognizes the need to remove larger lesions and observe smaller ones. While no size cutoff for intervention has been established, the biology of type I papillary RCC appears to be more indolent that ccRCC, suggesting the risk of death from kidney cancer in these patients is low. Again, PN with renal preservation is emphasized despite the diffuse micro- and macromultifocality of these lesions.
Birt-Hogg-Dubé Syndrome
BHD is characterized by cutaneous fibrofolliculomas, a 50-fold increased risk of pneumothorax, and bilateral multifocal solid renal tumors. It is an autosomal dominant disorder with an incidence of around 1:200,000. Linkage analysis has mapped the gene for BHD (folliculin), a tumor suppressor, to 17p11.2. Folliculin is thought to be a downstream effector of activated protein kinase and mammalian target of rapamycin (mTOR).39 Renal tumors associated with BHD are more indolent in nature, occurring in approximately 20% of individuals, with <5% developing metastases. While the histology of renal tumors associated with BHD is most often chromophobe RCC, oncocytoma, or hybrids of both, clear cell or papillary tumors may rarely occur as well.
Hereditary Leiomyomatosis Renal Cell Carcinoma
Hereditary leiomyomatosis RCC is also characterized by dermatologic manifestations. Patients with HLRCC exhibit cutaneous leiomyomas, early onset of uterine fibroids, macronodular adrenal hyperplasia, and kidney cancer. Linkage analysis has localized the HLRCC gene (fumurate hydratase), a key Kreb cycle enzyme, to 1p42.3 (FN).48 Renal tumors in HLRCC tend to be aggressive and lethal. The most common histology observed is type II (eosinophilic) papillary RCC.39 Unlike other hereditary forms of RCC, given the aggressive nature of these tumors, AS with delayed intervention is not recommended.
TREATMENT OF LOCALIZED RENAL CELL CARCINOMA
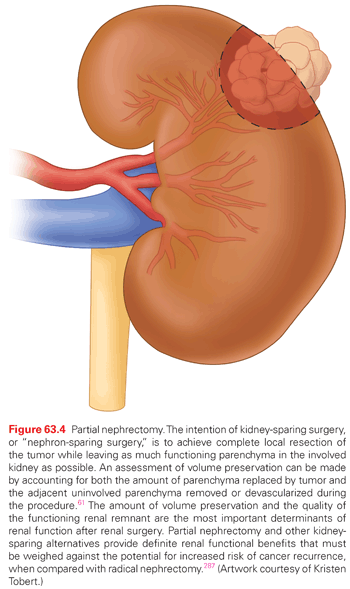
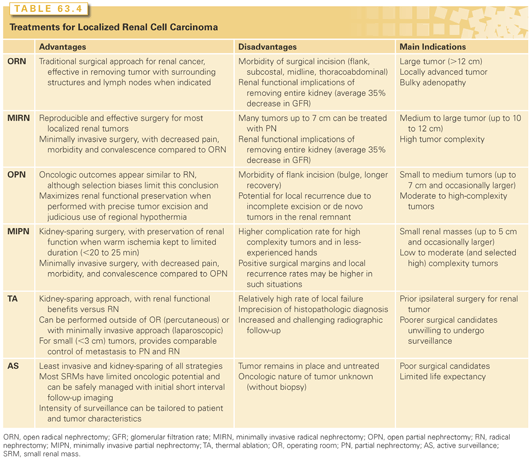
Greater use of cross-sectional imaging has contributed to earlier detection of RCC in many cases.2,49,50 Between 50% to 70% of RCC are detected incidentally,51 and the majority are “small renal masses (SRM),” or clinically localized renal cortical tumors up to 4 cm in size. Our perspectives about treatment of clinical T1 renal masses have changed substantially in the past 20 years. While all had been presumed to be malignant and managed aggressively, we now recognize the tremendous biologic heterogeneity of these lesions, and multiple management strategies are now available, including RN, PN, TA, and AS.52–57 Once controversial, elective PN is now accepted as a standard of care, based in part on the appreciation of the deleterious renal functional consequences of RN (Fig. 63.4).53,55,58 Ongoing analysis of the relative merits of PN, RN, and other management strategies has produced vibrant literature over the past few years.46,59–62 Both the American Urologic Association (AUA) and the European Association of Urology have released guidelines for the management of localized renal masses in recent years providing a robust analysis of the available studies.9,63–67 Each approach has associated risks and benefits, and no one approach is best in all circumstances (Table 63.4). The involvement of an urologist with expertise in the management of RCC is essential for selection of the optimal strategy based on the individual features of each patient and tumor.
Radical Nephrectomy for Renal Cell Carcinoma
The objective of surgical therapy for RCC is to excise all of the cancer with an adequate surgical margin. Simple nephrectomy was practiced for many decades, but was replaced by RN when Robson and colleagues (1969) established this procedure as the “gold standard” approach for localized RCC.68 “RN” as currently practiced may be better termed “total nephrectomy,” as it often omits several of the components of the original, “radical” nephrectomy, which always included extrafascial nephrectomy, adrenalectomy, and extended lymphadenectomy from the crus of the diaphragm to the aortic bifurcation. Perifascial dissection is still routinely practiced for larger tumors, as ≥25% of these tumors extend into the perinephric fat.69,70 Removal of the ipsilateral adrenal gland is no longer recommended, unless there is suspicion of direct invasion of the gland by tumor or a radiographically or clinically suspicious adrenal tumor, because of the similar propensity of RCC to metastasize to the ipsilateral or contralateral adrenal gland.71–73 Finally, extended lymphadenectomy has been shown to be of no benefit for patients with clinically localized RCC and remains controversial for those with higher-risk disease.74–77
RN is still a preferred option for many patients with localized RCC, such as those with very large tumors (most clinical T2 tumors) or the relatively limited subgroup of patients with clinical T1 tumors that are not amenable to nephron-sparing approaches.78 The surgical approach for RN depends on the size and location of the tumor, as well as the patient’s habitus and medical/surgical history. For locally advanced disease and/or bulky lymphadenopathy, an open surgical approach using either an extended subcostal, midline, or thoracoabdominal incision is generally used.79
Current minimally invasive approaches allow all of the essential steps of RN to be performed, with the associated benefits of shorter convalescence and reduced morbidity.80–83 Laparoscopic RN is now established as a preferred approach for moderate to large volume tumors (≤10 cm to 12 cm), without invasion of adjacent organs, with limited (or no) venous involvement, and having manageable (or no) lymphadenopathy. Therefore, a minimally invasive approach is suitable for most patients with renal tumors, even including some patients with features previously thought to mandate open RN.
On the other hand, RN has fallen out of favor for smaller renal tumors due to concerns about chronic kidney disease (CKD), and it should only be performed when necessary in this population.53,58,78,84 Several studies have shown an increased risk of CKD on longitudinal follow-up after RN.58,85,86 Huang et al. first reported that 26% of patient populations with a small renal mass, normal opposite kidney, and “normal” serum creatinine had preexisting grade 3 CKD (estimated glomerular filtration rate [eGFR] <60 ml/min per 1.73 m2). After surgery, stage 3b or higher CKD (eGFR <45 ml/min per 1.73 m2) was more common after RN than PN (36% versus 5%, p <0.001). CKD has been proven to lead to increased rates of cardiovascular events and death, with proportionally greater impact with higher CKD stage (and lower GFR). For example, in a population-based study of >1 million subjects, the relative death rates were 1.2, 1.8, 3.2, and 5.9 for eGFR (ml/min per 1.73 m2) of 45 to 60, 30 to 45, 15 to 30, and <15, respectively, even after controlling for hypertension, diabetes, and other potential confounding factors.87 Coupled with the biologic heterogeneity of small renal masses, many of which will never lead to compromised survival, the potential negative consequences of RN on renal function have highlighted the importance of nephron-sparing approaches.53,58,72,88–90
Partial Nephrectomy for Renal Cell Carcinoma
Kidney-sparing surgery for renal tumors was first described by Czerny in 1890; however, significant morbidity limited its use for the next half century.91 Vermooten (1950) revisited the concept of local excision with a margin of normal parenchyma for encapsulated and peripherally located renal tumors.92 The use of PN for RCC has subsequently been stimulated by experience with renal vascular surgery for other conditions, advances in renal imaging, growing numbers of incidentally discovered small renal masses, greater appreciation of the deleterious effects of CKD, introduction of minimally invasive techniques, and encouraging long-term survival in patients undergoing this form of treatment during the last 50 years.93–95 Kidney-sparing surgery entails complete local resection of the tumor while leaving the largest possible amount of normal functioning parenchyma in the involved kidney (see Fig. 63.4).
Initially described for patients with an “absolute” indication for kidney-sparing surgery or for the “elective” indication of a small renal tumor in the setting of a normal contralateral kidney, PN is now strongly considered whenever preservation of renal function is potentially important. Common indications include conditions that pose a threat to future renal function, such as hypertension, diabetes mellitus, peripheral or coronary artery disease, or nephrolithiasis, and patients with baseline CKD, an abnormal contralateral kidney, or those with multifocal or familial RCC.96,97 PN is generally considered feasible for the vast majority of localized renal masses <5 cm in size and often for tumors ≥7 cm by those with expertise with kidney-sparing surgery.98–101 Particularly for those with a strong rationale for nephron-sparing surgery, PN can even be performed for tumors that deeply invest the renal vascular structures or with limited venous thrombus, but such procedures clearly carry higher perioperative morbidity.95,102 Local recurrence rates after PN for imperative indications have averaged 3% to 5% or higher in historical series.103 The decision to perform a PN in such circumstances should be individualized, weighing the potential increased technical and oncologic risks of such an operation with the renal functional consequences of RN (see Fig. 63.4).
PN clearly leads to improved functional outcomes, when compared with RN, even for complicated situations.104,105 Temporary or permanent renal replacement therapy has been reported to be necessary in <5% of patients undergoing PN in a solitary kidney and is rarely needed for patients with a functioning contralateral kidney.106,107 In fact, the vast majority of patients will avoid permanent dialysis, even following multiple surgeries for multiple tumors in both kidneys, so long as at least 30% of a well-functioning remnant kidney is preserved.95 For situations in which PN is deemed impossible, RN with ensuing hemodialysis is sometimes necessary, but presurgical therapy with a tyrosine-kinase inhibitor is an alternative approach that has proven successful in some patients.108–111
For patients with clinical T1 renal masses, local recurrence rates are 1% to 2% after PN and most commonly located distant from the initial resection. Cancer-free survival is achieved in well above 90% of patients.104 The contralateral kidney is also at risk for metachronous disease, which also occurs in 1% to 2% of patients, even with contemporary imaging modalities. This provides further rationale to avoid unnecessary RN for tumors amenable to nephron-sparing surgery. The goal of PN is resection of all grossly appreciated tumor with negative microscopic surgical margins; this is generally performed with a thin rim of normal parenchyma based on prior literature indicating that margin width is immaterial.67,112–117 Some centers now routinely perform enucleation of renal tumors with excellent oncologic outcomes, although enthusiasm for more widespread use of this approach has been tempered by the somewhat higher recurrence rate among patients with RCC with positive margins and the propensity of some RCC subtypes to invade the pseudocapsule that is generally present.
Within the last decade, substantial progress has been made with minimally invasive PN, which is now the most commonly performed procedure for small renal masses. Laparoscopic PN, with or without robotic assistance, is performed according to the same principles as open PN. Margin status and oncologic outcomes with laparoscopic and open PN appear equivalent in series of patients in which patients were selected appropriately for each of these approaches.101,118 Although early to intermediate experience with laparoscopic PN suggested increased urologic complications compared with open RN,119 subsequent experience with pure laparoscopic PN and more prevalent use of robotic PN have substantially reduced perioperative morbidity.104,120–125 Tumor complexity remains a major predictor for intraoperative and postoperative complications, regardless of surgical approach, and open PN should be considered for particularly challenging situations.126
Thermal Ablation of Renal Cell Carcinoma
TA, including renal cryosurgery and radiofrequency ablation (RFA) have emerged as alternative kidney-sparing treatments for patients with small (<3 cm) renal tumors.127,128 Both can be administered percutaneously or with laparoscopic exposure and thus offer the potential for reduced morbidity and more rapid recovery compared with extirpative approaches.128,129 In general, the long-term efficacy of TA has not been well established when compared to surgical excision, and current data suggest that the local recurrence rates are somewhat higher than that reported with extirpative approaches.55,63 Another concern has been the lack of accurate histologic and pathologic information obtained with these modalities because the treated lesion is left in situ.
The ideal candidates for TA are patients with advanced age and/or significant comorbidities who prefer a proactive approach (over surveillance), but are not optimal candidates for conventional surgery, patients with local recurrence after previous kidney-sparing surgery, and patients with hereditary renal cancer who present with multifocal lesions for which multiple PNs might be cumbersome.55 Patient preference must also be considered as some patients not fitting these criteria may select TA after balanced counseling about the current status of these modalities.130,131 Tumor size is an important factor in patient selection because the current technology does not allow for reliable treatment of lesions >4 cm and success rates appear to be highest for tumors <2.5 cm to 3 cm.132–135
Clinical experience and follow-up of patients after renal cryosurgery suggests successful local control in about 90% of patients, although many studies provide limited and often incomplete, follow-up.136–140 Diagnosis of local recurrence after TA can be challenging because evolving fibrosis within the tumor bed can be difficult to differentiate from residual cancer. In general, central or nodular enhancement within the tumor bed on extended follow-up has been considered diagnostic of local recurrence and the clinical experience with cryoablation has thus far supported this.141,142 Other findings that suggest local recurrence include failure of the treated lesion to regress over time, a progressive increase in size of an ablated neoplasm, new nodularity in or around the treated zone, or satellite or port site lesions.143 If these features are found, biopsy and possible retreatment should be considered. The AUA guidelines for surveillance after TA include cross-sectional scanning (computed tomography or MRI) with and without intravenous contrast at 3 and 6 months following ablative therapy and annually with chest X-ray for 5 years thereafter.143
More mature data from a limited number of studies now provide encouraging outcomes for smaller tumors, particularly those <3 cm, yet the cumulative experience continues to suggest that local control after cryoablative therapy remains suboptimal when compared to surgical excision.55,138 The 5-year local recurrence rates in two series were 8% to 9%,52,144 which is significantly higher than the 1% to 2% consistently reported with surgical excision of analogous small renal masses.53 Other concerns with TA relate to surgical salvage and potential morbidity. Most local recurrences can be salvaged with repeat ablation, but some patients with progressive disease eventually require surgical extirpation. Nguyen and colleagues (2008b) have shown that PN and minimally invasive approaches are occasionally precluded in this setting due to the extensive fibrotic reaction induced by TA.78 As expected, the incidence of treatment failure or complications after TA correlates with tumor size and complexity.145
The experience with RFA has been even more variable, likely related to surgeon experience, the availability of different platforms for the procedure, and inability to monitor treatment progress.128 Local control after RFA is estimated to be 80% to 90%, although the definitions of local recurrence within the literature have been inconsistent.53,55 Although loss of enhancement on cross-sectional imaging within the lesion has generally been accepted as an indicator of success, viable cancer cells have been detected at biopsy of the tumor bed even in the absence of enhancement on the MRI 6 months after treatment.142 The issue of potential false-negative and false-positive imaging findings after TA remains a concern, and more strict definitions of local control after TA were recently advocated by an AUA guidelines panel.143,146 The technology for RFA continues to improve with most contemporary series reporting relatively low rates of local recurrence. Some patients require repeat treatments to achieve local control, which is an infrequent event with cryoablation and rarely required with conventional surgical treatments for localized RCC.128 One recent series documented local control in 91% of 179 patients with biopsy proven RCC at median follow-up of 27 months.135 Some RFA series report even more encouraging results, particularly for tumors <3 cm diameter,132 but others have reported 5-year local recurrence rates as high as 39%.147
Complications from ablation are uncommon, but have included acute renal failure, stricture of the ureteropelvic junction, necrotizing pancreatitis, and lumbar radiculopathy; therefore, careful and judicious selection of patients is essential.128 Direct comparisons between cryoablation and RFA are inevitable, but perhaps unfair because RFA is earlier in its development and recent reports suggest great promise.128,132 Other new technologies, such as high-intensity focused ultrasound and frameless, image-guided radiosurgical treatments (CyberKnife), are also under development and may allow extracorporeal treatment of small renal tumors in the future.148–150 However, at present cell kill with these modalities is not sufficiently reliable and they should still be considered developmental.151
Active Surveillance of Clinically Localized Renal Cell Carcinoma
The concept of overdiagnosis and overtreatment of kidney cancers is a relatively new concept. The risks and consequences associated with unnecessary treatment of low-risk RCC are an unintentional, yet underappreciated harm associated with incidental detection of these tumors. While early detection leads to “cure,” lead time biases in reported surgical series and the growing recognition that some localized renal tumors exhibit an indolent natural history have challenged the “find it, excise it” practice pattern. As the data emerge, AS remains a rational therapeutic option, particularly in the elderly or infirm where competing health, surgical, or renal functional risks may exceed that of the tumor’s biology.152,153
Objectifying and comparing the risks of treatment (excision/ablation) versus AS remains difficult as the data upon which competing risks models are based remain largely retrospective and incomplete.28 Nonetheless, data have emerged to suggest that radiographically localized small renal masses, most of which are RCC,154,152 exhibit slow linear/volumetric growth (0.3 cm/year on average) with a low metastatic potential (1.1% to 1.4%) over the first 24 to 36 months following diagnosis.154,152 Moreover, in patients with localized small renal tumors at diagnosis, the risk of metastases appears to be related to both the size of the primary tumor and perhaps more importantly the growth kinetics of the lesion.155 Interestingly, as many as 20% to 30% of small renal tumors exhibit zero radiographic growth over the initial 24 months following diagnosis.55 Given these data, the practice of AS with delayed intervention informed by an objective assessment of risk in the elderly, infirm, and/or well-informed is emerging.156 This practice is a calculated risk accepted by the patient and managed by the physician. Percutaneous biopsy and emerging biologic and genetic markers will continue to improve the decision-making process. In the meantime, AS remains a viable option for highly motivated patients and highly engaged physicians.
Follow-Up for Localized Renal Cell Carcinoma
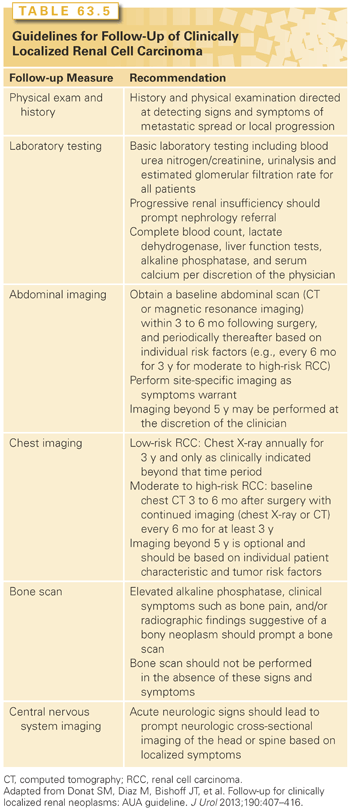
Follow-up for cancer survivors focuses broadly on early detection of cancer recurrence. With earlier diagnosis of many cancers and a longer length of life after diagnosis and treatment, an increasing number of survivors remain under the care of cancer specialists and primary care physicians. Wide variations in recommended practice have led to the development of guidelines by various organizations. The AUA released guidelines for follow-up of clinically localized RCC in 2013 that reflect a consistent approach that also takes into account the heterogeneity of the population of cancer survivors (Table 63.5).143 Clinicians should be aware that in managing adult cancer survivors they are not only looking for RCC recurrence, but also monitoring for secondary malignancy and the effects of cancer treatment, implementing therapies to prevent recurrences or new tumors, understanding the consequences of cancer and its treatment effects, and coordinating the overall care between cancer specialists and the primary care physician to meet each individual’s needs.
TREATMENT OF LOCALLY ADVANCED RENAL CELL CARCINOMA
Surgery for Tumor Thrombus in the Inferior Vena Cava
Renal tumors are unique in their ability to form tumor thrombi that can propagate from the ipsilateral renal vein into the IVC and extend as far as the patient’s right atrium. Approximately 4% to 10% of patients who present with renal masses will have a concomitant tumor thrombus. The level of tumor thrombus is classified as level 0 (thrombus limited to the renal vein), level I (thrombus extending into the inferior vena cava ≤2 cm above the renal vein), level II (thrombus extending into the inferior vena cava >2 cm above the renal vein, but below the hepatic veins), level III (thrombus extending into the inferior vena cava to or above the level of the hepatic veins, but still remaining below the diaphragm), and level IV (thrombus extending into the inferior vena cava and above the level of the diaphragm) (Fig. 63.5). A tumor thrombus should be suspected in patients with a renal tumor who also have new onset lower extremity edema, an isolated right-sided varicocele or one that does not collapse with recumbency, dilated superficial abdominal veins, proteinuria, pulmonary embolism, right atrial mass, or nonfunction of the involved kidney.
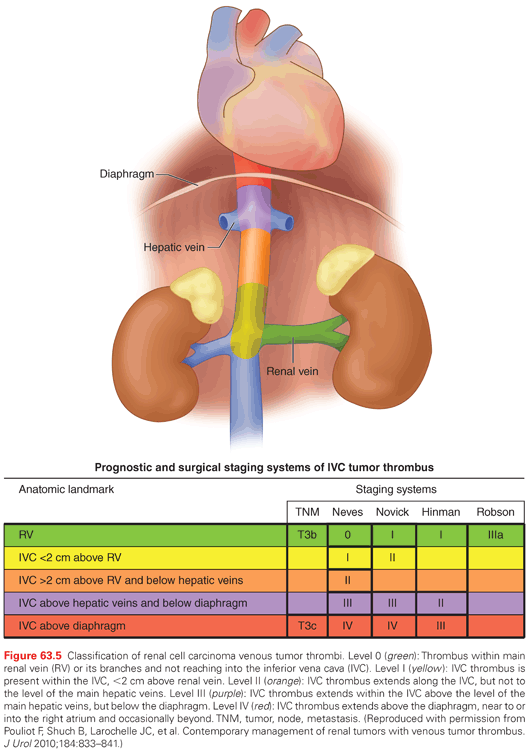
Five-year cancer-specific survival for patients with RCC and venous extension ranges from 45% to 70%, and surgical therapy in the form of RN and IVC thrombectomy can be curative. Interestingly, many patients with vena cava extension will present without metastatic disease.157,158 The prognostic significance of IVC thrombus level has been controversial. Most studies suggest that the incidence of locoregional or systemic progression is higher in patients with level III-IV IVC thrombus, which may account for the reduced survival reported in this subgroup in some series.159–162 Other series have shown that any IVC involvement is worse than renal vein involvement without distinction with regard to IVC level; in these series, other factors, such as nodal or metastatic involvement and tumor grade, have more impact on overall survival (OS).163,164 Despite this debate, patients with any tumor thrombus level can be cured with surgical resection, even level IV, in the absence of metastases and other adverse features.165–168
More recent series have re-evaluated the clinical variables predictive of survival after surgery for patients with tumor thrombi. In a single institutional series of 99 patients, median survival for patients with level I/II tumor thrombus was 6.6 years compared to 1.4 years for patients with level III/IV tumor thrombi. Higher level of tumor thrombus ([III/IV versus I/II], hazard ratio [HR] = 1.84 95% confidence interval [CI] = 1.03 to 3.30, p = 0.041) and the presence of metastatic disease at the time of surgery (HR = 2.97, 95% CI = 1.65 to 5.36, p <0.001) both portended a worse OS on multivariate analysis.169 Other investigators have examined the impact of tumor histology on clinical and pathologic outcomes in patients with venous tumor extension. Authors at the Mayo Clinic found that patients with non–clear cell histology presented with a significantly larger tumor size, greater rate of lymph node disease, higher nuclear grade, and more frequent sarcomatoid differentiation.170 As a result of these clinical and pathologic variables, these patients had a considerably worse 5-year cancer-specific survival as compared with patients with clear cell histology (p = 0.03).170 Similarly, a recent international multi-institutional retrospective study analyzed the role of tumor histology on survival in patients undergoing RN and caval thrombectomy. In this series of 1,774 patients, the overall 5-year cancer-specific survival was 53.4%.171 In this series’ multivariable analysis, papillary histology (HR = 1.62, 95% CI = 1.01 to 2.61, p <0.05), fat invasion (HR = 1.49, 95% CI = 1.10 to 2.03, p <0.01), and thrombus level (p <0.01) were all independent predictors of a poor cancer-specific survival.171 In contrast to the Mayo series, when the authors restricted their analysis to only N0M0 patients, thrombus level and papillary histology were still significantly associated with a decreased cancer-specific survival.170,171
Surgery remains an integral part in the treatment paradigm for patients with tumor venous extension because of the sequelae of such vascular involvement. The surgical approach and technique to treat these challenging tumors are tailored to the level of IVC thrombus, but uniformly begin with careful mobilization of the kidney and early ligation of the arterial blood supply.164,172,173 With an increasing tumor thrombus level, more advanced surgical techniques are required for vasculature control and complete tumor extirpation, including veno-venous bypass and cardiopulmonary bypass potentially with hypothermic circulatory arrest for some cases. Specifically for level III and level IV thrombi, a multidisciplinary surgical team is often required for advanced surgical maneuvers, including a liver surgeon to aid in mobilization of the liver and exposure of the intrahepatic IVC and a vascular and/or cardiac surgeon if bypass is required.
Despite the surgical ability to resect these tumor thrombi, perioperative mortality rates associated with RN and IVC thrombectomy have been reported to be as high as 5% to 10% in some series, depending on patient comorbidities and tumor characteristics.164,166 Although there may be a palliative role for surgery in some patients with metastasis who experience severe disability from intractable edema, ascites, cardiac dysfunction, or associated local symptoms such as abdominal pain and hematuria, most such patients will not benefit due to risk of perioperative morbidity and limited life expectancy.174,175
Lymphadenectomy
The need for extensive lymphadenectomy in patients undergoing RN remains a subject of debate. Despite the fact that multiple prior studies have shown a survival benefit with a lymph node dissection performed at the time of nephrectomy,75,176,177 a recent randomized trial failed to show a distinct advantage.74 Although this trial represents level I evidence, its generalizability is limited since the trial included many patients at low risk for nodal metastasis (81% of patients had grade 1 or 2 tumors and 72% had organ-confined disease).74 Furthermore, in the entire cohort, lymph node metastases were present in only 4% of patients undergoing complete lymph node dissection.74 Based on this trial, a compelling argument for lymph node dissection in patients with clinically localized RCC cannot be supported.
Of greater impact is the study from Blute and colleagues164 who elucidated pathologic features associated with increased risk for nodal metastases: tumor grade (grade 3 or 4), presence of a sarcomatoid component, tumor size ≥10 cm, tumor stage pT3 or pT4, and histologic tumor necrosis. Based on this study and a subsequent prospective evaluation of this approach, patients with two or more of these risk factors should be considered for extensive lymph node dissection incorporating the ipsilateral renal hilar region, the ipsilateral great vessel, and the interaortocaval region. This dissection should extend from the crus of the diaphragm to the common iliac artery. In a retrospective study, 45% of patients had positive lymph nodes outside of the renal hilar region, mandating a broader template.177 Finally, a recently published retrospective study of 1,983 patients treated for RCC assessed the factors predictive of lymph node invasion and/or progression. In this study, the overall prevalence of lymph node invasion was 6.1% and the clinical factors that were independently predictive of lymph node involvement were tumor stage (T3-T4), clinical nodal status, the presence of metastatic disease, and tumor size as a continuous variable.178 Using these variables, the authors created a nomogram that calculates a patient’s probability of having lymph node disease at the time of nephrectomy or in disease follow-up.178 It appears fair to conclude that a lymph node dissection is not routinely needed for organ-confined disease; however, the presence of multiple adverse pathologic features (e.g., large tumor size, locally advanced disease, etc.) seems to favor performing a lymph node dissection that is more extensive than simply the hilar region.
ADJUVANT THERAPY FOR RENAL CELL CARCINOMA
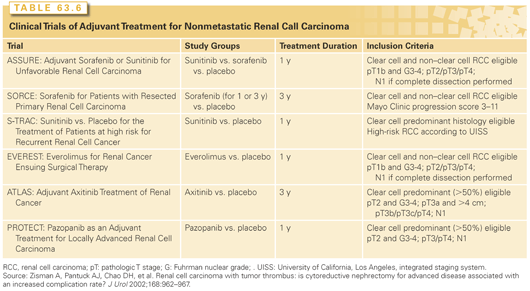
Although a significant proportion of patients can be considered to be cured or in remission after surgical treatment for nonmetastatic RCC, distant metastases are detected in 20% to 35% and local recurrence in 2% to 5% of patients.54,94 Patients with locally advanced RCC and other high-risk features are at greater risk of recurrence and various predictive tools can be used to provide an individualized estimate.54 Despite the significant likelihood of recurrence in patients with poor-risk features, no therapy has ever been shown to be of benefit in the adjuvant setting. Prior trials have evaluated hormone therapy, radiotherapy, immunotherapy, and tumor vaccines, all with essentially negative results. Based on the significant antitumor activity of targeted molecular therapies in advanced RCC, a number of randomized trials evaluating the ability of these agents to prevent metastasis have completed enrollment and results of these trials can be expected as early as 2015 (Table 63.6).
SURGICAL MANAGEMENT OF ADVANCED RENAL CELL CARCINOMA
Cytoreductive Nephrectomy
Approximately 30% to 40% of patients will present with metastatic or advanced RCC.179 For these patients, multimodal therapy, which includes surgery, has produced improved progression-free survival (PFS) and OS. The National Comprehensive Cancer Network Guidelines for kidney cancer list cytoreductive nephrectomy (CN) with or without metastasectomy prior to systemic treatment as the primary treatment option for patients with stage IV RCC.180 The data supporting this recommendation come from three randomized trials demonstrating a survival benefit for patients who received systemic immunotherapy with interferon (IFN)-alfa after surgical removal of the primary tumor.181–183 Flanigan et al.181 found the median survival of 120 patients assigned to surgery followed by IFN-alfa to be 11.1 months compared to 8.1 months in 121 patients assigned to IFN-alfa alone (p = 0.05). Similarly, Mickisch et al.183 found time to progression (5 months versus 3 months) and median duration of survival to be better in patients randomized to surgery plus IFN-alfa compared to those randomized to IFN-alfa alone. Combining the survival data from all these trials resulted in a median survival of 13.6 months versus 7.8 months for patients undergoing surgery in addition to IFN-alfa as compared to IFN-alfa alone.181,183–185
More recent data accounting for the current use of targeted therapies as first-line systemic therapy for patients with metastatic RCC have confirmed the survival advantage associated with CN. In one retrospective study, patients (n = 201) who underwent CN had an independent statistically significant survival advantage as compared to patients (n = 113) who did not (HR = 0.68; 95% CI = 0.46 to 0.99, p = 0.04).186 This survival advantage was present even when adjusting for contemporary adverse prognostic risk factors (e.g., performance status, time from diagnosis to therapy initiation, anemia, hypercalcemia, neutrophilia, and thrombocytosis).187 Despite these promising results, other studies have shown that CN may not confer a survival advantage in patients with non–clear cell histology, especially those with sarcomatoid features.172,188,189 Fortunately, a prospective study evaluating the benefit of surgery in combination with sunitinib versus sunitinib alone is currently ongoing in patients with metastatic RCC.
Although the use of laparoscopic/minimally invasive techniques in patients with advanced disease can potentially provide a less invasive and less morbid method for cytoreduction as preparation for administration of systemic therapies, CN is still not without risk, and surgical risk assessment needs to be considered preoperatively. According to prior reports, patients that are most likely to benefit from CN are those patients with lung-only metastatic disease, good prognostic features as defined by Motzer (or other) criteria, and a good performance status.175,190 Conversely, predictors of short survival for patients with advanced RCC include an elevated serum lactate dehydrogenase (LDH; >1.5 times upper limit of normal), a hemoglobin level <lower limit of normal, a corrected serum calcium level >10 mg/dl, an interval of <1 year from original diagnosis to the start of systemic therapy, a Karnofsky performance score ≤70, and two or more sites of organ metastasis.191 However, the challenge associated with these risk criteria is that they mostly account for the risk associated with the disease and do not consider the perioperative risk to the patient, which is not insignificant.
For example, Abdollah et al.192 identified 17,688 patients within the Florida Inpatient Database that underwent nephrectomy between the years 1999 to 2008. They identified 1,063 (6%) patients who underwent a CN and found that these patients were more likely to have a longer length of stay (8.4 versus 5.7 days, p <0.001), a secondary surgical procedure (28.3% versus 10%, p <0.001), an in-hospital mortality (2.4% versus 0.9%, p <0.001), and a postoperative complication (26.5% versus 18.9%, p <0.001). In this report, increasing age was predictive of increasing in-hospital mortality and complications for patients undergoing CN. A similar analysis was conducted by Trinh et al193 using the Nationwide Inpatient Sample registry. Thirty one percent of the study population (n = 16,285) experienced a perioperative complication, and patients ≥75 years old who had a comorbidity score ≥3 were more likely to experience a complication after CN (both p <0.001).193 Of the patients (n = 4,974) that experienced an adverse event, 5% (n = 245 patients) died during their hospitalization.193 Finally, it is worth noting that despite the survival advantage garnered from a CN followed by targeted therapy, it is not universally received. In a single institutional study from the Fox Chase Cancer Center, only 69.5% of patients actually received systemic therapy after surgery.194 The most common reason in this study for patients not to receive systemic therapy was rapid disease progression, which occurred in 30% of patients. Also, there were eight perioperative deaths, accounting for 19% of patients who did not receive systemic treatment.194
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








