Provocative assessments of population groups suggest a dominantly inherited susceptibility to colorectal adenomas and cancer, which may account for the majority of sporadic CRC, but this may have variable inheritance based on the degree of exposure to environmental factors.31 What are these susceptibility factors? The answer has yet to emerge. Nonetheless, genetic polymorphisms may be of paramount importance, such as in glutathione-s-transferase,32 ethylene tetrahydrofolate reductase,33,34 and N-acetyltransferases, especially NAT1 and NAT2.35 In fact, genetic polymorphisms can vary among different racial and ethnic groups, which may provide clues to the geographic variation of CRC as well.
Environmental Factors
Seminal studies have underscored the importance of environmental factors as contributing to the pathogenesis of CRC. One has to take population-based studies into the context of methodologies employed, lead-time bias, time-lag issues, definition of surrogate and true end points, and the role of susceptibility factors.
One such population-based study recently evaluated risk factors for CRC from the Women’s Health Initiative, a comprehensive prospectively collected database of 150,912 postmenopausal women, in which 1,210 developed colon cancer and 282 developed rectal cancer. Eleven risk factors were independently associated with colon cancer, some which have little or no previous support in the literature (age, waist girth, use of hormone therapy at baseline [protective], years smoked, arthritis [protective presumably due to medications used], relatives with CRC, lower hematocrit levels, fatigue, diabetes, less use of sleep medication, and cholecystectomy). Three of these factors were also significantly associated with an increased risk of rectal cancer (age, waist girth, and not taking hormone therapy).36
Diet
Total Calories
Obesity and total caloric intake are independent risk factors for CRC as revealed by cohort and case-control studies.37,38 Increased body mass may result in a two-fold increase in CRC risk, with a strong association in men with colon but not rectal cancer. Weight gains during early to middle adulthood have also recently been linked with increased risk of colon but not rectal cancer. This relationship too seems more prominent in men than women in a large prospective study.39
Meat, Fat, and Protein
Ingestion of red meat but not white meat is associated with an increased CRC risk,40,41 and as such, per capita consumption of red meat is a potent independent risk factor. Whether the total abstinence from red meat leads to a decreased CRC incidence has not been clarified, as there are studies with opposing results.42 Also unclear is whether the type of red meat or the degree of processing or cooking method make any difference. While Probst-Hensch et al.43 found fried, barbecued, and processed meats to be associated with CRC risk, especially for rectal cancer, with odds ratio (OR) of 6, follow-up reports do not consistently support these claims. In the population-based Norwegian Women and Cancer cohort including 84,538 participants, highly processed meat intake (especially sausage) was associated with increased CRC risk but meat cooking methods and total meat intake were not.44 A second study of 53,988 participants reported no difference with processed meat intake either. The authors did find that cancer risk was associated with different meat subtypes (i.e., animal of origin) which varied by tumor location—specifically, colon cancer risk was significantly elevated in the setting of high lamb intake (incidence rate ratio = 1.07) and rectal cancer risk was affected by pork (incidence rate ratio = 1.18).45 However, McCullough et al.46 recently reported a positive association in patients with nonmetastatic CRC between red and processed meat consumption before cancer diagnosis with higher risk of death after definitive surgery.
Coffee
Coffee contains numerous bioactive compounds that may modulate cancer risk but previous epidemiologic studies investigating its role in CRC have yielded ambiguous results. In a recent meta-analysis of 41 studies (25,965 patients), Li et al.47 found a significant inverse association from case-control data for CRC (OR = 0.85) and colon cancer (OR = 0.79), but not rectal cancer. This was particularly true among females and in Europe.47 Stronger evidence comes from the National Institutes of Health–AARP Diet and Health Study, a large prospective US cohort including 489,706 members. In this report, both caffeinated and decaffeinated coffee drinkers had a decreased risk of colon cancer, particularly of proximal tumors (hazard ratio [HR] for more than six cups a day = 0.62), and decaffeinated coffee drinkers also had a decreased risk of rectal cancer. While known confounders such as smoking and red meat consumption were adjusted for, further investigation is warranted to confirm and clarify this association.48
Fiber
Classically, a high-fiber diet was associated with a low incidence of CRC in Africa,49 with numerous studies substantiating this premise.50 Protection was believed to be afforded from wheat bran, fruit, and vegetables.41 A high-fiber diet was believed to dilute fecal carcinogens, decrease colon transit time, and generate a favorable luminal environment. The European Prospective Investigation into Cancer and Nutrition is an ongoing multicenter prospective cohort study, which was one of the largest and most influential studies to initially report an inverse association between dietary fiber and CRC. More long-term data, with a mean follow-up of 11 years and a near three-fold increase in CRC cases, further supports this claim while providing a more precise estimation by fiber food source as well. After multivariable adjustments, total dietary fiber was found to be inversely associated with both colon and rectal cancers (HR per 10 g/day increased in fiber = 0.87), and this did not differ by age, sex, lifestyle, or other dietary factors.51 However, other large, well-controlled studies show no inverse relationship between CRC and fiber intake.52 In a study of nearly 90,000 women from ages 34 to 59 who were followed for 16 years, no protective effect was noted between fiber and incidence of either adenomatous polyp or CRC.52 This was further corroborated by two large randomized controlled trials that evaluated high-fiber diets for moderate duration and discovered a lack of effect on the number, size, and histology of polyps found on colonoscopy.53,54 At this point, therefore, it is unclear whether dietary fiber plays any substantial role in the risk of developing CRC.
Vegetables and Fruit
A protective effect of vegetables and fruits against CRC is generally believed to be true.40 This has been observed with raw, green, and cruciferous vegetables. Whether certain agents such as antioxidant vitamins (E, C, and A), folate, thioethers, terpenes, and plant phenols may translate into effective chemopreventive strategies requires further investigation, although the data for folate intake are sound.55
Taking this nutritional data a step further, Bamia et al.56 recently evaluated the impact of the Mediterranean diet on CRC risk in a large European cohort. This diet, introduced in the 1960s as “health-protecting,” includes a high intake of vegetables, fruits, nuts, fish, cereals, and legumes with moderate alcohol consumption and low consumption of dairy and meat. The authors found an 8% to 11% decreased CRC risk when comparing patients with the highest to lowest diet adherence rates (HR = 0.89). The association was strongest for women and colon tumors.56
Other dietary factors under recent investigation include calcium, magnesium, and vitamin D. Calcium has been historically implicated as having a protective effect, perhaps due to its ability to bind injurious bile acids with reduction of colonic epithelial proliferation.57 This is supported through cell culture models. However, population-based studies are not definitive.
A recent meta-analysis evaluating the influence of magnesium intake demonstrated a modest risk reduction, with pooled RRs of 0.81 for colon cancer and 0.94 for rectal cancer. This association persisted even after results were adjusted for calcium intake in six of the analyzed studies.58
Vitamin D has been shown to inhibit cell proliferation and increase apoptosis in vitro, and its deficiency is considered an important risk factor for many types of solid cancers. In a meta-analysis of 18 prospective studies, vitamin D intake and blood 25 (OH)D levels were found to be inversely associated with the risk of CRC as well (RR = 0.79 and 0.62 for colon cancer, respectively; RR = 0.78 and 0.61 for rectal cancer, respectively). While this report offers only preliminary observational data, larger randomized trials for vitamin D supplementation are warranted59 and would be needed before routine vitamin D supplementation could be recommended for the purpose of CRC prevention. It is noteworthy that the Institute of Medicine, while supporting vitamn D supplementation to maintain bone health, found the evidence insufficient to support vitamin D as being protective against colorectal or any other cancer.60
Lifestyle
Physical inactivity has been associated with CRC risk, for colon more than rectal cancer. A sedentary lifestyle may account for an increased CRC risk, although the mechanism is unclear. Data suggest that physical activity after the diagnosis of stages I to III colon cancer may reduce the risk of cancer-related and overall mortality, and that the amount of aerobic exercise correlates with a reduced risk of recurrence following resection of stage III colon cancer.61 More recently, positive associations have been established between increased amounts of recreational physical activity before and after CRC diagnosis and lower mortality.62
Most studies of alcohol have demonstrated at most a minimally positive effect. Associations are strongest between alcohol consumption in men and risk of rectal cancer. Perhaps interference with folate metabolism through acetaldehyde is responsible.63
Prolonged cigarette smoking is associated with the risk of CRC.40 Cigarette smoking for >20 pack-years was associated with large adenoma risk and >35 pack-years with cancer risk. To examine the impact of smoking cessation on the attenuation of this risk, Gong et al.64 conducted a pooled analysis of eight studies, including 6,796 CRC cases and 7,770 controls. The authors found that former smokers also remained at increased risk for up to 25years after quitting. However, this varied substantially by cancer subsite with risk declining immediately for proximal colon and rectal cases but not until 20 years after smoking cessation for distal colon tumors.64
Diabetes
Type 2 diabetes has previously been implicated in the development of CRC, but it has been difficult to separate this association from other confounding lifestyle factors such as smoking and obesity. Two recent meta-analyses provide further evidence that this condition is in fact a significant indepent risk factor. Yuhara et al.65 identified 14 studies, most of which controlled for smoking, obesity, and physical exercise, and demonstrated that diabetes was associated with increased risk of both colon and rectal cancer (RR = 1.38 and RR = 1.20, respectively).65 A second report, analyzing 24 studies, found a similar association (RR = 1.26) with even higher risk for those patients on insulin therapy (RR = 1.61).66
Drugs
Nonsteroidal Anti-Inflammatory Drugs
Population-based studies strongly support inverse associations between use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAID) and the incidences of both CRC and adenomas.67–69 As a result, NSAIDs and selective cyclooxygenase 2 (COX-2) inhibitors have been investigated intensively in hereditary and sporadic CRC.
Long-term results have just been reported from the CAPP2 study, the first double-blind randomized controlled trial of aspirin chemoprevention with cancer as the primary end point. In this study, 861 carriers of Lynch syndrome were randomly assigned to aspirin or placebo. With a mean follow-up of 55.7 months, the authors report a significantly decreased incidence of CRC in the treatment group as well as a trend toward reduction in extracolonic Lynch syndrome–associated cancers. Importantly, there was no significant difference in adverse events such as gastrointestinal (GI) bleeding, ulcers, or anemia during the intervention period. These data provide strong rationale for the routine use of aspirin chemoprevention in Lynch syndrome and establish a foundation for further study in sporadic neoplasia. In a combined analysis of four large randomized trials of lower-dose aspirin (75 to 300 mg/day) involving 14,033 patients, aspirin taken for 5 years or more was associated with a reduced 20-year incidence and mortality due to CRC (absolute reduction = 1.76%; 95% confidence interval [CI] = 0.61 to 2.91; p = 0.001). Reduction was largely confined to right-sided tumors.70 In addition to generalized chemoprevention, the question of aspirn and other NSAIDs in patients with a diagnosis of CRC has been addressed. Liao et al.71 have reported evidence that suggests that aspirin therapy after CRC diagnosis may be beneficial to those patients whose tumors have a PIK3CA mutation, but not in those with wild-type PIK3CA.71 However, PIK3CA mutation status had no impact on the influence of the COX-2 inhibitor rofecoxib on cancer recurrence.72
Bisphosphonates
In addition to being one of the most commonly used medications for osteoporosis, bisphosphonates have been shown to have various antiproliferative, antiangiogenic, proapoptotic, and antiadhesive effects in preclinical studies. Practical impact on malignant disease, however, has been inconsistent. Singh et al.73 performed a recent meta-analysis demonstrating a statistically significant 17% reduction in CRC incidence with bisphosphonate use. This finding was observed independently for both proximal and distal colon cancers as well as rectal cancers, highlighting another potential pathway for chemoprevention.73
Biomarkers
In an effort to improve screening protocols and advance understanding of colorectal carcinogenesis, investigators are focusing on a variety of biomarkers for increased risk as well.
Toriola et al.74 evaluated the role of C-reactive protein and serum amyloid A, two common inflammatory mediators, in the Women’s Health Initiative Observational Study. With over 900 case-control pairs for each marker, the authors found that elevated concentrations of both C-reactive protein and serum amyloid A conferred significantly increased risk of colon cancer (OR = 1.50, p = 0.006). This is not surprising given the role inflammation plays in colorectal carcinogenesis as well as the new promising data surrounding NSAID chemoprevention.74
Leptin, a peptide hormone produced by adipocytes, is also thought to contribute to CRC pathogenesis. A recent prospective analysis found that soluble leptin receptor levels, which may regulate leptin function, was strongly inversely associated with both CRC and colon cancer risk (RR = 0.55 and RR = 0.42, respectively). This finding was independent of leptin levels and other circulating biomarkers.75 Chi et al.76 performed a similar investigation of insulin-like growth factor peptides, also implicated in CRC carcinogenesis, and found that high levels of insulin-like growth factor I and insulin-like growth factor II significantly increased cancer risk (OR = 1.25 and OR = 1.52, respectively).76 Along these lines, high circulating levels of C-peptide, a direct marker of hyperinsulinemia, may also be a predictive factor for increased CRC risk, as indicated in a recent meta-analysis.77
Human Papillomavirus
While human papillomavirus is well-established as the critical pathogenic force behind cervical and anogenital cancer, its role in colorectal malignancy is less clear. An association between the two was first reported in 1990 and since then, a growing number of studies have detected the virus in colon adenocarcinoma specimens. In the first meta-analysis to address this topic (including 16 articles and 1,436 patients), Damin, Ziegelmann, and Damin78 not only reported a high prevalence of human papillomavirus (31.9%) in affected patients, but also found a strong correlation between human papillomavirus positivity and increased CRC risk (OR = 10.04; 95% CI = 3.7 to 27.5). These results may indicate an alternative pathway of colorectal carcinogenesis that could have vast implications for treatment and prevention.78
Familial Adenomatous Polyposis
Familial adenomatous polyposis (FAP) constitutes 1% of all CRC incidence (Table 57.2). Hallmark features include hundreds to thousands of colonic polyps that develop in patients in their teens to 30s, and if the colon is not surgically removed, 100% of patients progress to CRC. Extracolonic manifestations include benign conditions—congenital hypertrophy of the retinal pigment epithelium, mandibular osteomas, supernumerary teeth, epidermal cysts, adrenal cortical adenomas, desmoid tumors (although these tumors may lead to obstruction)—and malignant conditions—thyroid tumors, gastric small intestinal polyps with a 5% to 10% risk of duodenal or ampullary adenocarcinoma, and brain tumors.79 The brain tumors may be of two types—glioblastoma multiforme or medulloblastoma—and the particular association of brain tumors and colonic polyposis is called Turcot syndrome.80 The colonic polyps in Turcot syndrome are fewer and larger than in classic FAP. An attenuated form of FAP harbors up to 100 colonic polyps and has a predisposition to colorectal cancer in patients when they are in their 50s or 60s.81

FAP is an autosomally dominant disorder with nearly 100% penetrance. However, about 30% of patients have de novo mutations and are without an ostensible family history. Based on karyotypic analysis that reveals an interstitial deletion on human chromosome 5q and subsequent genetic linkage analysis to 5q21, the gene responsible for FAP was identified as APC. Patients with FAP inherit a mutated copy of the APC gene, thereby predisposing them to early onset polyposis. During life, patients with FAP acquire inactivation of the remaining APC gene copy, which accelerates the progression to CRC. Interesting genotypic-phenotypic associations exist between the location of the APC gene mutation and certain clinical manifestations, such as congenital hypertrophy of the retinal pigment epithelium, desmoid tumors, and classic FAP versus attenuated FAP.
The APC gene comprises 15 exons and encodes a protein of nearly 2,850 amino acids (310 kDa). Nearly all germline mutations in the APC gene lead to a truncated protein, which can be detected through molecular diagnostic assays that can be integrated into genetic counseling and genetic testing of affected patients and at-risk family members.82,83 The functions of the APC protein and the interrelated pathways and regulatory molecules will be discussed later.
Hereditary Nonpolyposis Colorectal Cancer
Hereditary nonpolyposis CRC (HNPCC) accounts for about 3% of all CRCs. Salient features include up to 100 colonic polyps (hence the term nonpolyposis), preferentially, albeit not exclusively, in the right or proximal colon.84 There is an accelerated rate of progression to CRC in these diminutive, at times flat, polyps with mean age of onset of CRC being 43 years. This is designated HNPCC type I. HNPCC type II is distinguished by extracolonic tumors that originate in the stomach, small bowel, bile duct, renal pelvis, ureter, bladder, uterus and ovary, skin, and perhaps the pancreas. The lifetime risk of CRC in HNPCC is 80%, up to 50% to 60% for endometrial cancer, and 1% to 13% for all other cancers.84,85 Of note, a variant of HNPCC involves skin tumors and is designated as Muir-Torre syndrome. HNPCC is defined classically by the modified Amsterdam criteria (Table 57.3).
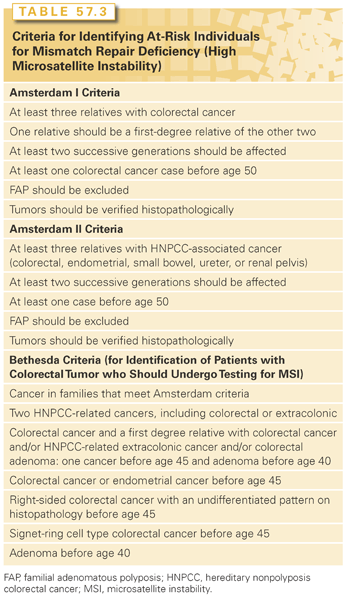
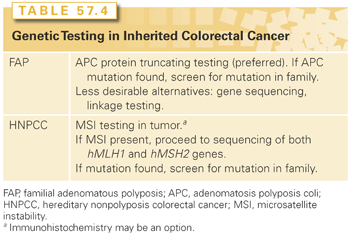
HNPCC is an autosomally dominant disorder with about 80% penetrance. Genetic and biochemical approaches led to the discovery of the involvement of human DNA mismatch repair genes in HNPCC. Recognized as the human orthologues of mismatch repair genes described in bacteria and yeast, human mismatch repair genes encode enzymes that repair errors during DNA replication that may occur spontaneously or upon exposure to an exogenous agent (e.g., ultraviolet light, chemical carcinogen). Mutations in one of these mismatch repair genes results in MSI, which creates a milieu of somatic mutations of target genes—TGF-β2 receptor, bax, IGF type I receptor, among others—in HNPCC-associated tumors.86 About 60% of germline mutations in HNPCC are found in either the hMLH1 gene or the hMSH2 gene, but mutations in other members of this family—hMSH6, hPMS1, hPMS2—are rare, thereby indicating that other genes are involved but have yet to be discovered. Genetic testing is not facile for HNPCC as it is for FAP, but it involves sequencing both the hMLH1 and hMSH2 genes (Table 57.4). If a germline mutation is found, then the remaining at-risk family members can be genetically screened. MSI testing and hMLH1/hMSH2 immunohistochemistry (IHC) can be performed on tumor specimens as a possible prelude to genetic testing.
Hamartomatous Polyposis Syndromes
Hamartomatous polyposis syndromes are rare syndromes, mostly affecting the pediatric and adolescent population, and represent <1% of CRCs annually. Peutz-Jeghers syndrome involves large but few colonic and small bowel polyps that can manifest by GI bleeding or obstruction and an increased risk of CRC. The polyps are distinguished by a smooth muscle band in the submucosa. Hallmark clinical features on physical examination include freckles on the hands, around the lips, in the buccal mucosa, and periorbitally. Associated characteristics include sinus, bronchial, and bladder polyps, and about 5% to 10% of patients have sex cord tumors. Patients can also develop lung and pancreatic adenocarcinomas. The gene responsible for this syndrome is LKB1, a serine threonine kinase.
Juvenile polyposis have overlapping clinical manifestations with Peutz-Jeghers, but the polyps tend to be confined to the colon, although cases of gastric and small bowel polyps have been described and there is an increased risk of CRC. Extracolonic manifestations are not prevalent. This is a polygenic disease, involving germline mutations in PTEN, SMAD4, BMPR1, or other genes yet to be identified.
Cowden syndrome harbors hamartomatous polyps anywhere in the GI tract, and surprisingly, there is no increased risk of CRC. However, about 10% of patients will have thyroid tumors and nearly 50% of patients have breast tumors. Germline PTEN mutations have been reported.
It is estimated that about 20% to 30% of CRCs are compatible with an inherited predisposition, independent of known syndromes.87 The identification of other responsible genes will have great clinical impact. Intensive approaches are being pursued through sibling-pair studies and other familial studies. As previously mentioned, patients may be predisposed to an increased risk of adenomatous polyps as well in the context of a family history of sporadic adenomatous polyps.
The colon and rectum make up the segment of the digestive system commonly referred to as the large bowel. Defined as the portion of intestine from the ileocecal valve to the anus, the large bowel is approximately 150 cm in length. It is divided into five segments defined by its vascular supply and by its extraperitoneal or retroperitoneal location: the cecum (with appendix) and ascending colon, the transverse colon, the descending colon, the sigmoid colon, and the rectum. The anatomy of the rectum will be discussed in detail in the chapter on rectal cancer. The large bowel has a muscular wall and can be distinguished from the small intestine by its increased diameter, the presence of haustra, appendices epiploicae, and tenia coli. The tenia consist of condensations of longitudinal muscle fibers starting near the base of the appendix and continuing throughout the abdominal colon to form a continuous longitudinal muscle coat in the upper rectum. Haustra are outpouchings of bowel wall separated by folds that give a classic appearance on radiography or barium enema.
The right colon is made up of the cecum (with appendix) and ascending colon. It is anterior to the right kidney and the duodenum. Its vascular supply is from branches of the superior mesenteric artery (SMA). The SMA divides into the middle colic artery and the trunk of the SMA. The middle colic artery immediately forms two to three large arcades in the transverse mesocolon. The SMA ileocolic arterial branches then extend from the SMA. The right colic artery arises as a separate branch from the SMA in 10.7% of cases.88 The ileocolic artery gives off a right colic artery to the upper ascending colon and forms an anastomosis with branches from the middle colic artery. The ileal branch of the ileocolic artery gives off branches to the distal small bowel and cecum, whereas the colic branch supplies the ascending colon. An anastomosis occurs between the distal SMA and the ileal branch of the ileocolic artery at the junction of the terminal ileum and cecum. The right colon is a retroperitoneal structure.
The transverse colon is supplied by branches of the middle colic artery. It is the first portion of the colon considered to be intraperitoneal, and its length can vary. Its boundaries are defined by the hepatic flexure on the right and the splenic flexure on the left. Both of these points are fixed. The hepatic flexure abuts the gallbladder fossa, while the splenic flexure lies anterior to the splenic hilum and the tail of the pancreas. The descending colon is where the colon once again becomes a retroperitoneal structure, and it is defined as the segment of colon from the splenic flexure to the sigmoid colon. The descending colon is the first segment of the left side of the colon and receives its blood supply from the inferior mesenteric artery. The inferior mesenteric artery arises from the aorta and gives off the left colic artery. It also gives off three to four sigmoidal arteries, which supply the intraperitoneal sigmoid colon. The anastomosis between the vessels of the middle colic artery and those of the left colic artery and right colic artery is known as the marginal artery of Drummond. The arcade, which effectively connects the left and right circulations, is known as the arc of Riolan. The arterial supply to the colon is depicted in Fig. 57.1.
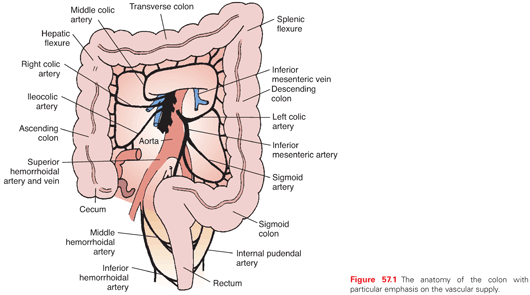
The venous and lymphatic drainage of the colon parallels the arterial supply, and all three vessels course and divide within the colonic mesocolon (Fig. 57.2). The mesocolon therefore contains the regional lymph nodes (LN) for the segment of colon it supplies and drains. The efferent lymphatic channels pass from the submucosa to the intramuscular and subserosal plexus of the bowel to the first tier of LNs lying adjacent to the large intestine and known as epicolic nodes.89 Paracolic nodes lie on the marginal vessels along the mesenteric side of the colon and are frequently involved in metastases. Intermediate nodes are found along the major arterial branches of the SMA and inferior mesenteric artery in the mesocolon. The principal nodes are found around the origin of these vessels from the aorta, and they drain into retroperitoneal nodes. The drainage of the superior and inferior mesenteric veins, which drain the ascending, transverse, descending, and sigmoid colon, is to the portal vein. The rectum is drained by rectal tributaries to the vena cava.
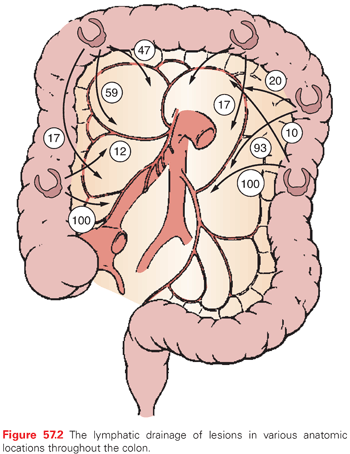
The extent of resection of the colon is defined by the vascular supply and by the need to take the regional draining LNs.90,91 A careful understanding of the colonic anatomy, structure, location, and vascular supply is therefore critical in order to perform a safe and effective cancer operation. The segmental resections important for removal of lesions in various locations within the colon will be described in greater detail in later sections.
DIAGNOSIS OF COLORECTAL CANCER
Symptoms associated with CRC include lower GI bleeding, change in bowel habits, abdominal pain, weight loss, change in appetite, and weakness, and in particular, obstructive symptoms are alarming.92 However, apart from obstructive symptoms, other symptoms do not necessarily correlate with stage of disease or portend a particular diagnosis.93
Physical examination may reveal a palpable mass, bright blood per rectum (usually left-sided colon cancers or rectal cancer) or melena (right-sided colon cancers), or lesser degrees of bleeding (hemoccult-positive stool). Adenopathy, hepatomegaly, jaundice, or even pulmonary signs may be present with metastatic disease. Obstruction by colon cancer is usually in the sigmoid or left colon, with resulting abdominal distention and constipation, whereas right-sided colon cancers may be more insidious in nature. Complications of CRC include acute GI bleeding, acute obstruction, perforation, and metastasis with impairment of distant organ function.
Laboratory values may reflect iron-deficiency anemia, electrolyte derangements, and liver function abnormalities. The carcinoembryonic antigen (CEA) may be elevated and is most helpful to monitor postoperatively, if reduced to normal as a result of surgery.94
Evaluation should include complete history, family history, physical examination, laboratory tests, colonoscopy, and pan-body computed tomography (CT) scan.95 For rectal cancer, additional imaging techniques, such as magnetic resonance imaging or endoscopic ultrasound, are utilized to further characterize the primary tumor prior to therapy (see Chapter 60). Upon completion of diagnosis and staging for both colon and rectal tumors, it is essential to incorporate the expertise from medical, radiation, and surgical oncologists in order to formulate and implement an optimal treatment plan.
With the advent of molecular biologic techniques, attention has been drawn to stool-based tools and new blood-based tests. Technology now exists to extract genomic DNA or protein from stool and assay for evidence of genetic alterations.96,97 Large-scale validation studies are in progress, one of which has just been published, describing an automated multitarget sDNA assay (fecal immunochemical testing) with a 90% specificity and 98% sensitivity for the detection of CRC, as well 83% sensitivity for advanced adenoma with high-grade dysplasia.98 In addition, Epi proColon (Epigenomics AG, Berlin, Germany), a blood-based test, was shown to be noninferior to fecal immunochemical testing in preliminary results from a multicenter double blind comparative study (press release from Epigenomics AG, December 4, 2012). One particularly attractive pathway for stool-based diagnostics would be able to stratify patients as high, moderate, or low risk for CRC and thus influence screening modalities and frequency of screening. In a complementary fashion, functional genomics are being applied to pair-wise comparisons of normal colon and CRCs to sample the entire human genome of nearly 30,000 genes to discover those genes, known and novel, that may be upregulated or downregulated and possibly linked to detection, prognosis, and therapy.
SCREENING FOR COLORECTAL CANCER
Debate is vigorous as to the best approaches for screening, and multiple factors influence that decision: simplicity and rapidity so as to enhance patient compliance, benefit to risk ratio, sensitivity, specificity, cost-effectiveness, and other economic factors. To that end, currently, optical colonoscopy likely offers the most effective approach when one considers all of these factors.
The average-risk patient is defined as a man or woman above the age of 50 without personal or family history of adenomatous polyps or CRC and absence of any occult or acute GI bleeding. Screening recommendations or guidelines for average-risk and high-risk individuals are presented in Table 57.5.
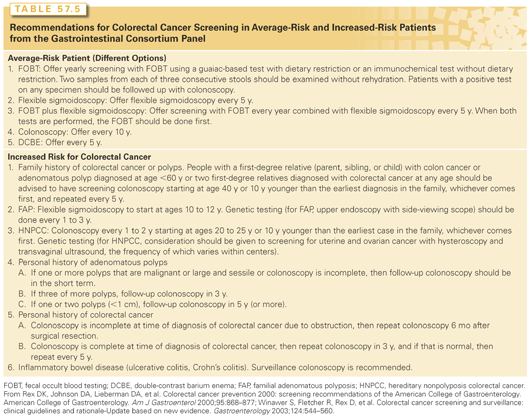
Optical colonoscopy is currently the most sensitive method for screening. Advantages include direct visualization, with the ability to remove polyps (with rate-limiting factors of size and anatomic location) and to obtain biopsies. Disadvantages involve the preparation, invasive nature of the procedure, and potential side effects that include perforation (although this is <1%).
The digital rectal examination should be part of the general physical examination. Anorectal masses may be palpated. Flexible sigmoidoscopy does not require conscious sedation and hemodynamic monitoring, and will typically allow visualization of the rectum, sigmoid colon, and descending colon to the splenic flexure. Flexible sigmoidoscopy should not be considered as a single screening measure but requires coupling with barium enema. Barium enema allows visualization of the entire colon, and experience is necessary to ensure proper visualization of the rectum. Barium enema affords advantages of ease of preparation, lack of conscious sedation and hemodynamic monitoring, and ability to visualize polyps and masses. However, small polyps may be missed. Furthermore, if a luminal polyp or mass is identified, then colonoscopy will be necessary for polypectomy or biopsies.
New noninvasive technologies, such as CT and magnetic resonance colonography, are receiving increased attention in clinical studies, which demonstrate overall feasibility, as well as some advantages.99
Two meta-analyses published in 2011 provide strong support for the implementation of CT colonography as a viable alternative to optical colonoscopy in both average and high-risk populations. In a review of 4,086 asymptomatic patients, de Haan et al.,100 estimates sensitivities of 82.9% and 87.9% and specificities of 91.4% and 97.6% for adenomas ≥6 mm and ≥10mm, respectively.
In a complementary analysis looking exclusively at cancer detection, Pickhardt et al.101 concludes that CT colonography is not only clinically equivalent to colonoscopy but perhaps even more suitable for initial investigation given consistently high sensitivity (96.1%) without heterogeneity across 49 studies, and 11,151 patients, despite wide variation in technique.
Other reports suggest advantages in long-term costs and patient compliance, although these issues remain controversial.102,103
Lastly, CT colonography may also offer improvements in preoperative staging as one study found this technique to be highly predictive of T3-4 tumors. Whether this information will prove as clinically relevant in colon cancer as it is for the rectum remains to be seen.104
STAGING AND PROGNOSIS OF COLORECTAL CANCER
This discussion will focus primarily on those prognostic and predictive indicators that are best supported by available data and are appropriate for use and consideration in current practice. The reader should remain aware of the potential for rapid changes and advances in this area, however.
Staging
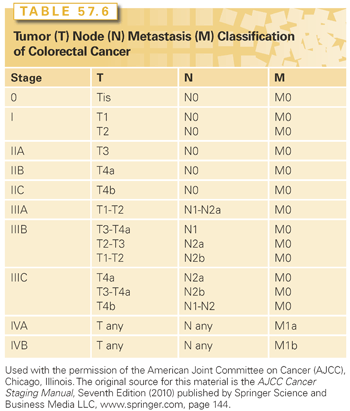
Although many factors have been identified that have an impact on recurrence and survival, none exceeds stage in terms of prognostic significance.105 Staging of CRC should be done using the current TNM (tumor, node, metastasis) classification of the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) staging system (Table 57.6).106 Other systems should be regarded as of historical significance only and must be comprehended solely for the purposes of understanding the studies that were performed and reported in the past using these older classifications.
The Dukes Classification and Its Modifications
In the 1930s, Cuthbert Dukes, a Scottish pathologist working predominantly on a classification scheme for rectal cancer, developed the classification system that bears his name. The system, and the several modifications to it made by Dukes and others, is at this time of historical interest only, and the reader is referred to chapters in earlier editions of this book for further details.
Tumor, Node, Metastasis Classification
The current AJCC/UICC staging system for CRC is now the only classification system that should be used.106 The TNM system classifies colorectal tumors on the basis of the invasiveness (not size) of the primary (T stage), the number (not size or location) of local-regional LNs containing metastatic cancer (N stage), and the presence or absence of distant metastatic disease (M stage) (see Table 57.6).
T Stage. A designation of Tx refers to the inability to describe the extent of tumor invasion due to incomplete information. In situ adenocarcinoma (Tis) includes cancers confined to the glandular basement membrane or lamina propria. The terms high-grade dysplasia and severe dysplasia are synonymous with in situ carcinoma and are also classified as Tis. T1 tumors invade into but not through the submucosa. T2 tumors invade into but not through the muscularis propria, and T3 tumors invade through the muscularis propria into the subserosa or into nonperitonealized pericolic or perirectal tissue. T4 tumors perforate the visceral peritoneum (T4a) or invade other named organs or structures (T4b). Tumors invading other colorectal segments by way of the serosa (i.e., carcinoma of the cecum invading the sigmoid) are classified as T4b. A tumor that is adherent to other structures or organs macroscopically is classified clinically as T4b; however, if the microscopic examination of the adhesions is negative, then the pathologic classification is pT3. The V and L substaging should be used to identify the presence or absence of vascular or lymphatic invasion. The “p” prefix denotes pathologic (rather than clinical) assessment, and the “y” prefix is attached to those tumors that are being reported after neoadjuvant (presurgical) treatment. For example, the pathologic T stage of a tumor showing only penetration into the submucosa after preoperative therapy would be ypT1. Recurrent tumors are reported with an “r” prefix (rpT3).
N Stage. Because of the prognostic significance associated with increased numbers of LNs inspected (see the following discussion), the current TNM classification scheme calls for at least 12 LNs to be analyzed, and both the number of nodes that are positive for tumor and the total number of nodes inspected should be reported. The term Nx is applied if no description of LN involvement is possible because of incomplete information. A pN0 designation may be made even if fewer than the recommended number of nodes are present; however, the prognostic significance of this pN0 designation is weaker. N0 denotes that all nodes examined are negative. N1a includes tumors with metastasis in one regional LN. N1b refers to involvement of two or three nearby LNs. N1c defines the presence of cancer cells found in areas of fat near LNs, but not in the LNs themselves. N2a indicates metastasis in four to six regional LNs. N2b denotes involvement of greater than seven nodes. Metastatic nodules or foci found in the pericolic, perirectal, or adjacent mesentery without evidence of residual LN tissue are regarded as being equivalent to a regional node metastasis and are counted accordingly.
Stage I disease is defined as T1-2N0 in a patient without distant metastases (M0). Stage II disease is defined as T3-4N0M0. The T-stage carries prognostic significance for stage II, and therefore T3N0 is classified as IIA, and T4a-bN0 is classified as IIB and IIC, respectively.
Node positivity in the absence of M1 disease defines stage III CRC. Recently, the prognostic significance of tumor invasiveness (T stage) has been reincorporated into the assessment of risk in stage III patients. In an exhaustive review of over 50,000 patients, Greene et al.107 demonstrated the prognostic significance of T stage within node-positive patients. Within the N1 category T stage was found to be highly prognostic, with T1-2 patients fairing significantly better than T3-4. Within the N2 population, the prognosis was worse than either subgroup of N1 patients, with T stage no longer carrying prognostic significance. Thus T stage is prognostic in patients with N0 and N1 but not N2 disease. The current TNM staging system takes these findings into account and now stratifies stage III patients into IIIA (T1-2N1), IIIB (T3-4N1), and IIIC (T any, N2). Stages IIIA, B, and C are highly prognostic for survival.
M Stage. Patients are designated M0 if no evidence of distant metastases is present. Identification of distant metastases denotes a classification of M1. Involvement of the external iliac, common iliac, para-aortic, supraclavicular, or other nonregional LNs is classified as distant metastatic (M1) disease. The M1 category is subdivided into M1a, defined as spread of tumor to one distant organ or set of distant LNs, and M1b, where spread has occurred to more than one distant organ or sets of LNs or spread has occurred to the peritoneum. Although the TNM staging system is regarded as the most comprehensive tool for prognostic and predictive purposes, a major criticism of the last two revisions is that survival of stage IIIA patients continues to be superior to stage IIB. This disparity, which is actually more pronounced in the seventh edition of AJCC manual, has been attributed to inadequate LN assessment and understaging. However, a recent review of SEER data showed this problem persists even in a subset analysis of patients with >12 LNs, highlighting the need for additional refinement and perhaps the incorporation of nonanatomic prognostic factors.108
Residual Tumor (R Stage) at Margins of Resection. Tumors that are completely resected with histologically negative margins are classified as R0. Tumors with a complete gross resection but with microscopically positive margins are classified as R1, the positive margin indicating that at least microscopic tumor remains in the patient. Patients who have incomplete resections with grossly positive margins are classified as having had an R2 resection. The R0, R1, and R2 designations carry strong prognostic implications.
Identification of the proximal and distal margins of resection is relatively straightforward, and definitions of these margins are well understood. A more complex and often misunderstood (as well as underreported) margin of resection is the circumferential radial margin (CRM). All three margins (proximal, distal, and CRM) should be specifically commented upon in the pathology report, as all three have prognostic significance.
The CRM is, by definition, a surgically dissected surface. It is defined as the cut retroperitoneal or perineal soft tissue margin closest to the deepest penetration of tumor. It is considered positive if tumor is present microscopically (R1) or macroscopically (R2) on a cut radial or lateral aspect of the surgical specimen. For the ascending colon, descending colon, and upper rectum, which are incompletely encased by peritoneum, the CRM is created by dissection of the retroperitoneal aspect of the bowel. In the case of the lower rectum, which is not encased by peritoneum, the CRM is created by sharp dissection of the mesorectum.
A tumor simply penetrating into pericolonic or perirectal fat does not necessarily constitute a positive CRM, but rather is simply a description of a T3 primary. A tumor that involves a peritonealized surface of the bowel and not a surgically cut surface does not constitute a positive CRM, but rather constitutes a T4a primary. If, however, the cut surface at the deepest penetration of the tumor is positive, then the CRM is positive and the resection is staged R1 (microscopic) or R2 (macroscopic). A positive CRM is highly predictive of local recurrence and should prompt consideration of adjuvant treatment.
Prognosis
Histologic Grade
Although histologic grade has been shown to have prognostic significance, there is significant subjectivity involved in scoring of this variable, and no one set of criteria for determination of grade are universally accepted.105 The majority of staging systems divide tumors into grade 1 (well differentiated), grade 2 (moderately differentiated), grade 3 (poorly differentiated), and grade 4 (undifferentiated). Many studies collapse this into low grade (well to moderately differentiated) and high grade (poorly differentiated or undifferentiated). Greene et al.107 demonstrated that this two-tiered split has important prognostic significance.
College of American Pathologists Consensus Statement. The College of American Pathologists (CAP) has published an expert panel consensus statement outlining their interpretation of the validity and usefulness of a large number of putatively prognostic and predictive factors in CRC.109 Variables were categorized as belonging to categories I through IV. Category I was defined as those factors proven to be of prognostic import based on evidence from multiple, statistically robust, published trials and generally used in patient management. Category IIA included factors intensively studied biologically or clinically and repeatedly shown to have prognostic value for outcome or predictive value for therapy that is of sufficient import to be included in the pathology report, but that remains to be validated in statistically robust studies. Category IIB included factors shown to be promising in multiple studies but lacking sufficient data for inclusion in category I or IIA. Category III included factors felt to be not yet sufficiently studied to determine their prognostic value, and category IV included those factors that are adequately studied to have convincingly shown no prognostic significance. A number of these factors are discussed in further detail in the following.
The T, N, and M categories of the current AJCC/UICC staging system were all classified as category I. Other category I inclusions were blood or lymphatic vessel invasion and residual tumor following surgery with curative intent (the R category). Although not assessed pathologically, an elevation of the preoperative CEA level was also felt to merit category I inclusion. Factors in category IIA included tumor grade, radial margin status (for resection of specimens with nonperitonealized surfaces), and residual tumor in the resection specimen following neoadjuvant therapy. Factors in category IIB (many of which are discussed in further detail in the following) included histologic type, histologic features associated with MSI (i.e., host lymphoid response to tumor and medullary or mucinous histologic type), high degree of MSI (MSI-H), loss of heterozygosity (LOH) of 18q (DCC [deleted in colon cancer] gene loss), and tumor border configuration (infiltrating versus pushing border). Factors grouped in category III included DNA content, all other molecular markers except for LOH of 18q/DCC and MSI-H, perineural invasion, microvessel density, tumor cell–associated proteins or carbohydrates, peritumoral fibrosis, peritumoral inflammatory response, focal neuroendocrine differentiation, nuclear organizing regions, and proliferation. Those factors in category IV (proven to be of no significance) included tumor size and gross tumor configuration.
Total Number of Lymph Nodes
It has been well established that an adequate number of LNs must be sampled before a patient can be considered node negative, and careful pathologic technique has been demonstrated to be crucial to adequate nodal interpretation. Failure to adequately dissect and display the mesentery will lead to underreporting and understaging.110,111 It should be noted that an insufficient number of LNs reported could be due to a suboptimal nodal dissection at operation, a less than thorough search for nodes by the pathologist, or some combination of the two. Additional patient- and tumor-related factors may also affect LN count independent of pathologist or surgeon. Belt et al.112 found a significant association between MSI phenotype and high LN yield in both stage II and III colon cancers, with the strongest effect in the latter group. The authors postulate that this may be due to a more prominent lymphocytic antitumor response known to be exhibited by MSI-H cancers.112 Another report suggests that low body mass index is associated with increased LN yield, although it did not affect relapse-free or overall survival in stage III cancers. Proximal tumor location, well- or moderately differentiated histology, and stage IIIC cancer were also significant variables for adequate LN recovery.113 Finally, a multivariate analysis of two large prospective US cohort databases (121,701 women and 51,529 men) demonstrated that specimen length, tumor size, ascending tumor location, T3N0M0 stage, and year of diagnosis were positively associated with negative node count (p <0.002). Mutation of KRAS was borderline significant and requires further study. The authors recommend that these variables be taken into account when judging adequacy of LN harvest and devising individualized treatment plans in the future.114 An analysis was reported on outcome versus nodal sampling in the patients who participated in an Intergroup trial (INT-0089), a large four-arm trial of different 5-fluorouracil (5-FU)–based adjuvant chemotherapies in patients with colon cancer. Multivariate analyses were performed on the node-positive (2,768 patients) and node-negative (648 patients) groups separately. The median number of LNs reported in the assessable patients on this trial was 11 (range, 1 to 87). Survival (overall, cancer-specific, and disease-free [DFS]) was found to decrease with an increasing number of involved LNs (p = 0.0001 for all three survival end points). However, after controlling for the number of involved nodes, survival increased with the total number of nodes (positive plus negative) reported (p = 0.0001 for overall survival, cancer-specific survival, and DFS). Even in patients who were node negative, overall survival (p = 0.0005) and cancer-specific survival (p = 0.007) were significantly increased as the number of reported LNs increased.
In a different secondary analysis of the Intergroup trial (INT-0089), a mathematical model was created to estimate the probability of a true node-negative result on the basis of the number of LNs examined in a subset of patients who had at least 10 LNs reported in their resection specimen.115 A total of 1,585 patients with stage III or high-risk stage II colon cancer were evaluated. This model concluded that when 18 nodes are examined, there is a <25% probability of true node negativity in T1 and T2 tumors. However, examination of <10 LNs was needed in T3 and T4 tumors to achieve the same probability. The overall conclusions of this analysis were that a very significant proportion of patients are understaged, and that such understaging could have important implications for decisions regarding adjuvant therapy and for overall prognosis.
The CAP consensus statement suggests that a minimum of 12 to 15 LNs should be examined in order to determine node negativity.109 Availability of fewer nodes should therefore be regarded as a relative high-risk factor in terms of prognosis and should be factored into decisions regarding adjuvant therapy. Further support for this recommendation comes from a newly published Danish cohort study that indicates that the advantage of larger LN harvest extends beyond more accurate staging. In addition to improved outcomes for node-negative patients, the authors found a significant increase in overall survival for stage III patients with >12 LNs removed as well (58.6% versus 45.2% for <12 LNs), despite a higher prevalence of N2 disease in this group. This may be related to better surgical technique or an underlying benefit of wider lymphadenectomy in general. LN ratio was also shown to be an important independent prognostic indicator and, in fact, superior to N-stage in predicting survival for stage III patients. This finding is consistent with a number of previous reports, many of which have advocated for incorporation of this parameter into the AJCC staging system.116–120
Microscopic Nodal Metastases
The advent of improved pathologic techniques and sensitive methods such as IHC or polymerase chain reaction may have an impact on the number of positive LNs detected and may have important prognostic significance.121,122 However, the prognostic value of these positive LNs, which otherwise would not be detected, remains controversial. In a recent review of 16 studies with survival data, Sirop et al.123 found only 8 papers that reported definitely poorer outcomes, whereas the remainder were either equivocal in their conclusions or demonstrated no influence on outcome at all. Jeffers et al.124 evaluated LNs from 77 patients who were found to have negative LNs by routine examination with immunocytochemical staining for cytokeratin AE1:AE3. Nineteen patients (25%) were found to have immunohistochemical evidence of micrometastases; however, there was no difference in survival between the microscopically positive and negative patients. A larger trial by Faerden et al.,125 on the other hand, did demonstrate adverse prognostic impact. In this study, 39 of 126 patients with stage I/II colon cancer were noted to have micrometastases or isolated tumor cells (MM/ITC+) on IHC staining. Prospective median 5-year follow-up of MM/ITC+ compared to MM/ITC- patients revealed recurrence rates of 23% versus 7% (p = 0.010) and 5-year DFS of 75% versus 93% (p = 0.012), respectively.125 If micrometastases are reported, the methodology by which they are detected should be specified, as it is likely that differences in reliability and reproducibility of different techniques will emerge. Although the actual TNM staging is not altered by the presence of micrometastases, many clinicians choose to regard the presence of such a finding as a poor prognostic variable in their consideration of adjuvant treatment.
Sentinel Node Analysis
Sentinel node analysis is an approach that has received attention in the management of cutaneous melanoma and breast cancer.126,127 This technique has been proposed as a means of increasing the yield and the diagnostic information for colon cancer.91,92 The technique for sentinel node mapping and biopsy for colon cancer has been described by Saha et al.128 Unlike sentinel node approaches for melanoma and breast, where the goal is to potentially limit the extent of an unnecessary formal dissection of a node basin, the goal of the sentinel node in colon cancer is to focus the pathologic analysis on fewer nodes so a more extensive study can be performed. The same extent of node dissection is performed regardless of the sentinel node procedure. The initial studies of sentinel node biopsy demonstrated it was technically feasible, with accuracy rates >80% and upstaging in 15.4% of patients according to a recent prospective trial.129–131 In addition, Saha et al.132 suggest that sentinel LN mapping may not just improve staging accuracy but influence the extent of nodal dissection as well. In this study, sentinel LN mapping detected aberrant lymphatic drainage in 22%, which in turn led to a change in operation (i.e., more extensive resection). In two patients, the aberrant sentinel nodes were the only positive nodes identified.132 However, not all subsequent studies have shown positive results. False-negative rates as high as 60% have been reported, and some studies have failed to demonstrate any change in the stage determination of the lesion.133 Based on the available data, two conclusions can be reached. First, from a technical standpoint, sentinel node dissection at the time of a colon resection can be performed and the sentinel node accurately identified. Second, the utility of this technique has not yet been established and further large-scale trials are required to establish its role in the staging of patients with CRC.
Blood or Lymphatic Vessel Invasion
Although there have been conflicting reports in the literature, the CAP consensus statement gave blood and lymphatic vessel invasion category I status, indicating that the preponderance of evidence strongly supports the reliability of these findings as indicators of poorer prognosis.109 Unfortunately, considerable heterogeneity exists in the methodology for examining and reporting of vessel involvement. The finding of vessel involvement increases with the number of sections examined, and differentiation of postcapillary venules from lymphatics is often not possible. These aspects can make interpretation of some older data on this topic potentially problematic. Current recommendations are that at least three blocks of tumor (optimally five or more) each have a single section examined using hematoxylin and eosin stain to look for tumor invasion of vessels. Vessels not definitively interpreted as venules or lymphatics should be reported as angiolymphatic vessels.
Histologic Type
Several histologic types of CRC carry specific independent prognostic significance. Signet ring carcinomas are characterized by >50% of cells demonstrating the “signet ring” morphology in which intracellular mucin accumulation displaces the nuclei and cytoplasm toward the cellular periphery. This histology carries an adverse prognosis.134,135 The prognostic significance of the finding of mucinous (>50% mucinous) carcinoma remains controversial. Although some reports list mucinous type as an adverse histology, this has not been consistently demonstrated. Most findings of adverse prognosis with mucinous histology are based on univariate analyses. The one finding in a multivariate analysis of a poor prognostic outcome with mucinous tumors was based on a study of tumors presenting with obstruction, a presentation that is in itself high risk. Some reports have lumped mucinous and signet cell tumors together and found this to be a negative prognostic factor; however, this may simply reflect the negative impact of the signet cell tumors, and its meaning regarding the risk of a mucinous histology is unclear. Small cell (extrapulmonary oat cell) tumors are high-grade neuroendocrine tumors with clearly adverse prognostic features. The prognostic significance of focal neuroendocrine differentiation is, however, unclear (CAP category III). Most data indicate that extensive neuroendocrine differentiation is associated with a poorer prognosis.136 Medullary carcinoma is a subtype characterized by an absence of glands and distinctive growth pattern that previously would have been classified as undifferentiated. It is typically infiltrated with lymphocytes. This histologic subtype is tightly associated with MSI-H and carries a more favorable prognosis.137 Histologic types other than signet ring, small cell, and medullary carcinomas are routinely designated in the pathology report; however, the majority of these other histologic types carry no established independent prognostic significance.
Microsatellite Instability
As discussed earlier in this chapter, there are two distinct mutational pathways that can give rise to CRC: the MSI pathway or the chromosomal instability pathway. Microsatellites are sections of DNA in which a short sequence of nucleotides (most commonly a dinucleotide) is repeated multiple times.138 MSI is a situation in which a microsatellite has gained or lost repeat units and so has undergone a change in length, resulting in frame shift mutations or base-pair substitutions. Approximately 15% of CRCs display these mutations. This form of genetic destabilization is typically associated with defective DNA mismatch repair function. Studies of HNPCC tumor specimens demonstrated mutations in mismatch repair genes such as MLH1 and MSH2. These genes encode proteins that repair nucleotide mismatches. The phenotype of tumors with this defect is termed the MSI-H–instability phenotype.
The majority (approximately 85%) of patients with CRC have cancers characteristic of the chromosomal instability pathway, typically having genetic alterations involving LOH, chromosomal amplifications, and chromosomal translocations. These are known as the microsatellite-stable (MSS) tumors. MSI-H tumors have a number of different features relative to low MSI (MSI-L) or MSS colorectal tumors.139,140 MSI-L and MSS tumors tend to behave and present similarly. MSI-H tumors are more frequently right-sided, high grade, and mucinous type.24,141 They are characteristically associated with increased peritumoral lymphocytic infiltration and are characteristically diploid, whereas MSS tumors are more likely to be aneuploid.142,143 MSI-H CRCs are more likely to have a larger primary at the time of diagnosis but are more likely to be node negative. Patients with MSI-H CRCs have a better long-term prognosis than stage-matched patients with cancers exhibiting MSS.144
Watanabe et al.145 evaluated MSI status as well as allelic loss from chromosomes 18q, 17b, and 8p, as well as cellular levels of p53 and p21waf1/c1p1 proteins as potential prognostic markers. Tumors were analyzed from 460 stage III and high-risk stage II patients who had been treated with 5-FU–based adjuvant therapy. A total of 62 of 298 tumors evaluated for MSI status (21%) were found to be MSI-H. Of the MSI-H tumors, 38 (61%) had a mutation of the gene for type II receptor of transforming growth factor (TGF)-β1. In this analysis, MSI-H was a favorable prognostic indicator for 5-year DFS (p = 0.02) and trended toward being a favorable independent prognostic indicator, but did not reach statistical significance for overall survival (p = 0.20). However, the 5-year survival among patients with MSI-H was 74% in the presence of a mutated gene for the type II receptor of TGF-β1 and 46% in patients whose tumors lacked this mutation (RR = 2.90; 95% CI = 1.14 to 7.34; p = 0.04). MSI-H cells are relatively resistant to 5-FU in vitro.146 All of the patients in Watanabe et al.’s145 analysis received 5-FU–based chemotherapy. The TGF-β1 pathway inhibits tumor proliferation by causing a late G1 cell cycle arrest. Therefore, a mutated and presumably nonfunctional TGF-β1 gene could favor increased proliferation, which would be anticipated to confer increased susceptibility to cytotoxic chemotherapy. A recent evaluation of the prognostic significance of MSI in the N0147 adjuvant trial147 demonstrated a more nuanced result. When looking at the colon overall, MSI was not found to be predictive. However, when divided by side, MSI was found to carry a favorable prognosis for right-sided colon lesions, but was a negative prognostic factor in left-sided colon lesions. The reasons for this difference is not clear; however, the different embryologic origins of the left and right colon may play a role in these observations.
BRAF
BRAF mutation, present in 10% to 20% of CRCs, is linked to a subset of MSI-H tumors that are sporadic and generally have poorer prognosis. Ogino et al.148 confirmed this relationship in comparative analysis of 506 stage III patients enrolled in the Cancer and Leukemia Group B (CALGB) 89803 trial. BRAF-mutated patients had significantly worse overall survival (HR = 1.66) compared to wild-type, a finding that was most pronounced in the setting of MSS.148 In a follow-up study, Lochhead et al.149 also identified combined BRAF/MSI status as a powerful prognosticator and recommends stratification of all patients into poor (MSS/BRAF mutant), intermediate (MSS/BRAF wild-type), and favorable (MSI-H/BRAF wild-type) groups in order better inform treatment strategies. Douillard et al.150 confirmed BRAF mutation to be a poor prognostic factor in patients with stage IV disease as well.
Allelic Loss of 18q (DCC Gene Loss)
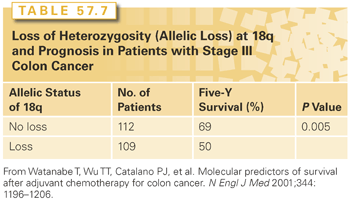
Allelic LOH that involves chromosome 18q occurs in half or more of all CRCs. Allelic loss of 18q typically involves the DCC gene; however, other genes in this region, such as Smad2 and Smad4, may also be relevant to CRC development. DCC expression is greatly reduced or absent in many colorectal carcinomas, and loss of DCC is associated with metastasis and an adverse prognosis.151 The specific product of the DCC gene has been shown to be the netrin-1 receptor. In the nonpathologic state, this receptor guides the migration of neuronal axons. DCC induces apoptosis in the absence of netrin-1 binding. DCC is cleaved by caspase, and mutation of the site at which caspase 3 cleaves DCC suppresses the proapoptotic effect of DCC completely. Binding of netrin-1 to DCC blocks apoptosis.152 Loss of DCC as a result of allelic loss in 18q could therefore be anticipated to impair apoptosis, thereby resulting in greater resistance to chemotherapy. This hypothesized mechanism of action of 18q LOH is attractive; however, it should be emphasized that it is not at all clear to what extent DCC is the active moiety in the setting of 18q allelic loss. Watanabe et al.145 evaluated allelic loss from chromosome 18q as a potential prognostic indicator in archived specimens of tumors from patients who were treated in one of two national Intergroup adjuvant trials (INT 0035 or INT 0089). MSI status was also evaluated, as were 17p, 8p, and cellular levels of p53 and p21waf1/c1p1 proteins. Tumors were analyzed from 460 stage III and high-risk stage II patients who had been treated with 5-FU–based adjuvant therapy. Allelic loss of 18q was present in 155 of 319 cancers (49%). Allelic loss in 18q was highly prognostically significant in this analysis (Table 57.7). In the stage III patients with allelic loss of 18q, 5-year overall survival was 50%, while in those with retained 18q alleles, 5-year survival was 69% (p = 0.005). Other markers evaluated in this analysis were not shown to be prognostically significant.
Host Lymphoid Response
Lymphocytic infiltration has been identified as a favorable prognostic indicator. Whether this is a truly independent predictor of outcome is not clear, however, as this finding is tightly associated with MSI-H, a favorable prognostic factor. Along these lines, the prognostic value of neutrophil-to-lymphocyte ratio has also been recently evaluated. Chiang et al.153 found that elevated preoperative neutrophil-to-lymphocyte ratio (>3) was associated with significantly worse DFS in stage I to III colon but not rectal cancers on multivariate analysis. In another study, neutrophil-to-lymphocyte ratio (>5) was also found to be an independent risk factor for recurrence. While the direct impact of this parameter is difficult to explain, the authors of the first study postulate that it may represent a measure of innate-to-adaptive immunity under stress with relative lymphopenia, as a marker of depressed cell-mediated immunity, conferring survival disadvantage.153,154
Tumor Border Configuration
The configuration of the tumor border (infiltrating versus pushing border) has been shown to have independent prognostic significance. An infiltrating border, characterized by an irregular, infiltrating pattern at the tumor edge (also known as focal dedifferentiation or tumor budding), has been shown in multivariate analyses to portend a poorer prognosis than tumors with smooth, pushing borders.
Carcinoembryonic Antigen
An elevated preoperative CEA is a poor prognostic factor for cancer recurrence. Although there is variability in the available data regarding the level that denotes a prognostic cutoff, a preoperative CEA level >5 ng/ml is considered a category I poor prognostic indicator by the CAP consensus panel.109 Patients in whom the elevated CEA fails to normalize after a potentially curative operation are at particularly high risk. Several authors have presented evidence that indicates that CEA is an independent prognostic factor. In a report of 572 patients who underwent curative resection for node-negative colon cancer, the preoperative CEA level and the stage of disease predicted survival by both univariate and multivariate analyses.155 Given the prognostic significance of the preoperative CEA, it is reasonable to recommend that all patients who undergo operation for CRC have a serum CEA drawn prior to operation.
No other serum markers have been demonstrated to be reliably prognostic or predictive in CRC. Cancer antigen (CA) 19-9, a factor that has become widely used for pancreas cancer, has no role at this time in the routine management of CRC.
Obstruction and Perforation
Carcinoma of the colon that is complicated by obstruction or perforation has been recognized as having a poorer prognosis. Data obtained from 1,021 patients with Dukes stage B and C CRC, who were entered into randomized clinical trials of the National Surgical Adjuvant Breast and Bowel Project (NSABP) showed that the presence of bowel obstruction strongly influenced the outcome. The effect of bowel obstruction was more pronounced when the obstruction was located in the right colon. The larger-sized tumor needed to block the ascending colon completely might allow a longer time for these tumors to grow and spread when compared with tumors located in the descending colon.
A review of the Massachusetts General Hospital records compared patients who presented with obstruction or perforation with a control group who underwent curative resection. The actuarial 5-year survival rate seen in patients who presented with obstruction was 31%, in contrast to 59% in historical controls. For patients with localized perforation, the 5-year actuarial survival rate was 44%. The Gastrointestinal Tumor Study Group (GITSG) multivariate analysis concluded that obstruction was an important indicator of prognosis, independent of Dukes stage. Bowel perforation was a poor prognostic factor only for DFS.
Category III Factors
Multiple factors, while of investigational interest, are at this time not appropriate for routine clinical use and have so been designated as category III (defined as not sufficiently studied to prove their prognostic value) by the CAP consensus panel. These include DNA content, or ploidy, and proliferation indices. Also included in category III are all molecular markers other than MSI and 18q deletions, such as thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD), and p53 mutational status. Perineural invasion, microvessel density, tumor cell–associated proteins or carbohydrates, peritumoral fibrosis, peritumoral inflammatory response, and focal neuroendocrine differentiation are also category III. The area of molecular prognostic markers is one of particular activity, however, and it is anticipated that clinical trials that are now ongoing will shed light on these important areas.
Perineural Invasion
The ability of CRCs to invade perineural spaces as far as 10 cm from the primary tumor has long been described. Early reports suggest an increased disease recurrence rate and worse 5-year survival. Multivariate analyses have failed to show the prognostic significance of this finding. The CAP consensus panel classified perineural invasion as category III (insufficient evidence of determine prognostic significance).
Tumor Size and Configuration
Studies have consistently shown that both the size and configuration of the primary tumor in CRC do not carry prognostic significance (CAP category IV). In a review of 391 patients, the mean diameter of Dukes stage B2 tumors was actually greater than the mean diameter of stage C2 tumors (p <0.001) and D tumors (p <0.05). The size of the primary tumor showed no relationship to 5-year adjusted survival. These results were confirmed by the NSABP experience.156 Tumor configuration is described as exophytic (fungating), endophytic (ulcerative), diffusely infiltrative (linitis plastica), or annular. The vast majority of studies have failed to show any of these configurations to have consistent independent prognostic significance. Linitis plastica has been related to a poor prognosis; however, this may be due to the signet cell and other high-grade features of the tumors that are typically associated with this morphology.
Hemorrhage or Rectal Bleeding
It has been speculated that tumors that present with bleeding might be found earlier and therefore might be associated with a better prognosis. This has not been confirmed by data. In the GITSG multivariate analysis, the presence of melena or rectal bleeding showed a trend as a prognostic factor for prolonged survival but failed to reach statistical significance (p = 0.08). One large study found bleeding to be a favorable prognostic indicator on univariate analysis; however, this finding disappeared on multivariate analysis. Bleeding at presentation does not appear to carry any significance.
Primary Tumor Location
Large retrospective reviews of data from the NSABP suggest that right-sided colon cancers carry a worse prognosis than left-sided ones. However, poorer prognosis for patients with disease in the left colon has also been reported. Several investigators report no difference based on the location of the primary tumor. The large GITSG colon cancer experience showed that tumor location (left, right, and rectosigmoid or sigmoid) was of low prognostic value. A recent analysis of SEER-Medicare data by Weiss et al.157 provides additionally ambiguous results. Of 53,801 patients, 67% had right-sided colon cancer and were more likely to be older, women, and diagnosed with more advanced stage and with more poorly differentiated tumors. However, on multivariate analysis, there was no significant difference in mortality for all stages combined or for stage I. Compared to left-sided lesions, right-sided cancers were associated with a lower mortality within the stage II subgroup (HR = 0.92; p = 0.001) but higher mortality within stage III (HR = 1.12; p = 0.001). Critics of this report point out that a less aggressive treatment approach was likely employed in this older study population, as at least 40% of stage III cases did not receive adjuvant therapy and nearly half underwent inadequate LN harvest. Regardless, these results further dispel the notion of a straightforward relationship between tumor location and mortality.157
Body Mass Index
While obesity is known to be a risk factor for the development of colon cancer, the prognostic impact of body mass index on long-term outcomes is controversial. In a cohort study conducted within a large randomized trial of 3,759 patients with high-risk stage II or III colon cancer (INT-0089), obese women had significantly worse overall mortality (HR = 1.34; 95% CI = 1.07 to 1.67); however, this finding was not apparent in men.158 Sinicrope et al.159 found the opposite gender correlation using the ACCENT database, a pooled resource of 25,291 participants in national and international adjuvant chemotherapy trials. On multivariate analysis, with a median follow-up of 7.8 years, obese and underweight men, but not women, had significantly poorer survival compared to overweight and normal weight patients.159 And in another prospective cohort of 913 patients with stage II and III colon cancer, Alipour et al.160 found no association between obesity (as measured by either body mass index or body surface area) and oncologic outcomes. Evidently, this topic warrants further study before any conclusions can be drawn.160
Diabetes Mellitus
The influence of diabetes mellitus on outcome is also unclear. In the INT-0089 cohort, diabetes conferred a strong disadvantage with affected patients experiencing a significantly worse DFS (48% versus 59%; p <0.0001), overall survival (57% versus 66%; p <0.0001), and recurrence-free survival (56% versus 64%; p = 0.012) at 5 years. Median survival for diabetics was 6 years, whereas for nondiabetics it was 11.3 years.158 Other reports, however, have generated less consistent results. Among 2,278 subjects from the Cancer Prevention Study-II Nutrition Cohort, patients with CRC and type 2 diabetes were at higher risk of all-cause mortality (ACM; RR = 1.53), but only those without insulin use were at higher risk for CRC-specific mortality. These results are in line with previous evidence that hyperinsulinemia (as in poorly controlled diabetes) plays an important role in tumorigenesis and metastasis of CRC.161 Another population-based study did not find any such an association in 6,974 patients with colon cancer. Disease-specific mortality was only significantly increased for patients with rectal cancer (n = 3,888, 10% of whom were diabetic; HR = 1.30). While hyperinsulinemia is again implicated, the authors call for additional study to clarify specific pathways responsible for these rectum-specific findings.162
Gender
Female sex has generally been considered a favorable prognostic factor, but data is limited and inconclusive. In the first study to examine the impact of gender in the era of oxaliplatin-based therapy, Cheung et al.163 performed a prospectively planned, pooled analysis of 33,345 patients participating in the ACCENT database of randomized trials. The authors found a significant but very modest survival advantage for women with early stage disease that persisted across all ages, stages, and types of adjuvant therapy. Sex was not a predictive factor for treatment efficacy, however, suggesting that chemotherapy regimens should be not be altered based on this parameter.163
Smoking
As discussed earlier, prolonged cigarette smoking appears to be a moderate risk factor for CRC with continued effect even after smoking cessation. Increasing evidence indicates that this association differs not just by tumor site but also by molecular features, such as the presence of MSI-H and BRAF mutations, which cumulatively seem to confer the strongest risk. Impact on survival has now also been reported in a recent study analyzing data from a large multicenter randomized adjuvant chemotherapy trial (N0147). The authors found that smokers experienced significantly shorter 3-year DFS (74% versus 70%; HR = 1.21) that was most evident in BRAF wild-type and KRAS-mutated tumors.164
Blood Transfusions
Considerable controversy has surrounded the question of an association between perioperative blood transfusions and the recurrence rate of CRC. Some investigators have reported worse DFS in patients who require transfusions. By multivariate analysis in a large prospective study, however, no negative influence of transfusion on survival could be detected, and it does not appear that perioperative blood transfusions carry negative prognostic value. A retrospective analysis evaluating 1,051 patients treated with curative surgery for stage II or III colorectal adenocarcinoma at the Mayo Clinic demonstrated that the use of blood components probably had no impact on disease recurrence, and the documented adverse impact of transfusions is more likely due to other variables or to the underlying illness necessitating the transfusion.165
Oncogenes and Molecular Markers
Oncogenes and molecular markers are discussed extensively in another chapter. At present, none of the markers under investigation has achieved adequate validity to permit routine clinical use. However, the study of molecular markers continues to progress and continues to advance the understanding of the development and treatment of CRC. TS continues to be a major area of investigation. Data are conflicting on its prognostic significance; however, preliminary studies suggest that high TS levels may be predictive for resistance to 5-FU–based therapies.166 At present, there is no role for TS determinations in routine clinical practice. The p53 gene located on chromosome 17p is a well-known tumor suppressor gene. The abnormal p53 appears to be a late phenomenon in colorectal carcinogenesis. This mutation may allow the growing tumor with multiple genetic alterations to evade cell cycle arrest and apoptosis. In a retrospective review of 141 patients with resected stage II and III colon carcinoma, a p53 mutation increased the risk of death by 2.82 times in patients with stage II disease and by 2.39 times in patients with stage III colon carcinoma. The Southwest Oncology Group assessed the prognostic value of p53 in 66 patients with stage II and 163 stage III colon cancer. p53 expression was found in 63% of cancers and was associated with favorable survival in stage III but not stage II disease. Seven-year survival with stage III disease was 56% with p53 expression versus 43% with nop53 expression (p = 0.012).167 Overall, the data are conflicting on the utility of p53 as a prognostic variable, and it does not have a use at this time in standard practice.
Epidermal growth factor receptor (EGFR) is an important molecular target for antibody-based therapy in various cancer types and is ubiquitous in colonic tissue. The prognostic impact of this biomarker was recently addressed in a meta-analysis demonstrating worse postoperative survival in patients with high compared to low EGFR expression (HR = 2.34).168
Genetic Polymorphisms
Extensive preliminary work is indicating that genetic polymorphisms can potentially have important predictive implications in terms of both efficacy and toxicity with chemotherapy. For example, the UGT1A1 polymorphism has been correlated with CPT-11 toxicity, and TS and XRCC1 polymorphisms may predict efficacy for oxaliplatin or 5-FU combinations.169 Although a commercial assay is currently available for measurement of UGT1A1 polymorphisms, it is not, at this time, clear how, or if, this assay should be used in routine practice. Currently, there are no specific guidelines for dose modifications on the basis of UGT1A1polymorphism, and the 7/7 mutation, associated with higher toxicity, has also been associated with greater antitumor activity. These approaches will require considerable more validation and exploration before they can be considered for standard management.170
APPROACHES TO SURGICAL RESECTION OF COLON CANCER
The management of colon cancer is best understood as a multimodality approach tailored to the stage of disease. However, there are certain basic tenets of surgical management for the resection of the primary lesion that can be applied across various pathologic stages. Therefore, in order to provide a clear description of these techniques, they will first be described based on the type of surgical resection. These procedures will then be referred to throughout the discussion of stage-specific treatment.
Colonoscopic Resection of Polyps
Many lesions of the colon are first detected during endoscopic procedures. These lesions can range from small hyperplastic polyps to large fungating invasive carcinomas. The appearance of these lesions often indicates their relative potential for malignancy. However, the only definitive way to make a diagnosis is through a pathologic examination of the tissue. Therefore, the goal of a colonoscopic biopsy or resection is to, whenever feasible, remove the lesion in its entirety and preserve a tissue architecture in order to achieve both a therapeutic resection and an accurate pathologic diagnosis. Various techniques can be employed for the removal of lesions in the colon depending on their size and location. Biopsy forceps and snares are the two most commonly employed instruments used during a colonoscopy. These devices are fashioned from flexible coated wires that can conform to the shape of the colonoscope and can also conduct electrical current in order to achieve coagulation and hemostasis.
Bleeding and perforation, while uncommon, are seen at an increased frequency during a therapeutic as opposed to a diagnostic colonoscopy.171,172 Small polypoid lesions (up to 5 to 8 mm) that are found during the course of a colonoscopic examination can often be removed in their entirety along with a small amount of normal mucosa using a biopsy forceps. Bleeding is usually minimal but can be controlled by electrocautery if persistent. Larger well-pedunculated polyps can often be removed using a technique employing a snare and electrocautery. The snare is placed over the polypoid lesion and cinched down at the base of the polyp. Once tightened, an electrical current is applied and the polyp is resected. If the lesions are too large to be retrieved through the working port of the colonoscope, they can be held in place with a snare just beyond the tip of the colonoscope where they can be kept in view and withdrawn with the scope from the patient. It is important, when sending these specimens to pathology, to properly orient the polyp so as to indicate the base where the resection took place as well as the other positions of the lesion. This will allow the pathologist to provide important information as to the margin status for the resection. Carcinoma in situ as well as stage I invasive carcinomas found in a well-pedunculated polyp can be treated with colonoscopic resection, as described previously, and no further surgical management is needed as long as there is a negative margin >2 mm and the tumor is well-differentiated without lymphovascular invasion or extension of malignant cells beyond the stalk (Haggitt levels 1 to 3).173 If these criteria are not met, further therapy is required. It is for this reason that it is often helpful to mark the site of the polyp resection with an agent that will leave a “tattoo” to guide additional intervention.
Larger lesions with a broad base or sessile lesions are best biopsied to make a diagnosis rather than resected using the colonoscope. The risk of perforation or inadequate resection margins is greatly increased with broad-based and sessile lesions. Multiple biopsies should be taken in order to determine whether the lesion harbors an invasive cancer, and further resection decisions are made based on the pathologic findings. In cases where there is low suspicion for malignancy, an endoscopic mucosal or submucosal resection may be attempted, usually by a gastroenterologist with advanced interventional endoscopic expertise. However, if such a lesion is left behind, it is of critical importance to note the position of the lesion in order that it might be more easily found if a subsequent procedure is required. In addition to determining the depth of insertion of the scope, which can be highly inaccurate with flexible instruments, other landmarks including the appendiceal orifice or ileocecal valve in the cecum and the liver/splenic shadows at the flexures should be noted. The most important step however is to properly mark the polyp site with 1 ml of tattoo injected submucosally in each of four quadrants for definitive intra- and extraluminal recognition at a later date.174
For lesions that cannot be resected through the scope or are found to be invasive carcinomas that are sessile or broad based, a variety of surgical resections can be employed depending on the position of the lesion and its T stage. It is important to keep in mind, however, that the formal staging of the lesion does not occur until after the resection is completed; therefore, if there is any suspicion of an invasive carcinoma being present, a definitive oncologic resection should be performed.175
Bowel Preparation
An important part of the preoperative regimen for a colon resection is the proper cleansing of the bowel in order to reduce the risk of postoperative complications as well as to allow for easier visualization during the procedure, particularly with the laparascopic approach. A variety of regimens have been described, and there are many that have demonstrated efficacy.176,177 Although there are several choices described in the literature, the basic components of a bowel preparation are a mechanical cleansing of the bowel using a cathartic or volume-displacing agent and appropriate antibiotic prophylaxis.178,179 Recently, some studies have suggested that mechanical bowel preparation may be unnecessary; however, this remains controversial.180,181
For rectal and low sigmoid tumors, a number of surgeons also perform distal rectal washout prior to resection, with the professed intention of eliminating exfoliated intraluminal cancer cells that may increase local recurrence risk. There has been little evidence to support this theory, and washout has not been routinely recommended as standard practice. However, a recent meta-analysis of nine studies and 5,395 patients is the first to demonstrate a significant benefit to this maneuver with a nearly two-fold reduction in local recurrence rates (5.79% versus 10.05%; p <0.00001). While the lack of randomized controlled trials limits the strength of this data, the authors conclude that distal washout should be reconsidered in all patients given the minimal cost, time, and risk it entails.182
Anatomic Resection
For invasive carcinomas of the colon, stages I through III, the surgical approach will be dictated by the size and location of lesions in the colon.183,184 The location will determine what region of bowel is removed, and the extent of its resection is dictated by its vascular and lymphatic supply.
Resection of the Right Colon
Lesions in the cecum and ascending colon are managed with a right hemicolectomy (Fig. 57.3A,B). The right colon is mobilized from the retroperitoneum by incising its retroperitoneal attachments, taking care to avoid injury to the ureter, inferior vena cava, duodenum, and gonadal vessels. The colon is mobilized from the ileum to the transverse colon, taking care at the hepatic flexure not to injure the gallbladder or duodenum. The ileocolic, right colic, and right branch of middle colic vessels are then ligated and divided. A proximal ligation in order to allow for the removal of colonic mesentery along with LNs is performed for staging purposes. Once the vascular supply is divided and the intervening mesenteric tissue ligated and divided, attention can be addressed to the resection of the colonic tissue.
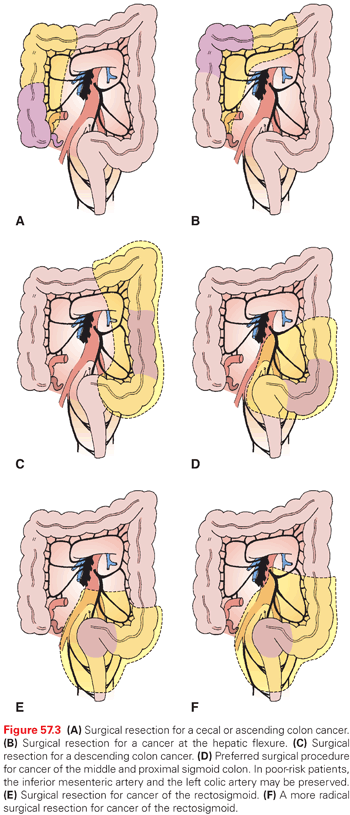
There are a variety of techniques for dividing the colon. This can be done between clamps using scalpel or using a variety of stapling devices. One method would be to use a linear GI anastigmatic stapler. After making a small hole just below the colonic wall though the mesentery at the point chosen for resection, the stapler can be positioned across the colon and fired, thus dividing the tissue. This is then repeated across the ileum just proximal to the ileocecal valve. Once divided, all remaining mesenteric tissue is carefully ligated and divided, and the colonic specimen can be removed. Although a no-touch “technique” has been advocated in the past, studies have demonstrated that this has no influence on recurrence or seeding of distant disease.185 Once the right colon has been removed, intestinal continuity can be re-established by creating an anastomosis between the terminal ileum and the remaining transverse colon using either a hand-sewn or stapled technique.
Resection of the Transverse Colon
For lesions located in the transverse colon, a variety of approaches can be undertaken. Those lesions that are proximal and near the hepatic flexure can be resected with an extended right hemicolectomy. This extension should encompass up to and include the middle colic vessel. The advantage of such a resection over a true transverse colectomy is that the anastomosis performed to restore intestinal continuity involves an anastomosis between the ileum and the remaining colon. Due to the improved blood supply delivered by the small bowel mesentery, there is a decreased risk of an anastomotic leak in an ileocolic as opposed to a colocolic anastomosis.175 Likewise, a lesion in the distal transverse colon can be resected with an extended left hemicolectomy, which will be described in more detail in the following section. For those lesions that are in the midportion of the transverse colon, however, a transverse colectomy can be performed. This procedure requires mobilization of the right colon in order to allow this tissue to be brought over for an anastomosis following the resection.
The omentum is divided from the greater curvature of the stomach up to and including its attachments at the splenic helium. The omentum can often be a source of micrometastatic disease and therefore its resection at the time a transverse colectomy is indicated. After dividing the omentum and mobilizing the right and transverse colon up to and including the splenic flexure, the middle colic artery is ligated at its trunk and smaller vessels from the right and left colic artery branches can be ligated and divided as required. A linear stapler can once again be used to divide the colonic tissue, and then the mobilized right colon can be anastomosed to the descending colon in an end-to-end fashion using a hand-sewn anastomosis or using a side-to-side stapled technique. Depending on the size of the transverse colon, however, it is often safer and easier to resect the right and transverse colon and connect the ileum to the descending colon. This allows enough colonic reserve for water absorption and normal bowel movements.
Resection of the Descending and Sigmoid Colon
For lesions in the proximal descending colon, the splenic flexure is mobilized and the left colic artery can be ligated and divided with the portion of colon removed by mobilizing the splenic flexure and dividing the omentum (Fig. 57.3C,D). The transverse colon can be brought over to the region of the sigmoid colon for anastomosis. For lesions in the midportion of the descending colon, a left hemicolectomy can be performed, taking care to ligate the left colic vessel along with some sigmoidal branches and taking an adequate portion of mesentery for staging purposes.
For lesions that involve the sigmoid colon, a sigmoid colectomy can be performed with margins of resection on either side of the lesion. The descending colon is mobilized (together with the splenic flexure as needed) and connected to the rectum using either a hand-sewn anastomosis or stapling device. The mesentery can be divided either at the level of the sigmoidal branches with preservation of the left colic artery or at the origin of the inferior mesenteric pedicle (Fig. 57.3E,F). While the latter approach is preferred by some to achieve greater mobilization and higher lymph node counts, neither this nor a more extensive left hemicolectomy has resulted in improved survival.
The approaches to the resection of lesions below the peritoneal reflection will be discussed in the chapter on rectal cancer.
Total Abdominal Colectomy
For patients with ulcerative colitis or familial polyposis syndrome who either have evidence of invasive carcinoma or are at significant risk for the development of invasive carcinoma, a total abdominal colectomy may be required. This can be performed by mobilization of the right colon, transverse colon along the omentum, taking the omentum as part of the resection, the hepatic and splenic flexures, as well as the complete mobilization of the descending colon down to the peritoneal reflection. Ligation of the ileocolic, right colic, middle colic, left colic, and sigmoid branches will allow for removal of the colon down to the peritoneal reflection. For ulcerative colitis and familial polyposis syndromes without evidence of carcinoma below the peritoneal reflection, the operation can be terminated at this point with ileorectal anastomoses and careful surveillance of the remaining rectum via proctoscopy. However, in order to remove all tissue at risk for further lesions, a total protocolectomy is often advocated.186,187 Although this procedure can be performed as an abdominal perineal resection with a permanent end ileostomy, most surgeons now advocate one of many continent pull-through procedures in order to preserve fecal continence in a patient population that is often very young. Such procedures provide very good control of continence and a relatively normal lifestyle.188
SURGICAL MANAGEMENT OF COMPLICATIONS FROM PRIMARY COLON CANCER
Patients with primary lesions of the colon can present with obstruction, bleeding, and perforation. The surgical management of these patients can be complex, requiring intraoperative decisions tailored to the situation encountered. Blood per rectum can be one of the most frightening experiences for patient and physician alike. Bleeding from a CRC can occur anywhere from the cecum to the distal rectum. Although bleeding can be temporized with endoscopic fulguration and the patient supported with transfusion, definitive management of the lesion with either surgery or radiation therapy will ultimately be required. Other maneuvers such as angiographic embolization may provide only a temporary solution. Fortunately, life-threatening hemorrhage due to a colon cancer primary is a rare occurrence. More often, these lesions lead to a chronic blood loss, resulting in anemia.
Colonic obstruction due to a primary tumor is not uncommon. Obstructing colon lesions present several important issues. First, the acute obstruction must be managed. Ideally, an exploration with resection of the tumor and primary anastomosis with or without a diversion is ideal. However, given the fact that the operation will be performed on unprepared bowel and the patient’s physical condition may be less than optimal, resection without an anastomosis and an end colostomy should be considered. In some instances, the obstructing lesion may present significant technical hurdles for resection in the setting of an acutely dehydrated and ill patient. In these circumstances, a decompression maneuver that can be performed rapidly and with minimal morbidity such as a transverse loop colostomy or a colostomy and mucous fistula can be performed to temporize the situation and allow the patient to be prepared and resuscitated adequately for a definitive resection at a second exploration.
Bypass operations should be reserved only for the most extreme circumstances as complications following these procedures due to repeat obstructions and leakage with abdominal sepsis are not insignificant.
Another option is to place an endoscopic stent either for temporary decompression or for definitive palliation of unresectable lesions. Multiple studies over the past 10 years have demonstrated the feasibility and safety of this maneuver in selected patients.189–194 As a bridge to surgery, stenting can provide a minimally invasive means for converting an emergency situation into an elective one, allowing time for resuscitation, bowel prep, and adjuvant therapies. In a small randomized, controlled trial from 2009, Cheung et al.195 reported additional advantages including significantly reduced rates of perioperative morbidity and stoma creation.
While short-term data seem to support the use of self-expanding metallic stents (SEMS) as a bridge to surgery, other reports as well long-term results from a recent comparative trial do not.196,197 Sabbagh et al.198 performed a head-to-head, intention-to-treat analysis of 87 patients undergoing either stenting or emergency surgery, using a propensity score to correct for selection bias. Overall survival at 3 and 5 years was significantly better in the surgery group (66% versus 44%, p = 0.015, and 62% versus 25%, p = 0.0003, respectively) and remained superior even when patients with perforation and metastatic disease were excluded (74% versus 51%, p = 0.02, and 67% versus 30%, p = 0.001, respectively). Five-year cancer-specific mortality was significantly higher in the SEMS group (48% versus 21%, p = 0.02) and there were trends toward worse 5-year DFS and increased recurrence as well as mean time to recurrence. Based on these findings, the authors have markedly changed their management of left-sided malignant obstruction, now reserving SEMS strictly for palliative indications and patients with high postoperative mortality risk.198
Laparoscopic Colon Resection
Since its introduction to the field of general surgery for gallbladder resection, the use of laparoscopic surgery has found increasing applications.199 Laparoscopic surgery has become a particularly important addition to the armamentarium of the surgical oncologist. The use of laparoscopy for the staging of the extent of disease for peritoneal malignancies, pancreatic cancer, colon cancer, and gastric cancer is now widely accepted.200–202 Laparoscopic resection has also found a niche for the removal of adrenal tumors, the spleen, and distal pancreas.203,204 The use of laparoscopic approaches for the resection of malignant lesions in the colon is now becoming more common.
With the increasing application of laparoscopic techniques to colon cancer surgery, concerns ranging from inadequacy of resection margins, inadequacy of LN sampling, and the potential seeding of port sites with malignant cells have been raised.205–207 Although these concerns are important, there are several potential advantages for laparoscopic approaches to the surgical management of colon cancer. Issues regarding length of incision, patient recovery time, and return to bowel function are often cited as justification for a laparoscopic approach. However, just as important are the technical advantages of surgery utilizing laparoscopic systems. The improved visualization due to magnification provided by video laparoscopy allows much more intricate and careful dissections in the deep pelvis, which could potentially reduce postoperative morbidity from low anterior resections that utilize a mesorectal excision technique. The ability to carefully trace vessels in the mesentery under magnification could improve the ability to perform high ligations in order to retrieve a greater number of LNs for sampling.
The technical difficulties faced during laparoscopic resection of the colon relate, in general, to the size of the specimen being removed and the need to perform an anastomosis. Each of these can be overcome through careful placement of incisions for specimen removal as well as a judicious use of stapling devices in order to perform both intracorporeal as well as a combination of intracorporeal and extracorporeal anastomotic techniques. A number of studies have examined the relative risks and benefits of the laparoscopic resection of colon cancer.205,208–210 A prospective random assignment trial conducted by Clinical Outcomes of Surgical Therapy Study Group examined both the oncologic outcomes with respect to DFS and overall survival as well as the impact of laparoscopic versus open surgery on patient recovery, pain management, and time to return of bowel function. An initial report on quality of life showed only a modest short-term benefit for laparoscopic resection versus a conventional open procedure,211 but the overall results of the trial with respect to oncologic outcomes demonstrated equivalence between the laparoscopic and open approach.212
Long-term follow-up from the corresponding UK randomized study (CLASICC Trial Group), which was similarly designed to compare laparoscopic to conventional surgery for colon and rectal cancer, and that initially reported noninferiority results in 2007, lends further support to the laparoscopic approach. With a median follow-up of 62.9 (range, 22.9 to 92.8) months, the authors found no statistically significant differences in overall survival (82.7% versus 78.3%), DFS (77% versus 89.5%), or local recurrence.213,214
Over a period of 10 years, the group from the Colon and Rectal Clinic of Orlando, Florida, performed a prospective nonrandomized study comparing laparoscopic to open resection for colorectal carcinoma. Laparoscopic resection was offered selectively in the absence of a large mass, invasion into the abdominal wall or adjacent organs, or if the patient did not have multiple prior operations.205 All laparoscopic resections were performed with curative intent, and 20% of the patients whose procedures were converted to open resection were included in the laparoscopic resection group based on an intention-to-treat model. The study measured oncologic outcomes and compared them with a computerized case-matched open resection group, using case-matching variables consisting of age, gender, site of primary tumor (colon versus rectum), and TNM stage. The group who received laparoscopic resection was followed prospectively and the data were updated on a regular basis. Follow-up of these patients consisted of a combination of office visits, telephone calls, and a review of the US Social Security Death Index Database. There were 172 patients in each group, and the groups were well matched for age, TMM stage, prior chemo- or radiation therapy, and site of the primary tumor (colon versus rectum).
Thirty-day mortality was 1.2% in the laparoscopic resection group and 2.4% in the open resection group; however, this difference was not statistically significant. The local recurrence rate of the laparoscopic group was 3.5% compared to a local recurrence rate in the open group of 2.9%. The stage-for-stage overall 5-year survival rate between the two groups was similar, and the conclusion of the authors, while acknowledging drawbacks based on the nonrandomized nature of the study, was that there was no significant difference in outcomes between using laparoscopic approaches versus an open approach in the management of primary colon and rectal tumors. There was, however, no formal cost analysis in this study, and, therefore, although oncologic outcomes were no different between the two groups, it is impossible to determine whether one group was superior to the other with respect to other outcomes.
A case-matched comparison of clinical and financial outcomes following laparoscopic and open colorectal surgery has been performed.206 The group at the Cleveland Clinic studied patients from a prospective database who had undergone laparoscopic or open colectomy and were matched for age, gender, and disease-related groupings. A group of 150 patients undergoing laparoscopic colectomy was compared to a matched group of patients undergoing open colectomy. There was no difference found between the two groups for diagnosis, complications, or 30-day readmission rate. Although operating room costs were significantly higher after laparoscopic colectomy, this was offset by a decrease in the length of hospital stay with an overall significant reduction in total costs. This is attributed mainly to a lower cost for pharmacy, laboratory, and ward nursing expenses.
The ultimate role of laparoscopic resection in the management of CRC has yet to be determined. The studies discussed have shed some light on the relative risks and benefits as well as costs of these two procedures. The questions will remain, however, if the procedures are equivalent and whether deviating from the accepted gold standard of open resection for the management of CRC will be warranted. Longer follow-up will hopefully assist in this assessment.
In the meantime, exploration of other minimally invasive approaches to resection is ongoing. Early studies report the feasibility and safety of single port surgery, or “SILC” (single-incision laparoscopic colectomy), as well as equivalent oncologic outcomes at 2 years, and robotic colectomy has also been performed although its greatest potential is thought to lie with rectal dissection.215–221 Natural orifice surgery is another area of interest with data accumulating on transvaginal specimen extraction as well as a pure transrectal approach.222–224 Whether any of these novel modalities will offer real advantages (other than cosmesis) to offset the significant drawbacks of increased technical difficulty, operating time, and cost remains to be seen.
POLYPS AND STAGE I COLON CANCER
The management of polyps and stage I colon cancer is through surgical resection. Most cancer in polyps is not diagnosed until after the polypectomy is performed. Therefore, with respect to pedunculated lesions, care should be taken to resect the stalk completely, down to its base. Invasive early stage I cancers found in a polyp managed by polypectomy do not require further resection if there is a negative margin >2 mm and the tumor is well-differentiated without lymphovascular invasion or extension of malignant cells beyond the stalk (Haggitt levels 1 to 3).173,225 Sessile lesions that are biopsied and shown to harbor an invasive cancer should be managed with a segmental colon resection. Large polypoid lesions may also require a segmental resection.
Because the stage of the lesion will not be determined until after the resection, all colon cancer lesions managed with a segmental resection should be approached the same way. The type of resection will be dictated by the location of the lesion, as has been described. Following a complete resection of a stage I lesion, no further adjuvant therapy is required. Patients managed in this way can expect a 5-year survival of over 95%.225 Those that recur are most likely improperly classified stage II or III lesions.
STAGE II AND STAGE III COLON CANCER
Adjuvant Chemotherapy Considerations
The earliest clinical trials of adjuvant chemotherapy in colon cancer were conducted in the 1950s, utilizing the limited arsenal of anticancer agents that were available at that time. Many of these agents are now known to have no meaningful activity in metastatic CRC, and thus would not be studied in the adjuvant setting today.
The adjuvant trials of the 1950s through the mid-1980s tended to be small by current standards. Based perhaps on an unrealistically optimistic expectation of what magnitude of benefit might be achieved from the use of available chemotherapies, the size of the trials did not allow evaluation of more modest clinical benefits. A large meta-analysis of controlled randomized trials of adjuvant therapy published through 1986 indicated a nonsignificant trend toward an overall survival benefit, with a mortality OR of 0.83 in favor of therapy (95% CI = 0.70 to 0.98).226 This sobering analysis suggested that substantially larger trials would be needed to detect the modest advantages that available chemotherapies might afford.
Large-Scale Randomized Trials
The large-scale 5-FU trials have been well summarized previously, and the reader who is interested in the details is referred to subject-relevant chapters in the previous edition of this book.227 The outcome of numerous trials performed largely in the 1990s can be briefly summarized as follows. Trials comparing 5-FU–based therapy to surgery only demonstrated a clear benefit in terms of 5-year DFS (essentially, an increased cure rate) for stage III patients who received chemotherapy.228,229 Six months of chemotherapy was sufficient, and no further benefit was provided by extending treatment to either nine or twelve months. Levamisole, an agent initially thought to be active, was, in fact inactive, and high-dose leucovorin did not confer superior efficacy over low-dose leucovorin, so comparisons of various 5-FU/leucovorin schedules did not demonstrate clear superiority of one schedule over the other in terms of efficacy. However, the Mayo Clinic daily times five schedule was substantially more toxic than either weekly bolus of biweekly infusion schedules. Alfa interferon conferred substantial toxicity and provided no benefit.230–234
Oral Fluoropyrimidine Therapies
Oral administration of 5-FU proved to be problematic secondary to erratic bioavailability. This was likely due in large part to variable effects of DPD, the rate-limiting enzyme in catabolism of 5-FU, on the first pass clearance of oral 5-FU by the liver. Two oral 5-FU prodrugs, capecitabine and uracil/tegafur (UFT), have demonstrated efficacy in metastatic disease that is comparable to the Mayo Clinic schedule of parenteral 5-FU/leucovorin. Both of these agents have now been studied in the adjuvant setting in comparison to the now defunct Mayo Clinic 5-FU schedule. In a study designed to assess for noninferiority in 3-year DFS, Twelves et al.235 randomly assigned 1,987 patients with resected stage III colon cancer to receive either oral capecitabine (1,004 patients) or Mayo Clinic bolus 5-FU plus leucovorin (983 patients). Each treatment was planned for 24 weeks. DFS in the capecitabine group was at least equivalent to that in the 5-FU/leucovorin group (in the intention-to-treat analysis [p <0.001] for the comparison of the upper limit of the HR with the noninferiority margin of 1.20), and capecitabine resulted in significantly fewer adverse events than Mayo Clinic bolus 5-FU/leucovorin (p <0.001). Overall, this trial demonstrates that capecitabine is a reasonable alternative to intravenous 5-FU/leucovorin in the adjuvant treatment of colon cancer in reliable, motivated patients who are able to comply with a complex schedule of oral medication. However, as discussed in the following, while possibly appropriate for some stage II patients, 5-FU/leucovorin alone is no longer the standard postsurgical adjuvant treatment for stage III colon cancer. As such, the role of single-agent capecitabine in the adjuvant management of resected colon cancer remains limited at this time. Data supporting its use with concurrent intravenous oxaliplatin are discussed subsequently.
The NSABP C-06 trial assessed the use of oral UFT plus oral leucovorin in the treatment of stage II and III colon cancer.236 A total of 1,608 patients with stage II (47%) and stage III (53%) colon cancer were randomly assigned to receive either oral UFT with leucovorin or intravenous 5-FU with leucovorin. With a median follow-up of 62.3 months, there were no significant differences in DFS or overall survival between the treatment groups. Toxicity and primary quality of life end points were similar in the two groups. As such, similar to the situation with capecitabine, the combination of oral UFT with leucovorin is an acceptable alternative to parenteral 5-FU/leucovorin; however, use of fluoropyrimidine plus leucovorin alone is no longer routine standard practice (see the following) in the adjuvant treatment of at least stage III disease. Furthermore, UFT is not commercially available in the United States.
Combination Adjuvant Therapies
Clinical trials in the metastatic setting have established the antitumor activity of combinations of agents, including irinotecan, oxaliplatin, bevacizumab, cetuximab, and panitumumab (see discussion of treatment of metastatic disease for more details). Although it had been assumed that activity in the metastatic setting would translate into an increased cure rate in the adjuvant setting, this assumption has turned out to be overly simplistic and often untrue. Of the agents listed previously, only the addition of oxaliplatin to fluoropyrimidines has resulted in benefit in the adjuvant setting.
Oxaliplatin
Oxaliplatin plus biweekly infusional 5-FU/leucovorin was first evaluated in the adjuvant setting in the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial.237 The results of this trial are summarized in Table 57.8. A total of 2,246 stage II and III patients were randomized to the LV5FU2 regimen, a biweekly infusional and bolus 5-FU/leucovorin regimen that has been demonstrated to have comparable efficacy to the Mayo Clinic daily times five bolus schedule in the adjuvant setting, or to the FOLFOX-4 regimen, which is LV5FU2 plus oxaliplatin on day 1.230 For the combined stage II and III study population, the 5-year DFS rates were 73.3% and 67.4% in the FOLFOX-4 and LV5FU2 groups, respectively (HR = 0.80; 95% CI = 0.68 to 0.93; p = 0.003).237 Six-year overall survival rates were statistically significantly improved by 2.5% (78.5% versus 76.0% in the FOLFOX-4 and LV5FU2 groups, respectively; HR = 0.84; 95% CI = 0.71 to 1.00; p = 0.046). For the stage III population, the 6-year overall survival rates were improved by 4.2% (72.9% versus 68.7%, respectively; HR = 0.80; 95% CI = 0.65 to 0.97; p = 0.023), whereas for the stage II population, the addition of oxaliplatin conferred no survival benefit (6-year survival 85.0% and 83.3%, respectively; p = 0.65). A more recent update showed that even amongst the stage II patients with high-risk factors, an improved outcome with the addition of oxaliplatin was not evident. While toxicity was regarded as manageable, the FOLFOX-4 regimen resulted in 41% grade 3 or 4 neutropenia versus 5% in the control arm, and 11% grade 3 or 4 diarrhea versus 7%. All-cause mortality in the first 60 days was 0.5% in each arm. Peripheral sensory neuropathy, a toxicity not present in the LV5FU2 control arm, was a frequent occurrence on the FOLFOX-4 arm. Grade 2 neuropathy was reported in 32% of the patients, and grade 3 occurred in 12%. In some cases, the duration of the neuropathy was substantial. One year after completion of therapy, 30% of patients still experienced some grade of neuropathy (0.8% grade 2 and 1.3% grade 3). Four years after completion of therapy, 15.4% still had some degree of neuropathy, and 0.7% still had grade 3 neuropathy. It is reasonable to assume that the toxicity still present at 4 years out from the last treatment is essentially permanent.
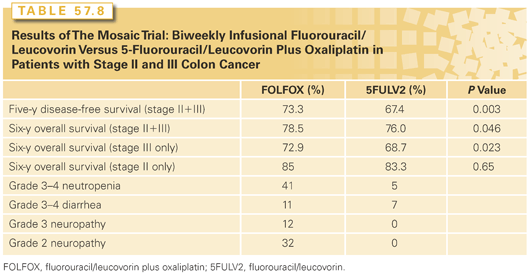
Oxaliplatin has also been combined with a weekly bolus 5-FU regimen in an adjuvant trial. The NSABP C-07 trial studied the FLOX regimen of oxaliplatin given on weeks 1, 3, and 5 plus weekly bolus 5-FU/leucovorin on weeks 1 through 6, repeated at 8-week cycles, versus the standard weekly Roswell Park regimen of 5-FU/leucovorin.238 A total of 2,409 patients were randomized to FLOX or to 5-FU/leucovorin. A total of 29% of patients had stage II disease and 71% had stage III. With a median follow-up of 8 years, FLOX showed a superior DFS, with 69.4% versus 64.2% alive and free of disease at five years (HR = 0.82; 95% CI = 0.72 to 0.93; p = 0.002). However, the overall survival difference was not statistically significantly different between the two arms. Treatment-related deaths were 1.3% versus 1.1% in the FLOX and 5-FU/leucovorin arms, respectively. Grade 2 or higher neurotoxicity was reported in 30.4% of patients on the FLOX arm versus 3.6% on 5-FU/leucovorin. Grade 3 diarrhea was 38.1% and 32.4% in the two arms, respectively, reflecting the higher incidence of serious diarrhea with the weekly bolus 5-FU regimen. Kidwell et al.239 published long-term data regarding the persistent neurotoxic side effects of oxaliplatin beyond 4 years from this same NSABP C-07 trial, and found that there was a statistically but not clinically significant increase in total neurotoxicity for those who received the agent, with initial differences between the two groups dissipating by 7 years. However specific symptoms of numbness and tingling in the hands and feet did remain substantially elevated over time.239
Another recent study examined the effect of diabetes and other comorbidities on oxaliplatin-induced neuropathy. With symptoms identified in 65% of patients, hypertension, smoking, and diabetes were associated with higher trends although not statistically significant differences in severe neuropathy. Additionally, patients with diabetes developed oxaliplatin-induced neuropathy at a significantly lower cumulative dose, highlighting the importance of tailoring patient-specific regimens to minimize toxicity.240
In an exploratory analysis, however, the authors noted significant age-related differences in response to oxaliplatin, finding statistically improved overall survival in patients <70 years old, whereas older patients actually fared worse with increased grade 4-5 toxicity (OR = 1.59) and a 4.7% decrease in 5-year overall survival.
This age-treatment interaction has also been supported by a 2012 pooled analysis of 5,489 patients >75 years old from four large data sets that demonstrated minimal benefit of oxaliplatin in this group.241 Moreover, post hoc analysis of MOSAIC data as well as revised findings from the ACCENT study (which initially showed no age-related difference) further indicate the limited clinical utility of this agent in older patients.241,242 Taking all this into account, the 2013 National Comprehensive Cancer Network (NCCN) guidelines now recommend individualizing the decision to add oxaliplatin to adjuvant regimens in the elderly.243
More recently, a 1,866-patient study comparing capecitabine plus oxaliplatin (Cape/Ox) with bolus 5-FU/leucovorin in the adjuvant treatment of stage III colon cancer has been reported.244 The Cape/Ox regimen had a statistically significant DFS advantage over 5-FU/leucovorin, with 66.1% of patients alive and disease-free at 5 years with Cape/Ox versus 59.8% with 5-FU/leucovorin. The difference between arms in overall survival at 5 years favored the Cape/Ox arm by 3.4%; however, this difference did not reach statistical significance at the time of this analysis (p
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree