FIGO recommendations for staging of disease associated with positive lymph nodes are somewhat ambiguous.2,31 Although clinical staging is recommended, with rules similar to those used for cervical cancer (e.g., disallowing use of imaging or surgical information about lymph node involvement in assignment of stage), the most recent FIGO manual quoted stage groupings based on nodal involvement. A more specific method of nodal evaluation is elaborated in the American Joint Committee on Cancer staging manual,31 which suggests options for pathologic or clinical TNM (tumor, node, metastases) staging. According to the American Joint Committee on Cancer, results of biopsy or fine needle aspiration of lymph nodes may be included in the clinical staging31; FIGO states that such results can be used for treatment planning only.2 Although the FIGO system could be interpreted to assign patients who have clinically evident inguinal involvement to stage IV, patients with inguinal metastases are sometimes cured with locoregional treatment; Kucera and Vavra30 reported uncorrected 5-year survival rates of 29% for patients with clinically suspicious inguinal nodes and 44% for patients with clinically negative groins.
Prognostic Factors
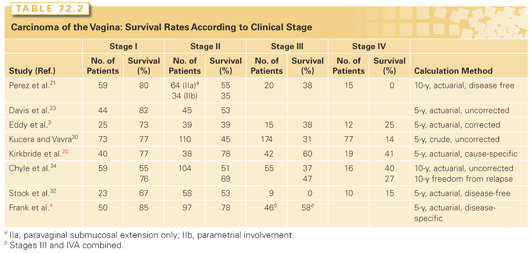
The rates of local control, distant metastasis, and survival in vaginal carcinoma are all correlated strongly with FIGO stage (Table 72.2).4,20,21,30 Tumor size also is an important predictor of outcome.4,20,21,32
Most investigators have been unable to find a correlation between tumor site and outcome.20,21,33 However, tumors that involve the entire vagina tend to be associated with a poorer prognosis, probably reflecting the larger size of these lesions. Exophytic tumors may be associated with a better prognosis than infiltrating or necrotic lesions.21
Frank et al.25 reported significantly poorer survival and pelvic disease control rates for patients with non–DES-related adenocarcinomas than for patients with squamous cell carcinomas; at 5 years, the overall survival and pelvic disease control rates were 34% and 39%, respectively, for patients with adenocarcinomas versus 58% and 81%, respectively, for patients with squamous carcinomas of the vagina. Although other investigators20,34 found no difference in outcome between patients with squamous carcinoma and those with adenocarcinoma, this may reflect inclusion of DES-related clear cell carcinomas, which appear to be associated with a better prognosis than other adenocarcinomas.15
Treatment
Technical aspects of the treatment of vaginal cancer are highly specialized and vary widely according to the site, size, and distribution of tumor within the vagina and adjacent structures. To achieve the best results for patients with these rare cancers, treatment should be delivered at a center that has a strong multidisciplinary team, including a radiation oncologist well versed in the specialized brachytherapy and external beam techniques used to treat these cancers.
Vaginal Intraepithelial Neoplasia
Patients with only HPV infection, or VAIN 1, do not require treatment. These lesions often regress spontaneously, are frequently multifocal, and recur quickly after attempts at ablative therapy. VAIN 2 may be treated with observation or topical estrogen. The malignant potential of VAIN 1–2 is uncertain. However, VAIN 3 may progress to an invasive lesion. Thus, when VAIN 3 is found, a careful evaluation should be done to rule out the presence of occult invasive disease.
VAIN 3 lesions that have been adequately sampled to rule out invasion can be treated with laser ablation. Cryosurgery should not be used in the vagina because the depth of injury cannot be controlled and inadvertent injury to the bladder or rectum may occur. Superficial fulguration with electrosurgical ball cautery may be used under careful colposcopic control. Local excision is an excellent method of treatment for small upper vaginal lesions. Intravaginal 5-fluorouracil (5-FU) has been used to treat patients who have persistent disease after resection.
Most authors report that about 5% to 10% of patients who undergo excision of VAIN develop subsequent invasive cancers,35,36 and Hoffman et al.5 reported finding occult invasive disease in upper vaginectomy specimens from 9 of 32 patients (28%) who had surgery for VAIN 3. These risks are sufficient to warrant close follow-up of patients treated for VAIN.
VAIN can also be treated effectively with intracavitary brachytherapy,20,21,34 but this treatment is usually reserved for patients with multifocal, multiply recurrent disease or high operative risk. Although most experiences have been with low-dose-rate (LDR) brachytherapy, MacLeod et al.37 reported a high control rate without major complications using high-dose-rate (HDR) intracavitary brachytherapy; the vaginal surface was treated with a total dose of 34 to 45 Gy in 4 to 10 fractions. In contrast, Ogino et al.38 reported adhesive vaginitis and rectal bleeding in two patients treated to the entire vagina with a less conservative HDR fractionation schedule.
Stage I Disease
Radiotherapy is often the treatment of choice for stage I disease because if surgery is used, total vaginectomy or even exenteration may be needed to obtain satisfactory resection margins. However, surgery has a definite role in selected cases.23,32 Early tumors that involve the upper posterior vagina can be removed with a radical hysterectomy and partial (proximal) vaginectomy if the uterus is intact or with a radical upper (proximal) vaginectomy if the patient has previously undergone hysterectomy; in both situations, bilateral pelvic lymphadenectomy is also performed. For patients with a prior history of pelvic irradiation, radical surgery (usually pelvic exenteration) is indicated and is often curative.
Disease-specific survival rates for patients with stage I disease treated with definitive irradiation range from 75% to 95%.4,21,30,39 Although some authors have suggested that selected patients with small, very superficial tumors may be treated with brachytherapy alone,21 others have noted unacceptable rates of paravaginal recurrence after treatment with brachytherapy alone and suggest that external beam irradiation should be used to treat at least the distal pelvis.4 Thicker stage I tumors always should be treated with a combination of external beam irradiation and brachytherapy with an aim to deliver 40 to 50 Gy to the pelvic nodes and 70 to 75 Gy to the tumor.
Stage II Disease
Because investigators rarely define their criteria for distinguishing stage I from stage II vaginal carcinoma or for selecting patients for various treatments, different institutional experiences cannot easily be compared. Disease-specific survival rates for patients with stage II disease range from 50% to 80%. To control possible regional disease, patients with stage II disease should receive 40 to 50 Gy to the whole pelvis delivered using conventional fields or intensity-modulated radiotherapy (IMRT). This should be followed by additional irradiation of sites of initial gross disease.4 In most cases, brachytherapy is used to supplement the dose to the primary vaginal tumor site. Perez et al.21 achieved pelvic tumor control in only 4 of 11 patients (36%) with stage II tumors treated with brachytherapy alone, compared with 54 of 81 patients (67%) treated with a combination of external beam irradiation and brachytherapy.21
Brachytherapy should be tailored to the volume and distribution of the tumor and its response to external beam irradiation. For apical tumors that flatten to <5 mm in thickness, the dose to the vagina may be boosted using intracavitary sources in a vaginal cylinder, although interstitial brachytherapy or conformal external beam techniques may still be useful in selected cases. Examination under anesthesia, transvaginal sonography, or MRI may be helpful in evaluation of disease extent for treatment planning. Larger tumors usually require a boost with interstitial brachytherapy or with additional external beam irradiation (taking into account the influence of internal organ motion on external beam radiation doses). Most authors emphasize the importance of brachytherapy in the treatment of vaginal cancer.21,39 However, brachytherapy must be designed to treat the entire vaginal tumor. Frank et al.4 argue that tumors that cannot be covered adequately with brachytherapy can often be cured with external beam irradiation alone using carefully designed conformal fields.
Selected patients with stage II disease may be cured with radical surgery.32 However, total radical vaginectomy or pelvic exenteration is often required to remove the tumor, and results with radical surgery do not appear to be better than those with radiotherapy alone. Primary radical surgery is usually indicated for patients who have previously had pelvic radiotherapy.
Stage III and IVA Disease
Reported 5-year disease-specific survival rates range from 30% to 60% for patients with stage III disease and from 15% to 40% for patients with stage IVA disease.4,20,21,30 Stage III and IVA tumors are usually bulky, highly infiltrative lesions involving most or all of the vagina as well as the pelvic wall, bladder, or rectum. The extent of these tumors and the proximity of critical normal tissue structures make their management a formidable technical challenge. Pelvic recurrence rates are high in many series; the risk of distant metastasis is also relatively high, although distant relapse is often accompanied by locoregional recurrence.
All patients require treatment with external beam irradiation. Most authors advocate the use of brachytherapy whenever possible. However, Frank et al.4 reported a high disease-specific survival rate (58% at 5 years) in a series of patients with stage III and IVA disease in which the majority of patients (31 of 46) were treated with external beam irradiation alone. Brachytherapy is undoubtedly an important part of disease management in some patients. However, in certain cases, interstitial brachytherapy does not provide adequate coverage of tumors that are very large and intimately associated with critical structures. In these cases, it may be appropriate to place greater emphasis on external beam treatment. Conformal radiotherapy techniques such as IMRT may help to increase the dose to tumor while limiting the dose to critical structures.
For selected patients with relatively small, mobile stage IVA cancers who are in otherwise good medical condition, pelvic exenteration with vaginal reconstruction using a gracilis myocutaneous flap or rectus abdominis myocutaneous flap may be the treatment of choice, particularly if a rectovaginal or vesicovaginal fistula is present.40 Radical radiotherapy is also curative in some cases; Frank et al.4 reported an 86% pelvic control rate in seven patients treated with radiotherapy for stage IVA disease.
Radiotherapy Technique
Pelvic external beam fields must be individualized according to the primary tumor site and potential sites of regional spread. Radiopaque markers placed at the distal edge of the tumor help to define the lower border and facilitate studies of internal organ motion. Treating the patient in an open (“frog-leg”) position can often reduce the severity of vulvar cutaneous reactions when coverage of distal lesions necessitates inclusion of the introitus in the radiation field.
When tumors involve the distal third of the vagina, pelvic fields should be designed to include at least the medial inguinal-femoral lymph nodes. When four fields are used to treat the pelvis, care must be taken to cover all the draining lymph nodes. Lateral fields should adequately cover posterior perirectal nodes, particularly when the primary lesion involves the posterior vaginal wall.
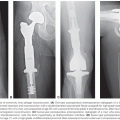
Intracavitary brachytherapy is of limited value in the treatment of locally advanced vaginal cancer because the dose falls off very rapidly from the surface of a vaginal cylinder. In general, the dose at a 5-mm depth is only 60% to 70% of the dose at the vaginal surface. Interstitial brachytherapy can provide better coverage of thick vaginal tumors. Vaginal implants can be inserted freehand. Successful use of this technique requires experience, but direct palpation during needle insertion permits excellent control of the position of sources with respect to the vaginal surface and rectal mucosa (Fig. 72.1). Vaginal implants may also be positioned using a perineal template. This technique provides a more homogeneous dose distribution because it facilitates parallel positioning of sources, but the template interferes somewhat with the ability of the brachytherapist to monitor the placement of needles with respect to the rectal and vaginal mucosa. When tumors involve the vaginal apex in patients who have had a hysterectomy, laparoscopic or laparotomy guidance may be needed to ensure accurate needle placement.
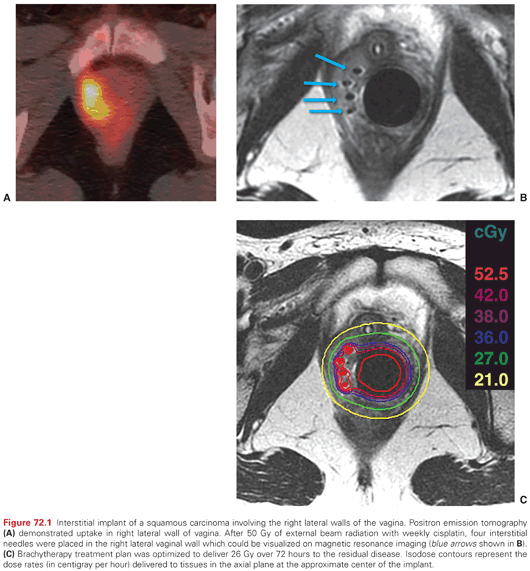
Interstitial needles can be placed transperineally while monitoring the position of the needles by direct palpation with fingers in the vagina and rectum. A plastic cylinder in the vagina can be used to displace uninvolved tissues away from the needles, which are loaded with iridium-192 (192Ir) sources. MRI after placement of MRI-compatible applicators allows visualization of residual tumor and can be used to shape the dose distribution.
Efforts to correlate radiation dose with tumor control have yielded inconsistent results and may be misleading because the total dose of radiation prescribed for a vaginal tumor is often influenced by the tumor’s size, extent, and initial response to irradiation, all of which determine the feasibility of delivering high-dose brachytherapy.4,21,39 When good brachytherapy coverage of the tumor can be accomplished, an effort should be made to treat the tumor to a dose of 75 to 85 Gy. When brachytherapy is not possible, some patients may be cured with external beam irradiation alone using shrinking pelvic fields or IMRT to deliver a tumor dose of 60 to 70 Gy. Treatment can usually be completed in <6 to 7 weeks and should not be protracted unnecessarily. Lee et al.41 reported a significantly lower pelvic recurrence rate in patients whose entire treatment course was completed in ≤9 weeks.
Complications of Radiotherapy
The close proximity of the bladder and rectum to the vagina makes them vulnerable to damage when invasive vaginal cancers are treated with radiotherapy. In their review of 193 patients treated with definitive irradiation, Frank et al.4 reported a 10% actuarial incidence of serious complications at 5 years. The most frequent complications were proctitis, hemorrhagic cystitis, and fistulae. Complication rates were significantly correlated with FIGO stage and with a history of smoking; major complications rates were 4%, 9%, and 21% for patients with stage I, II, or III–IVA disease, respectively. Other authors have reported similar major complication rates.20,21,30 There have been no comprehensive studies of vaginal function in women with vaginal cancer treated with radiotherapy, although some degree of vaginal stenosis or shortening is common.20 The severity of vaginal morbidity is probably related to the damage to vaginal mucosa and submucosa from tumor infiltration, ulceration, and infection; the age and menopausal status of the patient; and the radiation dose and the amount of vaginal tissue treated to high doses.
Role of Chemotherapy
Because primary vaginal carcinomas are rare, few reports have specifically addressed the role of chemotherapy in the treatment of this disease. Chemotherapeutic management is usually based on extrapolations from experience with the treatment of carcinomas of the cervix. For this reason, patients who have metastatic or recurrent vaginal carcinoma that is no longer amenable to local treatment are sometimes treated with cisplatin-based chemotherapy, even though the efficacy of this treatment is not well documented in the literature. Thigpen et al.42 noted several responses among 16 patients with vaginal cancers treated with cisplatin (50 mg/m2 every 3 weeks).
Because vaginal carcinoma resembles cervical carcinoma in its location, pattern of spread, histologic appearance, relationship to HPV infection, and response to radiotherapy, it may be reasonable to extrapolate from randomized trials demonstrating a benefit from concurrent chemoradiation in patients with locally advanced cervical cancer to justify a similar approach in selected patients with high-risk invasive vaginal cancer.43,44 A retrospective analysis of 71 patients treated with or without chemotherapy for vaginal cancer found that chemotherapy delivery concurrent with radiation was independently predictor of survival.45
Epidemiology
Invasive vulvar carcinoma is a rare disease that accounts for about 4% of gynecologic cancers.46,47 The American Cancer Society estimated that in the United States in 2014, 4,850 new cases of invasive vulvar cancer will be diagnosed and there will be 1,030 deaths due to vulvar cancer.1 The median age at diagnosis of patients with invasive vulvar cancer is about 65 to 70 years. In contrast, vulvar intraepithelial neoplasia (VIN) tends to occur in younger women; the median age at diagnosis of women with VIN is 45 to 50 years. The incidence of VIN has more than doubled since the early 1970s.46,47 This increase has been particularly marked in women younger than 55 years. In the past, the incidence of invasive vulvar cancer was thought to be stable; however, recent data suggest that the median age of women presenting with invasive vulvar cancer may be decreasing, probably reflecting an increase in HPV-related vulvar cancers in young women.48 This change has also been associated with an increase in the proportion of vulvar cancers involving the periurethral and clitoral region rather than the labia.48
Only 30% to 50% of invasive vulvar carcinomas are associated with evidence of HPV infection.49,50 However, 80% to 90% of VIN lesions contain HPV-16 or other HPV types. On the basis of these statistics, it has been estimated that HPV vaccination could prevent about half of the vulvar carcinomas in young women and about two-thirds of the VIN lesions.
HPV-positive vulvar cancers are usually basaloid or warty carcinomas with little keratin formation, are often associated with VIN, are frequently multifocal, and tend to occur in younger women (35 to 55 years).48,49,51 Patients with HPV-positive tumors are also more likely to have CIN and to have risk factors typically associated with cervical cancer.51 In contrast, HPV-negative tumors usually occur in older women (55 to 85 years), are often associated with vulvar inflammation or lichen sclerosis (but rarely with VIN), are generally unifocal, and are usually well differentiated with exuberant keratin formation.52,53 Although a number of investigators have reported this distinct grouping of patients with vulvar cancer, others have found greater overlap.54
Several investigators have reported a high incidence of TP53 mutations in HPV-negative tumors.55,56 Lee et al.55 found missense mutations of TP53 in 4 of 9 (44%) HPV-negative tumors but in only 1 of 12 (8%) HPV-positive tumors. They postulated that alteration in p53 activity, either through point mutations or through E6-mediated loss of p53 function in HPV-infected cells, could be important in the development of vulvar neoplasms.
Natural History and Pattern of Spread
The vulva includes the mons pubis, labia majora, labia minora, clitoris, vestibular bulb, vestibular glands (including Bartholin glands), and vestibule of the vagina. The region between the posterior commissure of the labia and the anus is termed the gynecologic perineum. About 70% of vulvar squamous carcinomas involve the labia majora or minora, most frequently the labia majora. Vulvar tumors may extend locally to invade adjacent structures, including the vagina, urethra, and anus; advanced vulvar tumors may invade adjacent pelvic bones.
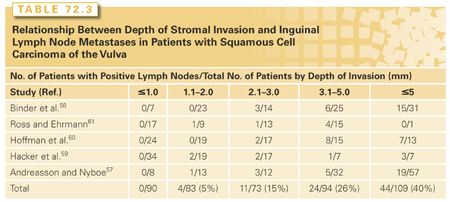
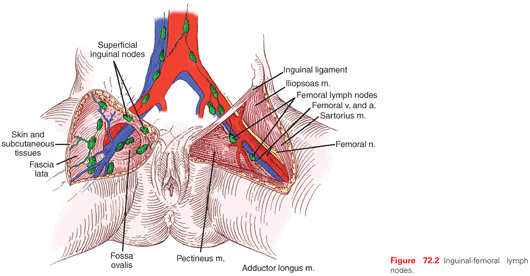
A rich network of anastomosing lymphatics that frequently cross the midline drains the vulva. Even minimally invasive vulvar tumors may spread to regional lymph nodes (Table 72.3).57–61 For most lesions, initial regional metastasis is to the inguinal lymph nodes that are superficial to Camper’s fascia; tumors may then metastasize secondarily to deeper femoral lymph nodes and to the pelvic lymph nodes (Fig. 72.2). However, some lesions, particularly those involving the clitoris and other medial structures, metastasize directly to medial femoral lymph nodes that lie in the region of the fossa ovalis, a gap in the cribriform fascia.62 Theoretically, tumors involving the clitoris can spread directly to the obturator nodes through lymphatics that follow the dorsal vein of the clitoris, although evidence of this route is rarely seen in practice. Despite the extensive anastomosis of lymphatics in the region, metastasis of vulvar carcinoma to contralateral lymph nodes is uncommon in patients with well-lateralized T1 lesions.The lungs are the most common sites of hematogenous metastasis.
Pathology
As classified by the International Society for the Study of Vulvar Disease, nonneoplastic epithelial disorders of the vulva (previously termed vulvar dystrophies) include lichen sclerosis, squamous hyperplasia, and other dermatoses.63 About 10% of these lesions have cellular atypia and are termed vulvar intraepithelial neoplasia. Histologically, VIN is characterized by disruption of the normal epithelial architecture, varying degrees of cytoplasmic and nuclear maturation, and giant cells with abnormal nuclei.49 VIN lesions are assigned a grade from 1 to 3 according to their degree of maturation. The most common VINs contain nuclear atypia throughout the epithelial layers and are frequently associated with HPV.49 A second subset of VINs have atypia that is largely confined to the basal layers of the epithelium. These lesions tend to occur in older women and are not usually associated with HPV but are commonly adjacent to areas of lichen sclerosis or hyperplasia. Buscema and Woodruff64 estimated that approximately 4% of patients treated for VIN develop a subsequent invasive cancer.
Paget disease of the vulva, a rare intraepithelial lesion located in the epidermis and skin adnexa, accounts for 1% to 5% of vulvar neoplasms. Histologically, vulvar Paget disease is characterized by large, pale, mucopolysaccharide-rich cells that are positive for periodic acid-Schiff. The lesions are usually negative for HPV.65 Electron microscopic studies have suggested that Paget cells derive from apocrine cells in the stratum germinativum of the epidermis.66 Paget disease usually occurs in postmenopausal women, who often present with symptoms of vulvar pruritus and discomfort.67 Grossly, Paget lesions appear eczematoid or, when extensive, may be raised and velvety with persistent weeping. About 5% to 10% of newly diagnosed Paget lesions are associated with underlying adenocarcinoma arising locally in a vulvar vestibular gland or skin appendage or from a distant site such as the breast or rectum.67
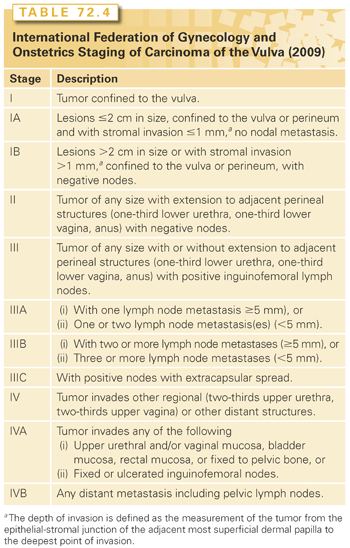
The term microinvasive carcinoma of the vulva should be used with caution. Stromal invasion by vulvar carcinomas is not measured in a uniform manner, and strict criteria for the diagnosis of microinvasive vulvar cancer have not been defined. VIN is not routinely seen adjacent to invasive vulvar cancer, and the transition from normal tissue to invasive cancer can be abrupt. Elongated rete pegs may extend 6 mm or more from the basement membrane and are sometimes misconstrued as invasive cancer. The International Society of Gynecologic Pathologists recommends that the depth of stromal invasion be measured vertically from the most superficial basement membrane to the deepest extent of tumor. Tumor thickness is defined as the distance between the granular layer of the epidermis and the deepest extent of tumor. Lymph node metastases from tumors <1 mm in depth or thickness are extremely rare (see Table 72.3). For this reason, FIGO now includes in its vulvar carcinoma staging system a stage IA subcategory for tumors that invade no more than 1 mm (Table 72.4).68 However, the risk of inguinal lymph node metastasis rises steeply as the depth of invasion exceeds 1 mm.
More than 90% of invasive vulvar cancers are squamous cell carcinomas. Atypical keratinization is the hallmark of invasive vulvar cancer. Most squamous cell carcinomas are well differentiated, but mitoses may be noted. About 5% of vulvar cancers are anaplastic carcinomas, which may consist of large immature cells, spindle sarcomatoid cells, or small cells. Vulvar carcinomas consisting of small cells may resemble small cell anaplastic carcinomas of the lung or Merkel cell tumors, and have demonstrated an aggressive biologic behavior in the few reported cases.69
The diagnosis of Bartholin gland carcinoma is based on clinical findings of a tumor arising in the anatomic location of Bartholin glands and on the histologic appearance. Biopsy of a tumor arising from a Bartholin gland usually reveals squamous cell carcinoma, but adenocarcinoma, transitional cell carcinomas (arising from the duct and histologically indistinguishable from transitional cell carcinoma of the bladder), and adenoid cystic carcinomas have also been reported.
Rare cases of primary mammary adenocarcinoma of the vulva have been reported, presumably arising in aberrant mammary tissue occurring along the embryonic milk line.70 Other rare carcinomas that may occur in the vulva include basal cell carcinomas,71 verrucous carcinomas,72 and sebaceous carcinomas.73
Malignant melanomas of the vulva account for approximately 2% to 4% of primary vulvar malignancies and 1% to 3% of melanomas arising in women.74 Vulvar melanoma occurs most frequently in women older than 60 years of age, but 10% to 20% of vulvar melanomas occur in women younger than 40 years. In a large Swedish series,75 57% of vulvar melanomas were of the mucosal lentiginous type, 22% were nodular, and 16% were superficial spreading or lentiginous. Most investigators have reported a correlation between higher depth of invasion or Breslow thickness and poorer outcome.75,76 However, because the vulvar epithelium sometimes lacks a well-developed papillary dermis, which makes it difficult to assign Clark’s levels of invasion, a modification of the Clark system is often used to categorize patients with vulvar melanoma.77 Other factors that have been associated with a poorer prognosis are ulceration, clinical amelanosis, and older age.75
Vulvar sarcomas constitute 1% to 2% of vulvar malignancies and include leiomyosarcomas, rhabdomyosarcomas, angiosarcomas, neurofibrosarcomas, and epithelioid sarcomas. The prognosis appears to depend on three main determinants: lesion size, tumor contour, and mitotic activity. Lesions >5 cm in diameter with infiltrating margins, extensive necrosis, and more than five mitotic figures per 10 high-power fields are the most likely to recur after surgical resection.78,79
Diagnosis, Clinical Evaluation, and Staging
Patients with VIN may complain of vulvar pruritus, irritation, or a mass, but many are asymptomatic at the time of diagnosis. Patients with invasive vulvar cancer usually complain of a vulvar mass and chronic vulvar pruritus. Advanced lesions may bleed and are often exquisitely tender.
Because VIN can have many manifestations, any new vulvar lesion should be biopsied. Once a diagnosis of high-grade VIN has been established, the entire vulva, cervix, and vagina should be carefully examined because patients often have multifocal or multicentric involvement.80 Colposcopic examination may help to define the extent of disease.
Diagnosis of invasive vulvar lesions requires a wedge biopsy of the lesion with surrounding skin and with underlying dermis and connective tissue so that the pathologist can adequately evaluate the depth of stromal invasion. This procedure can usually be performed in the physician’s office under local anesthesia. Excisional biopsy is preferred for lesions <1 cm in diameter.
All patients with invasive disease require a careful physical examination including a detailed pelvic examination, chest radiography, and a biochemical profile. Cystoscopy and proctoscopy should be performed in patients with tumors that are near the urethra or anus, respectively. CT, MRI, and FDG-PET/CT scans can be obtained to evaluate deep inguinal and pelvic lymph nodes and possible local extension of disease to adjacent structures. FDG-PET/CT has relatively poor sensitivity but high specificity in the prediction of lymph node metastases.81
The correlation between clinical assessment of the inguinal lymph nodes and pathologic findings is poor.82 Homesley et al.82 reported that 24% of patients with clinically negative nodes had inguinal lymph node metastases and 24% of patients with suspicious but mobile nodes had negative findings at lymphadenectomy. For this reason, in 1988, the FIGO staging system was changed from a clinical staging system to one that incorporates the more accurate information gained from surgical assessment of regional lymph nodes; a subsequent amendment provided a definition of minimally invasive vulvar cancer.83
Studies also suggest that, when corrected for the number of involved lymph nodes, lymph node bilaterality and local factors such as tumor size and early involvement of adjacent structures have little impact on survival.84,85 However, extracapsular nodal extension was found to be a powerful prognostic indicator.86,87 In 2009, to incorporate these findings and to improve the prognostic accuracy of the FIGO staging system, another major revision was implemented.84 In this revision, the role of tumor diameter was diminished; distal urethral, vaginal, and anal involvement were removed as indications for upstaging; and stage III was subdivided to include more detailed information about the number of positive lymph nodes and the presence of extracapsular nodal extension (see Table 72.4).68
Prognostic Factors
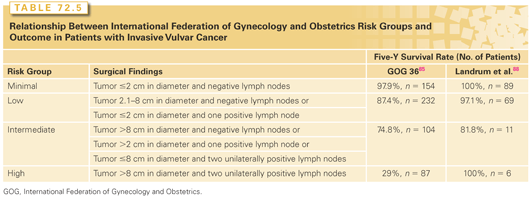
Our understanding of the importance of various prognostic indicators in vulvar carcinoma has shifted with the increased use of adjuvant radiotherapy and the accumulation of outcome data. In a 1991 retrospective review of 586 patients entered in Gynecologic Oncology Group (GOG) trials between 1977 and 1984, the presence and number of lymph nodes and tumor diameter were the only independent predictors of survival.85 Five-year survival rates were 91% for patients with negative inguinal lymph nodes and 75%, 36%, and 24%, respectively, for patients with one or two, three or four, or five or six positive nodes. Using these data, the authors suggested a classification system that categorized patients according to tumor size and number of involved lymph nodes (Table 72.5). Although treatment details were not given, postoperative radiotherapy was not standard in the years of this study, and it is likely that most patients did not receive radiotherapy; more recent reviews suggest that modern adjuvant radiotherapy may reduce the influence of tumor size and number of positive nodes on outcome (see Table 72.5).68 Nevertheless, the GOG risk classification was a major influence on recent modifications of the FIGO staging system. In addition to the number of lymph nodes, the presence of extracapsular extension has been found to be an important predictor of outcome.88,89 However, in a review of patients treated with radiation after lymphadenectomy, Katz et al.90 found no correlation between extracapsular extension and inguinal node recurrence if the dose of radiation was ≥56 Gy.
The presence of pelvic lymph node metastases is generally considered to be a predictor of very poor prognosis.91 However, this impression comes largely from decades-old studies of outcome in patients who had pelvic lymph node dissection without adjuvant radiotherapy. The generalizability of these data to current practice, in which most patients who have nodal involvement receive radiotherapy, is uncertain.
Other factors that have consistently been correlated with lymph node metastasis and outcome include depth of invasion, tumor thickness, and the presence or absence of lymph-vascular space invasion (LVSI).82,85,92 More than 75% of patients with LVSI have positive inguinal nodes.82 Studies of the relationship between tumor grade and outcome have supported various conclusions, possibly reflecting the inconsistent criteria used to grade vulvar tumors.82,89,93 Other factors that have been associated with poorer prognosis include high mitotic rate, aneuploidy, an infiltrative growth pattern, and a basaloid histologic pattern.93–95 Several authors have reported that tumors containing HPV DNA have a poorer prognosis than HPV-negative tumors.53,96 Some investigators have reported a worse prognosis for patients age 70 years or older, whereas others have found no correlation between prognosis and age.82,93
Studying the relationship between surgical margins and tumor recurrence, Heaps et al.92 reported no local failures in 91 patients whose narrowest tumor margin (deep or at the skin surface) was ≥8 mm in the fixed specimen. A total of 10 of 23 patients (43%) with margins of ≤4.8 mm experienced a local recurrence, as did 8 of 13 patients (62%) with margins between 4.8 and 8 mm. The risk of recurrence in patients with narrow margins may be diminished when postoperative radiotherapy is given.97
Treatment
During the last 30 years, the treatment of vulvar cancer has evolved away from radical en bloc surgical resection, which was standard before the 1980s, toward a multidisciplinary approach that emphasizes tissue-sparing operations and selective use of radiotherapy or chemoradiation to optimize local control, survival, and organ function.
High-Grade Vulvar Intraepithelial Neoplasia
After invasive carcinoma has been excluded by a sufficient number of excisional biopsies, the treatment of high-grade VIN (VIN 3) should be as conservative as possible. Focal lesions can be simply excised. Multiple lesions can be excised separately or, if confluent, with a larger single excision. This approach is generally well tolerated and provides material for histologic assessment. When there is more extensive high-grade VIN, the lesions can be vaporized with a CO2 laser. This method may provide an alternative to more extensive operations but does not yield a specimen for histologic inspection.
Extensive, diffuse VIN 3 may necessitate a wider excision, particularly if the lesion involves the perianal skin. These lesions are sometimes treated with a partial vulvectomy of the superficial skin (“skinning vulvectomy”). Whenever possible, the vulvar skin should be sutured primarily, but a split-thickness skin graft is sometimes needed to close the defect.
VIN 3 often recurs at or near the margins of resection, even when the histopathology analysis demonstrates that the initial lesions were completely resected. Presumably, this phenomenon reflects the multifocal nature of the condition.80 VIN 3 can also recur within the donor skin from split-thickness grafts.98
Women with HPV-16–positive high-grade VIN showed that vaccination with a mix of long peptides from the HPV-16 viral oncoproteins E6 and E7 induced clinical responses (including sustained complete responses in 47% of patients at 24 months of follow-up) and relief of symptoms.99 Complete responses were associated with induction of HPV-16–specific immunity.
Invasive Disease
The optimal treatment of invasive disease requires careful consideration of the potential benefits of various local and regional treatment options to find an overall treatment strategy that will maximize locoregional disease control with as little acute and long-term morbidity as possible.
Treatment of the Vulva. Most small lesions (approximately <4 cm) that do not involve the urethra, anus, or other adjacent structures can be controlled locally with a radical local excision. A wide and deep excision of the lesion is performed, with the incision extended down to the inferior fascia of the urogenital diaphragm. An effort should be made to remove the lesion with a 1-cm margin of normal tissue in all directions unless this would require compromise of the anus or urethra. Small lesions that invade ≤1 mm can be managed with local resection alone because the risk of regional spread is very small. Patients with more invasive tumors must also have surgical or radiation treatment of the inguinal nodes, as discussed in the next section.
Primary tumors that involve the anus, rectum, rectovaginal septum, or urethra pose a difficult problem because adequate surgical clearance can often be obtained only by sacrificing organ function. Some patients who have tumors that minimally involve the external urethra or anus can undergo initial vulvectomy without sacrifice of major organ function if close margins are accepted near critical structures. Postoperative radiotherapy can then be delivered to prevent local recurrence.100 Although local recurrences are frequently successfully controlled with additional surgery, Faul et al.101 reported an overall 5-year survival rate of only 40% after the first local recurrence and emphasized the importance of achieving local control. These authors reported a significant reduction in the local failure rate (from 58% to 16%) when tumors that were within 8 mm of the operative margins were treated with radiotherapy after surgery.101 Although some patients with more extensive organ involvement may be cured with ultraradical operations, in some cases with pelvic exenteration, the risks of acute and long-term complications of these procedures are substantial.102,103 For this reason, a number of investigators have explored the use of radiotherapy with or without surgery and chemotherapy to spare critical structures in patients with locally advanced disease.
In the 1980s, several investigators104–106 reported results of preoperative radiotherapy in small series of patients with locally advanced disease. These reports indicated that modest doses of radiation (45 to 55 Gy) produced dramatic tumor responses in some patients with locally advanced disease, permitting organ-sparing surgery without sacrifice of tumor control. Hacker et al.106 reported that four of eight patients with T3 or T4 tumors treated preoperatively with 44 to 54 Gy had no residual tumor in the vulvectomy specimen, and that seven of these eight had local control of their disease. More recently, investigators have emphasized the use of concurrent chemoradiation, as discussed later in this section.
Treatment of Regional Disease. Effective treatment of regional disease is the single most important element in the curative management of early vulvar cancer. Although patients with vulvar recurrences may have their disease successfully controlled with additional local treatment, patients who suffer inguinal recurrences are rarely curable.
All patients with primary tumors that invade >1 mm must have their inguinal lymph nodes treated. In the past, this treatment usually included a bilateral radical inguinal-femoral lymphadenectomy, which initially was combined with vulvectomy using a single incision and, more recently, was performed through separate groin incisions. At one time, pelvic lymphadenectomy was also performed in most patients with invasive vulvar cancer. When subsequent studies demonstrated that pelvic node metastases were found only in patients with positive inguinal nodes, use of the procedure was limited to patients found intraoperatively to have inguinal node metastases.
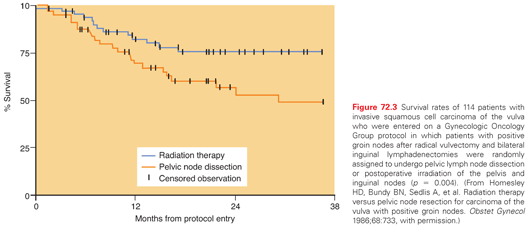
Then, in 1986, Homesley et al.91 published results of a prospective randomized study that compared pelvic lymphadenectomy with inguinal and pelvic irradiation in patients with inguinal node metastases from carcinoma of the vulva. All patients were initially treated with radical vulvectomy and inguinal-femoral lymphadenectomy. Patient randomization was done intraoperatively after frozen-section evaluation of the inguinal-femoral lymph nodes. This trial was closed prematurely, after 114 eligible patients had been entered, when interim analysis revealed a survival advantage for the radiotherapy arm (p = 0.03; Fig. 72.3). The difference was most marked for patients with clinically positive or multiple histologically positive groin nodes. The initial preliminary report was finally updated in 2009,107 confirming marked reductions in the risks of recurrence and cancer-related death in patients who had radiotherapy. There were 3 inguinal recurrences in the radiation arm versus 13 in the control arm. Although no differences were seen in the number of pelvic recurrences, competing risks and the lack of high-quality tomographic imaging in this early study may have led to underestimates of the risks of pelvic recurrence. In the updated report, the relative risk of disease progression with radiation was 39% (95% confidence interval = 0.17 to 0.88; p = 0.02); the relative risk of death was less impressive, with a hazard ratio of 0.61 (95% confidence interval = 0.3 to 1.3; p = 0.18), apparently because of a marked difference in the rate of deaths from other causes: 14 in the radiation arm versus 2 in the control arm. With the 1986 publication of this study, most practitioners abandoned routine pelvic lymphadenectomy, and postoperative radiotherapy became standard for most patients with inguinal lymph node metastases.
Although radical inguinal-femoral lymphadenectomy was historically considered the treatment of choice for regional management of invasive vulvar carcinoma, a number of groups have investigated the possibility that regional radiotherapy may be an effective and less morbid way of preventing recurrence in patients with clinically negative groins.90,108–110
In 1992, the GOG reported the results of a trial that randomly assigned patients with clinically negative inguinal nodes to receive inguinal lymph node irradiation or inguinal-femoral lymphadenectomy (followed by inguinopelvic irradiation in patients with positive lymph nodes) after resection of the primary tumor.110 The study was closed after entry of only 58 patients, when an interim analysis demonstrated a significantly higher rate of inguinal recurrence and death in the radiotherapy group. The authors concluded that lymphadenectomy was the superior treatment, although the morbidity rate of lymphadenectomy was greater than that of groin irradiation. However, the radiotherapy techniques used in this study have since been criticized. Preirradiation CT scans were not consistently obtained to verify the position and size of inguinal nodes. Patients were treated with anterior appositional fields, the dose was prescribed at a depth of 3 cm, and the use of electrons (usually 12 MeV) was emphasized. This method of treatment can lead to significant underdosage of the inguinal-femoral nodes, which frequently extend to a depth of >5 to 8 cm.111
In contrast, retrospective studies have indicated that patients who have negative inguinal nodes (by tomographic imaging) and careful radiotherapy treatment planning rarely experience a regional recurrence after inguinal-pelvic irradiation to 40 to 50 Gy.90,108,109 Katz et al.90 emphasized the importance of careful technique. They reported three recurrences in 29 patients treated with radiotherapy alone for clinically negative inguinal nodes; two of these recurrences occurred adjacent to radiation fields that had not fully encompassed the lateral inguinal nodes. It appears that, with careful radiotherapy technique, microscopic disease in the inguinal lymph nodes can be readily controlled with radiation alone. Radiation alone appears to be a reasonable treatment to prevent inguinal recurrence, particularly for patients who have clinically and radiographically negative groins but require radiation for locally advanced disease.
Some surgeons have tried to reduce the incidence and severity of surgical complications by reducing the extent of lymph node dissections. In the 1990s, several groups reported the use of a more limited “superficial” inguinal lymphadenectomy for patients with early disease; patients who had positive lymph nodes were referred for radiotherapy. Although many of the complications usually associated with radical lymphadenectomy were avoided, inguinal recurrence rates were higher than expected, ranging from 7% to 16% in patients who had negative dissections.90,112 It has been suggested that the procedure used in these studies did not remove medial inguinal-femoral nodes, which may be the primary site of drainage of some vulvar cancers113,114; for this reason, many gynecologic oncologists now recommend removal of at least the superficial and medial inguinofemoral nodes.
During the last decade, a number of investigators have explored the use of intraoperative lymphatic mapping to identify a “sentinel” node that would predict the presence or absence of regional metastases. A number of studies have evaluated the results from sentinel lymph node biopsy followed by regional lymphadenectomy. From the pooled results for 383 patients entered in 10 trials, the authors concluded that the negative predictive value of sentinel node biopsy was 99.3% and the false-negative rate was 2.4%.115 The GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS)-V116 study assessed the efficacy of sentinel lymph node evaluation alone in patients with invasive vulvar cancers <4 cm in diameter. Of 402 patients registered in this trial, 231 patients with negative sentinel nodes did not undergo lymphadenectomy; at the time of the analysis, groin recurrences had been observed in 9 (3.9%) of these 231 patients, and 7 patients (3.0%) had died. Patients with sentinel lymph node metastasis >2 mm had significantly lower disease-specific survival (69.5% versus 94.4%; p = 0.001).117 Robison et al118 reported long-term follow-up after sentinel lymph node evaluation. With a median follow-up of 58 months, only 3 of 57 patients who were observed after a negative sentinel lymph node developed an inguinal recurrence. A large GOG trial designed to estimate the sensitivity of sentinel lymph node biopsy in a community-based setting enrolled 452 women who underwent intraoperative lymphatic mapping, sentinel lymph node biopsy, and inguinal femoral lymphadenectomy. Ninety-two percent of patients had at least one sentinel node identified. The sensitivity was 91.7% and the false-negative predictive value (1-negative predictive value) was 3.7%, which was even lower in women whose tumors were <4 cm. These data suggest that sentinel lymph node biopsy is a reasonable alternative to inguinal femoral lymphadenectomy in selected women with squamous cell carcinoma of the vulva.119 On the subsequent observational (GROINSS)-VI study, patients with a positive sentinel nodes received postoperative radiation therapy without undergoing a full lymphadenectomy. The currently enrolling (GROINSS)-VII study uses the same approach for patients with <2 mm of disease in the sentinel node, but patients with >2 mm focus of disease undergo a lymphadenectomy followed by postoperative radiation.
Participants in a 2008 expert panel at an International Sentinel Node Society Meeting concluded that sentinel lymph node biopsy “is a reasonable alternative to complete inguinal lymphadenectomy when [it] is performed by a skilled multidisciplinary team in well-selected patients.”115 They concluded that patients who have tumors that invade >1 mm, no obvious metastatic disease, and a tumor diameter of <4 cm are good candidates for the procedure.
Radiotherapy Technique. Comprehensive regional radiotherapy for vulvar cancer requires adequate coverage of at least the inguinofemoral and distal pelvic lymph nodes. Patients who have extensive inguinal or pelvic disease may require larger fields that encompass the common iliac nodes. If the vulvar cancer has been excised with widely negative margins, some clinicians choose to not treat the primary site. Several techniques have been used to reduce the dose given to the femoral head and neck during treatment of the groin. One approach is to use a combination of photons and electrons; this technique requires careful image-based planning to assure that the treatment is delivering an adequate dose to the superficial and deep inguinal lymph nodes. Alternatively, IMRT can be used to spare soft tissue, bladder, bowel, and other critical structures, but this method is technically challenging and requires a sound understanding of the local and regional anatomy.120
Patients who have local risk factors for recurrence but pathologically negative lymph nodes may be treated with local radiation alone using conformal photon fields or, in selected cases, appositional electron beam techniques. Whenever photons are used to treat the vulvar surface, tissue-equivalent materials may need to be applied to ensure that the surface dose is adequate. Thermoluminescent dosimeters can be used to verify that the surface of the vulva is receiving the prescribed dose of radiation.
Chemoradiation in Locoregionally Advanced Disease. To reduce the need for morbid ultraradical surgery and to improve locoregional control rates, a number of investigators have explored combinations of chemotherapy with radiation and surgery in patients with locally advanced vulvar carcinoma.121–128 Most studies have used combinations of cisplatin, 5-FU, and mitomycin-C (Table 72.6), extrapolating from the high response rates observed with such combinations for locally advanced carcinomas of the cervix and head and neck and from studies that have demonstrated the efficacy of these drugs as radiosensitizers in the treatment of carcinomas of the anus. Although studies have usually included small numbers of patients with very advanced local or regional disease, most investigators have observed impressive responses that often appear to be better than would be expected with radiation alone. Randomized trials have not been done and may be difficult to perform because of the small number of patients with locally advanced vulvar cancer. However, results of trials in other types of cancer are encouraging: trials that demonstrated improved local control and survival when concurrent cisplatin-containing chemotherapy was added to radiation treatment of cervical cancers129,130 and improved colostomy-free survival when mitomycin-C and 5-FU were added to radiation treatment of anal cancer131 suggest that this approach may be also be useful in the treatment of women with vulvar cancer. In a single-arm phase 2 study, the GOG investigated the use of cisplatin with daily radiation therapy to a total dose of 57.6 Gy in locally advanced vulvar cancer.132 Overall, 50% of patients achieved a complete pathologic response with this regimen. The current GOG trial will utilize IMRT and deliver gemcitabine in addition to cisplatin.
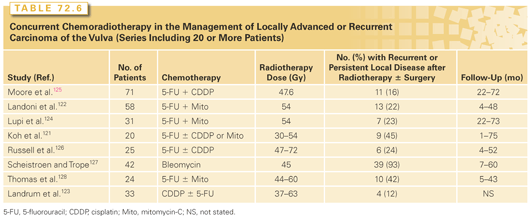
Several investigators have explored the use of neoadjuvant chemotherapy for locally advanced vulvar cancer.133,134 Although partial responses to multiagent chemotherapy have been observed, response rates appear to be lower than for cervical cancers, and survival rates have been discouraging.134
Caution is warranted in designing aggressive treatment protocols for patients with vulvar cancers, as these patients typically are elderly and often have concurrent medical problems. Serious pulmonary damage has been observed in a number of patients treated in studies that included bleomycin.127 In the largest published series of patients treated with mitomycin-C and 5-FU, hematologic tolerance was acceptable, but the administered dose of mitomycin-C was somewhat lower than that generally used in the treatment of anal cancers.128 Although chemotherapy may improve control rates, radiation alone can produce impressive responses and should be considered in patients who are poor surgical candidates and who cannot tolerate chemotherapy.
Complications of Treatment
Most of the serious acute and subacute complications of radical vulvectomy are related to the lymphadenectomy, although these risks have decreased somewhat with the use of separate groin incisions. Acute complications include wound seroma, disruption, or infection in up to 50% to 75% of cases, chronic lymphedema in 20% to 50%, and perioperative death in 2% to 5%.109,112,135,136 Other acute complications include urinary tract infection, wound cellulitis, temporary anterior thigh anesthesia from femoral nerve injury, thrombophlebitis, and, rarely, pulmonary embolus.91,137 The risk of chronic leg edema decreased from approximately 30% to 15% with the use of separate groin incisions and is rare after sentinel lymph node dissection only.116,138 Other chronic complications include genital prolapse, urinary stress incontinence, temporary weakness of the quadriceps muscle, and introital stenosis. These risks are less when radical local excision of the primary lesion is done instead of radical vulvectomy.139,140 Patients who undergo vulvectomy without inguinal lymphadenectomy have significantly shorter hospital stays and fewer complications.109,112
The most prominent acute complication of radical radiotherapy for vulvar carcinoma is radiation dermatitis. Moist desquamation is commonly seen in the final weeks of treatment but resolves within 2 to 3 weeks after completion; sitz baths and appropriate use of pain medications are helpful during the acute phase. Skin reactions that occur in the first 2 to 4 weeks of treatment are frequently due to superinfection with Candida albicans and should be treated presumptively with antifungal agents. Other acute side effects of radiation include diarrhea, dysuria, and painful defecation. Late complications result from a combination of radiation, surgery, and tissue destruction from locally advanced tumors. Introital or vaginal stenosis, tissue atrophy, and other effects of combined therapy may cause sexual dysfunction. Vulvar edema, tissue atrophy, hyperpigmentation, fibrosis, and telangiectasia may occur and are related to the dose of radiation and the volume of tissue irradiated. Combined effects of treatment may also cause bladder or rectal incontinence, urethral or anal stenosis, ulceration, or fistula.
Treatment of Metastatic Disease
Unfortunately, reports of chemotherapy activity in the treatment of metastatic or recurrent squamous cell carcinoma of the vulva are largely anecdotal. In the absence of reliable data specific to this cancer, clinicians often use single agents and combination regimens that have had some activity in the treatment of cervical cancer. However, there are, as yet, few data to indicate that chemotherapy can provide effective palliation for patients with metastatic or recurrent vulvar carcinomas that are not amendable to locoregional treatments. In terms of molecular-targeted agents, erlotinib has shown promising activity and may represent one of the most active agents for the management of squamous cell carcinoma of the vulva. Specifically, in a phase 2 study, erlotinib exhibited a 67.5% overall clinical benefit rate (i.e., 27.5% partial response and 40.0% stable disease by response evaluation criteria in solid tumors).141
Epidemiology
The American Cancer Society estimated that in the United States in 2014, 12,360 new cases of invasive cervical cancer would be diagnosed and there would be 4,020 deaths due to cervical cancer, representing approximately 1.5% of all cancer deaths in women.1 In the United States and other developed countries, age-adjusted death rates from cervical cancer have declined steadily since the 1930s. However, global cervical cancer incidence increased from 378,000 cases per year in 1980 to 528,000 new cases in 2012; during that period, the annual death rate increased by 7.5% with 266,000 deaths per year in 2012.142 The decrease in incidence in developed countries is primarily the result of the adoption of routine screening programs; however, the death rates from cervical cancer had begun to decrease before the implementation of Pap screening, suggesting that other, unknown factors may have played some role.143
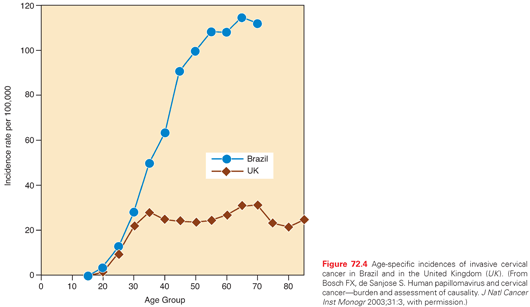
International incidences of cervical cancer tend to reflect differences in cultural attitudes toward sexual practices and differences in the penetration of mass screening programs. The highest incidences tend to occur in populations that have low screening rates combined with a high background prevalence of HPV infection.144 Rates of invasive cervical cancer are particularly high in Latin America, southern and eastern Africa, India, and Polynesia. In many of these developing countries, cervical cancer is the leading cause of cancer deaths among women. Differences in age-specific incidences between developed and medically underserved countries illustrate the probable impact of mass screening on the development of invasive disease. For example, a comparison between data from Brazil and the United Kingdom showed similar rates of cervical cancer in young women, suggesting similar levels of exposure to HPV, but rapidly diverging rates in older women, probably reflecting differences in the availability of mass screening in the two countries (Fig. 72.4).
Although the overall incidence of cervical cancer is low in the United States, the incidence in black Americans is about 30% higher than the incidence in white Americans, and the incidence in Hispanic women is about twice the incidence in white Americans.145 Barriers to cervical cancer screening, including lack of insurance, low income, and cultural factors, probably contribute to higher incidences and mortality rates in black and Hispanic women.146
Molecular and human epidemiologic studies have demonstrated a strong relationship between HPV, CIN, and invasive carcinoma of the cervix. HPV can be identified in >99% of cervical cancers, and infection with HPV is now accepted as a necessary cause of most cervical cancers.144 It appears that most of the covariables historically associated with an increased risk of cervical cancer are surrogates for sexually transmitted HPV infection. Women who have coitus at a young age, who have multiple sexual partners, have partners with multiple partners, or who bear children at a young age are at increased risk. A pooled analysis of 26 epidemiologic studies showed a strong inverse association between use of intrauterine devices and cervical cancer, perhaps due to a cellular immune response triggered by the device.147 Castellsague et al.148 have reported that circumcised males have a lower incidence of HPV infection than uncircumcised males and a correspondingly lower incidence of cervical cancer in their female partners.
A number of studies suggest that the incidence of cervical adenocarcinoma has been increasing, particularly among women in their 20s and 30s.149 In a study based on Surveillance, Epidemiology, and End Results program data, Smith et al.149 found that the age-adjusted incidence of cervical adenocarcinoma in the United States increased by 29.1% during a period (1973–1996) when the overall incidence of cervical cancer decreased by 41.9%. Several investigators have reported a correlation between cervical adenocarcinoma and prolonged oral contraceptive use.150 However, this relationship may not be causative, given the many potential confounding risk factors and possible changes in diagnostic criteria over time.149,151 Another possible explanation for the increase in incidence of cervical adenocarcinoma is that cytologic screening methods may be less effective in detecting preinvasive adenocarcinomas than they are in detecting preinvasive squamous lesions, resulting in a less dramatic reduction in the incidence of invasive adenocarcinomas.
In 1993, the Centers for Disease Control and Prevention added cervical cancer to the list of AIDS-defining neoplasms.152 However, the relationship between immunosuppression (particularly HIV-related immunosuppression) and the risk of HPV-related disease is complex and incompletely understood.153,154 Current data strongly suggest that women infected with HIV have an increased incidence and are more likely to have persistence of cervical HPV infection, even when studies are corrected for confounding risk factors; women infected with HIV also tend to have a faster rate of progression to high-grade CIN.153,154 Iatrogenic immunosuppression in organ transplant recipients is also associated with an increased prevalence of CIN.155 Although less definitive, evidence linking HIV infection with invasive cervical cancer has also been increasing.154 Some investigators156 have suggested that cervical cancer is a more aggressive disease in immunosuppressed patients, but other studies have failed to reveal an independent linkage.153,154 In most cases, antiretroviral therapy does not appear to affect HPV levels, nor does it appear to decrease the risks of high-grade squamous intraepithelial lesions (HSILs) or invasive cancer.154 Because of the increased risk of HPV infection in women infected with HIV, vigilant surveillance with Pap smears, pelvic examinations, and colposcopy (when indicated) should be part of the routine care of these women.
Human Papillomavirus
HPV is associated with nearly all cases of cervical cancer. Examination of tumor DNA from cervical cancers reveals integration of one the high-risk HPV subtypes, including 16, 18, 31, 33, and 45. The most common subtypes in human cancers are HPV-16 and -18, which are found in 70% of cases. Although cervical cancer is relatively rare in the United States, HPV is highly prevalent in the US population. Vaginal swab testing demonstrated that 26% of US women and 44% of women between 20 and 24 testing positive for HPV.157 However, in only a small percentage of women does HPV infection lead to the development of premalignant or malignant lesions. This suggests that viral infection is essential, but not sufficient, for the development of high-grade dysplasia or invasive cancers.
In the initial infection, viral DNA remains episomal in the basal cell of the epidermis. Viral DNA integration occurs preferentially into areas of genomic instatibility, such as fragile sites, and oncogenesis does not appear to require disruption of any critical tumor suppressor genes. Rather, expression of viral genes, E6 and E7, disrupts the function of critical tumor suppressor genes, p53 and pRB, respectively, leading to enhanced cell cycle proliferation, impaired apoptosis, and loss of genome maintenance leading to increased genomic instability.
The strong correlation between high-risk HPV types and cervical carcinoma has led to the development of prophylactic HPV vaccines that have proven highly effective in randomized trials.158 In response to these studies, the US Food and Drug Administration approved a prophylactic HPV vaccine for women between the ages of 9 and 26 years in 2006. Currently, two vaccines, Guardasil (Merck & Co, White House Station, NJ) and Cervarix (GlaxoSmithKline, Brentford, UK), are available. Both vaccines target HPV subtypes 16 and 18, which account for 70% of cervical cancers. Guardasil also includes antigen from HPV subtypes 6 and 11, which are associated with 90% of cases of benign genital warts. Both of these vaccines deliver viral-like particles composed of the L1 capsid protein that has assembled into the highly immunogenic viral-like particle form. The vaccines are DNA-free and thus carry no risk of infection.
Both vaccines are highly effective at generating a robust and durable immune response, including the production of neutralizing antibodies to the HPV viral capsid protein.159 The effect appears to be highly durable, lasting at least 5 years and likely significantly longer.159 The efficacy of these vaccines has been tested in large prospective randomized trials: FUTURE I160 and FUTURE II161,162 for Guardasil, and PATRICIA and the Costa Rica HPV vaccine trial160,163 for Cervarix. FUTURE II enrolled 12,167 women between the ages of 15 and 26 years who received three doses of either HPV-6/-11/-16/-18 vaccine or placebo. The study was designed to determine if the vaccine reduced the rate of malignant or premalignancy cervical lesions. In women with no previous infection, the vaccine eliminated 98% of CIN grade 2 or 3, adenocarcinoma in situ, or cervical cancer related to HPV-16 or -18. Among all women, including those with previous infection with HPV-16 and -18, the vaccine efficacy was significantly lower, just 44%. Other studies have confirmed that the HPV vaccine does not appear to speed clearance of the HPV virus for women who have previous exposure to HPV.164 As a result, the vaccine is recommended for girls aged 11 to 12 years, with the goal of vaccinating prior to exposure to HPV. Analysis of the Costa Rica HPV vaccine trial has demonstrated that HPV vaccination also prevents oral and anal infection with HPV-16 and -18. Future analysis will determine if this translates to a reduction of HPV-associated oral and anal cancers.
The impact of widespread vaccination programs on HPV infection and HPV-associated disease is now being reported. In Scotland, HPV testing performed prior to and after introduction of the HPV vaccine revealed a reduction in the rate of HPV-16 and -18 positivity on cervical swabs from 29.8% (95% confidence interval = 28.3 to 31.3) to 13.6% (95% confidence interval = 11.7 to 15.8). A reduction in other HPV subtypes, including -31, -33, and -45 was also seen, suggesting cross-protection.
Natural History and Pattern of Spread
Most cervical carcinomas arise at the junction between the primarily columnar epithelium of the endocervix and the squamous epithelium of the ectocervix. This junction is a site of continuous metaplastic change, which is greatest in utero, at puberty, and during first pregnancy, and declines after menopause. Long before the relationship between HPV and cervical cancer was known, Richart and Barron165 demonstrated that invasive squamous cell cancer of the cervix was the end result of progressive intraepithelial dysplastic changes within metaplastic epithelium of the transformation zone. The greatest risk of neoplastic transformation of virally induced atypical squamous metaplasia coincides with periods of greatest metaplastic activity. The approximately 15-year difference in the mean ages of women with CIN and women with invasive cervical cancer indicates a generally slow progression of CIN to invasive carcinoma.166
Once tumor has broken through the basement membrane, it may penetrate the cervical stroma directly or through vascular channels. Invasive tumors may develop as exophytic growths protruding from the cervix into the vagina or as endocervical lesions that can cause massive expansion of the cervix despite a relatively normal-appearing ectocervix. From the cervix, tumor may extend superiorly to the lower uterine segment, inferiorly to the vagina, laterally to the broad ligaments (where it may cause ureteral obstruction), or posterolaterally to the uterosacral ligaments. Large tumors may appear fixed on pelvic examination, although true invasion of the pelvic wall musculature is probably uncommon. Although the cervix is separated from the bladder by only a thin layer of fascia and cellular connective tissue, extensive bladder involvement is uncommon, occurring in <5% of cases. Tumor may also extend posteriorly to the rectum, although rectal mucosal involvement is a rare finding at initial presentation.
The cervix has a rich supply of lymphatics that drain the mucosal, muscularis, and serosal layers. The lymphatics of the cervix anastomose extensively with those of the lower uterine segment.167 The most important lymphatic collecting trunks exit laterally from the uterine isthmus in three groups.167 The upper branches, which originate in the anterior and lateral cervix, follow the uterine artery, are sometimes interrupted by a node as they cross the ureter, and terminate in the uppermost hypogastric nodes. The middle branches drain to deeper hypogastric (obturator) nodes. The lowest branches follow a posterior course to the inferior and superior gluteal, common iliac, presacral, and subaortic nodes. Additional posterior lymphatic channels arising from the posterior cervical wall may drain to superior rectal nodes or may continue upward in the retrorectal space to the subaortic nodes overlying the sacral promontory. Anterior collecting trunks pass between the cervix and bladder along the superior vesical artery and terminate in the internal iliac nodes.
The incidence of pelvic and para-aortic node involvement is correlated with tumor stage, size, histologic subtype, depth of invasion, and presence of LVSI. Reported rates of regional metastasis, which come primarily from series of patients who underwent lymphadenectomy as part of radical surgical treatment or before radiotherapy, vary widely. For patients with stage I disease treated with radical hysterectomy, most investigators report an incidence of positive pelvic nodes of 15% to 20% and an incidence of positive para-aortic nodes of 1% to 5%. For patients with stage I disease treated with radiation, reported rates of positive para-aortic nodes tend to be higher—usually 10% to 25%—reflecting the fact that stage I tumors selected for treatment with radiation are usually more advanced. Variations in the completeness of lymphadenectomies and histologic processing may lead to underestimates of the true incidence of regional spread from carcinomas of the cervix.168
Cervical cancer usually follows a relatively orderly pattern of metastatic progression, initially to primary echelon nodes in the pelvis and then to para-aortic nodes and distant sites. Initial presentation with hematogenous metastases is uncommon. FDG-PET scanning is probably the most accurate noninvasive method for the diagnosis of nodal metastasis, with a sensitivity of 82% and specificity of 95% as reported in a 2010 meta-analysis.169 PET-positive pelvic nodes are frequently identified along the lymphatics that extend from the obturator vessels to the bifurcation of the common iliac vessels. Nodes that lie along the common iliac vessels lie most frequently between the psoas muscle and the common iliac artery and vein.170 In the para-aortics, the nodes are closely associated with the aorta and are rarely identified to the left of the vena cava. The anatomic distribution of FDG-avid nodal metastasis in the pelvis and para-aortic nodes in a series of patients with cervical cancer demonstrates the areas where lymphatic metastasis are most commonly identified (Fig. 72.5).
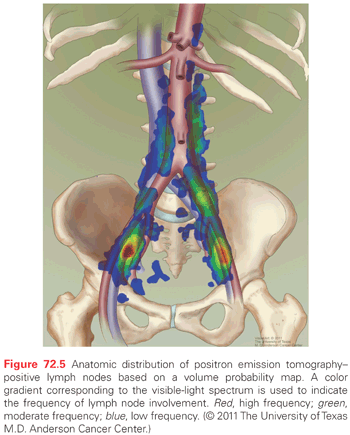
The most frequent sites of distant recurrence are lung, left supraclavicular and mediastinal nodes, liver, and bone.
Pathology
Cervical Intraepithelial Neoplasia
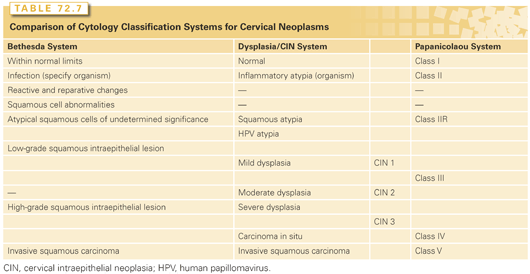
Several systems have been developed for classifying premalignant cytologic and histologic cervical findings (Table 72.7). Following a 1988 National Cancer Institute Consensus Conference, the Bethesda system of classification was developed in an effort to further standardize reporting. The Bethesda system divides cytologic specimens into two groups: low-grade squamous intraepithelial lesions (LSIL) and HSILs. LSILs have low-grade dysplasia or changes associated with HPV. They are typically associated with low-risk HPV types and have a low likelihood of progressing to invasive cancers. These are to be distinguished from HSILs, which have findings of moderate of high-grade dysplasia such as abnormal mitoses, coarse chromatin, and loss of polarity. HSILs are usually associated with high-risk HPV types and have a higher likelihood of progressing to invasive cancer. The Bethesda system was meant to replace the Papanicolaou system and is now widely used in the United States. However, its use is still controversial. Some groups171 argue that the new nomenclature has failed to improve diagnostic accuracy and believe that with dichotomization of the spectrum of atypical lesions, lesions that were formerly classified as CIN 2 (now HSIL) may be overtreated despite their relatively low risk of progression.
The Bethesda system also introduced the term atypical squamous cells of undetermined significance (ASC-US). This uncertain diagnosis is now the most common abnormal Pap smear result in United States laboratories,172 with 1.6% to 9% of Pap smears reported as having ASC-US. Although most cases of ASC-US reflect a benign process, about 5% to 10% are associated with an underlying HSIL and one-third or more of HSILs are heralded by a finding of ASC-US on a Pap smear.173 There has been considerable controversy about the evaluation and management of ASC-US, leading the National Cancer Institute to initiate the ASC-US-LSIL Triage Study.174 This multicenter, randomized trial compared three methods of management—immediate colposcopy, cytological follow-up, and triage by HPV DNA testing—in 5,060 patients who were recruited to the study following a community-based Pap smear report of ASC-US or LSIL. Preliminary analyses of this study174 demonstrated that in patients with LSIL, the prevalence of high-risk HPV was too high to permit useful triage based on HPV DNA testing, but that in the 3,488 patients with ASC-US, HPV DNA testing had a sensitivity in the detection of HSIL similar to that of immediate colposcopy and reduced the number of referrals for colposcopy by 50%. After exclusion of patients who had a diagnosis of CIN 2 or 3 at initial colposcopy, the cumulative risk of subsequent progression to CIN 2 or 3 was equivalent for women with LSIL (27.6%) or high-risk HPV-positive ASC-US (26.7%).175
Biopsy specimens that allow evaluation of tissue architecture can be scored as CIN I-III based on the Bethesda system.176 Although criteria for the diagnosis of CIN and degree of neoplasia vary somewhat between pathologists, the important features of CIN are cellular immaturity, cellular disorganization, nuclear abnormalities, and increased mitotic activity. If mitoses and immature cells are present only in the lower third of the epithelium, the lesion is usually designated CIN 1. Lesions involving only the lower and middle thirds are designated CIN 2, and those involving the upper third are designated CIN 3. The term cervical intraepithelial neoplasia, as proposed by Richart,177 refers only to a lesion that may progress to invasive carcinoma. Although CIN 1 and CIN 2 are sometimes referred to as mild-to-moderate dysplasia, the term CIN is now preferred over dysplasia.
Adenocarcinoma in Situ
Adenocarcinoma in situ is diagnosed when normal endocervical gland cells are replaced by tall, irregular columnar cells with stratified, hyperchromatic nuclei and increased mitotic activity but the normal branching pattern of the endocervical glands is maintained and there is no obvious stromal invasion. About 20% to 50% of women with cervical adenocarcinoma in situ also have squamous CIN.178 Because adenocarcinoma in situ is frequently multifocal, cone biopsy margins are unreliable.179 Although some investigators have described a possible precursor lesion termed endocervical glandular dysplasia, the reproducibility and clinical value of this designation are uncertain.180
Microinvasive Carcinoma
Microinvasive carcinoma is defined by FIGO as “invasive carcinoma which can be diagnosed only by microscopy, with deepest invasion ≤5 mm and largest extension ≥7 mm” (stage IA in Table 72.8). Thus, this diagnosis can be made only after examination of a specimen that includes the entire neoplastic lesion and cervical transformation zone. This requires a cervical cone biopsy. Following the advent of cytologic screening, the proportion of invasive carcinomas that invade <5 mm increased more than 10-fold to about 20% in the United States.181
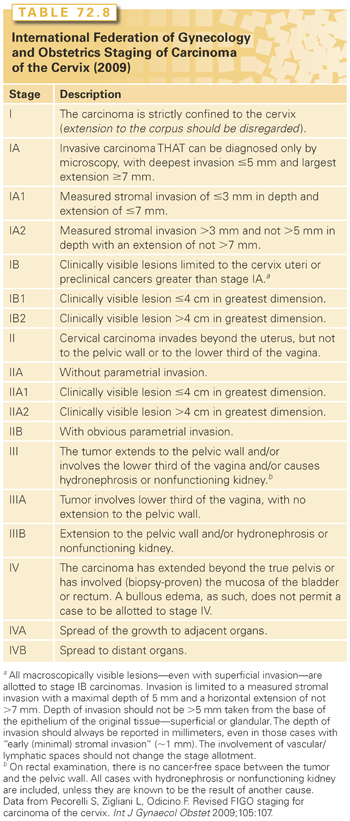
The earliest invasion appears as a blurring of the stromoepithelial junction with a protrusion of cells into the stroma. These cells are less well differentiated than the adjacent noninvasive cells and have abundant pink-staining cytoplasm, hyperchromatic nuclei, and prominent nucleoli. They also exhibit a loss of polarity at the stromoepithelial junction.181 Early microinvasion is usually characterized by a desmoplastic response in adjacent stroma with scalloping or duplication of the neoplastic epithelium or formation of pseudoglands (nests of invasive carcinoma that can mimic crypt involvement). In a study of cone specimens, Reich et al.182 reported that 12% of microinvasive carcinomas were multifocal. The depth of invasion should be measured with a micrometer from the base of the epithelium to the deepest point of invasion. Lesions that have invaded <3 mm (FIGO stage IA1) are rarely associated with metastases; 5% to 10% of tumors that have invaded 3 to 5 mm (FIGO stage IA2) are associated with positive pelvic lymph nodes.183 Until FIGO refined its definition of microinvasive carcinoma (see Table 72.8), most clinicians in the United States used a different definition of microinvasive carcinoma formulated by the Society of Gynecologic Oncologists: cancers that invaded <3 mm with no evidence of LVSI. The importance of LVSI remains somewhat controversial; the risk of metastatic regional disease appears to be exceedingly low for any tumor that invades <3 mm, even in the presence of LVSI.181 Although most clinicians have adopted the FIGO definitions, many think that the risk of regional spread from tumors that have invaded 3 to 5 mm is sufficiently high to warrant evaluation or treatment of the parametria and regional nodes.
For adenocarcinoma, in particular, measuring the depth of invasion can be difficult. Because invasive adenocarcinomas may originate anywhere along the profile of architecturally complex glands that course through the cervical stroma, no reproducible method has been found for measuring the depth of invasion of these tumors. Some authors have measured the extent of invasion from the basement membrane or from the nearest abnormal glandular epithelium; others have defined early adenocarcinomas according to the volume of tumor (in cubic millimeters).180 Despite these differences in measurement method, it is apparent that a subset of patients with very small adenocarcinomas have a low likelihood of lymph node metastasis or recurrence.180
Invasive Squamous Cell Carcinoma
Between 80% and 90% of cervical carcinomas are squamous cell carcinomas. Although squamous neoplasms are often subclassified as large cell keratinizing, large cell nonkeratinizing, or small cell carcinomas, these designations do not correlate well with prognosis.184 Small cell squamous carcinomas have small- to medium-sized nuclei, open chromatin, small or large nucleoli, and abundant cytoplasm, and are believed by most authorities to have a somewhat poorer prognosis than large cell neoplasms with or without keratin. However, small cell squamous carcinomas should not be confused with the much more aggressive anaplastic small cell neuroendocrine carcinomas discussed later. Papillary variants of squamous carcinoma may be well differentiated (occasionally confused with immature condylomata) or very poorly differentiated (resembling high-grade transitional carcinoma).181 Verrucous carcinoma is a very rare warty-appearing variant of squamous carcinoma that may be difficult to differentiate from benign condyloma without multiple biopsies or hysterectomy.185 Sarcomatoid squamous carcinoma is another very rare variant, demonstrating areas of spindle-cell carcinomatous tumor confluent with poorly differentiated squamous cell carcinoma; immunohistochemistry demonstrates expression of cytokeratin as well as vimentin. The natural history of this uncommon tumor is not well understood.186
Adenocarcinoma
Invasive adenocarcinoma may be pure or mixed with squamous cell carcinoma (adenosquamous carcinoma). About 80% of cervical adenocarcinomas are endocervical-type adenocarcinomas, which are composed predominantly of cells with eosinophilic cytoplasm, brisk mitotic activity, and frequent apoptotic bodies, although many other patterns and cell types have also been observed. Endocervical-type adenocarcinomas are frequently referred to as mucinous; however, although some have abundant intracytoplasmic mucin, most have little or none.187
Minimal-deviation adenocarcinoma (adenoma malignum) is a rare, extremely well-differentiated adenocarcinoma that is sometimes associated with Peutz-Jeghers syndrome.188 Because the branching glandular pattern strongly resembles normal endocervical glands and the mucin-rich cells can be deceptively benign-appearing, minimal-deviation adenocarcinoma may not be recognized as malignant in small biopsy specimens.187 Earlier studies reported a poor outcome for women with this tumor, but more recently, patients have been reported to have a favorable prognosis if the disease is detected early.188
Glassy cell carcinoma187 is a variant of poorly differentiated adenosquamous carcinoma characterized by cells with abundant eosinophilic, granular, ground-glass cytoplasm with large round to oval nuclei and prominent nucleoli. Adenoid basal carcinoma is a well-differentiated tumor that histologically resembles basal cell carcinoma of the skin and tends to have a favorable prognosis.189 Adenoid cystic carcinoma consists of basaloid cells in a cribriform or cylindromatous pattern; metastases are frequent, although the natural history of these tumors may be long.189 Rarely, primary carcinomas of the cervix are composed of endometrioid, serous, or clear cells; mixtures of these cell types may be seen, and histologically, some of these tumors are indistinguishable from those arising elsewhere in the endometrium or ovary. In a study of 17 cases, Zhou et al.190 found that serous carcinomas of the cervix have an aggressive course, similar to that of high-grade serous tumors originating in the other Müllerian sites.
Anaplastic Small Cell/Neuroendocrine Carcinoma
Anaplastic small cell carcinomas resemble oat cell carcinomas of the lung and are made up of small tumor cells that have scanty cytoplasm, small round to oval nuclei, and high mitotic activity; they frequently display neuroendocrine features.191 Anaplastic small cell carcinomas behave more aggressively than poorly differentiated small cell squamous carcinomas; most investigators report survival rates of <50% even for patients with early stage I disease, although recent studies of aggressive multimodality treatments have been somewhat more encouraging.192–195 Widespread hematogenous metastases are frequent, but brain metastases are rare unless preceded by pulmonary involvement.195
Other Rare Neoplasms
A variety of neoplasms may infiltrate the cervix from adjacent sites, and this makes differential diagnosis difficult. In particular, it may be difficult or impossible to determine the origin of adenocarcinomas involving the endocervix and uterine isthmus. Although endometrioid histology suggests endometrial origin and mucinous tumors in young patients are most often of endocervical origin, both histologic types can arise in either site.196 Metastatic tumors from the colon, breast, or other sites may involve the cervix secondarily. Malignant mixed Müllerian tumors, adenosarcomas, and leiomyosarcomas occasionally arise in the cervix but more often involve it secondarily. Primary lymphomas and melanomas of the cervix are extremely rare. Despite the different prognostic implications of the histologic subtypes of cervical cancer, the treatment approach generally remains the same.
Clinical Manifestations
Preinvasive disease is usually detected during routine cervical cytologic screening. Early invasive disease may not be associated with any symptoms and is also usually detected during screening examinations. The earliest symptom of invasive cervical cancer is usually abnormal vaginal bleeding, often following coitus or vaginal douching. This may be associated with a clear or foul-smelling vaginal discharge. Pelvic pain may result from locoregionally invasive disease or from coexistent pelvic inflammatory disease. Flank pain may be a symptom of hydronephrosis, which may be complicated by pyelonephritis. Patients with very advanced tumors may have hematuria or incontinence from a vesicovaginal fistula caused by direct extension of tumor to the bladder. External compression of the rectum by a massive primary tumor may cause constipation, but the rectal mucosa is rarely involved at initial diagnosis.
Diagnosis, Clinical Evaluation, and Staging
Diagnosis
The long preinvasive stage of cervical cancer, the relatively high prevalence of the disease in unscreened populations, and the sensitivity of cytologic screening make cervical carcinoma an ideal target for cancer screening. In the United States, screening with cervical cytologic examination and pelvic examination has led to a decrease of >50% in the incidence of cervical cancer since 1975.197 Only nations with well-developed screening programs have experienced substantial decreases in cervical cancer incidence.
The American Cancer Society, in conjunction with multidisciplinary working groups, recently updated the guidelines for cervical cancer screening.198,199 The guidelines are as follows: screening is recommended to begin at age 21 years; screening should be avoided before this age because screening at younger ages may lead to unnecessary and harmful evaluation and treatment in women at very low risk of cancer. Between ages 21 and 29, Pap tests are recommended every 3 years. Between ages 30 and 65, both Pap test and HPV testing is recommended every 5 years. A single negative test for HPV is sufficient to reassure against cervical cancer over 5 years, so women aged 30 years and older who are negative for HPV testing have normal cytology can now safely extend the screening interval.200,201 Women with a normal Pap test result who test positive for oncogenic HPV should be rescreened annually.The 5-year screening interval may be also recommended for women infected with HIV who are cytologically normal and are oncogenic HPV-negative based on a recent study showing that the 5-year cumulative incidence of HSIL and CIN-2 is similar in women infected with HIV and women not infected with HIV who are cytologically normal and oncogenic HPV-negative.202 Women who have had a total hysterectomy for benign conditions and who have no history of high-grade CIN may discontinue routine screening. It is also reasonableto discontinue screening for women older than 65 to 70 years who have three or more consecutive negative studies and have had no abnormal test results in the past 10 years. Women previously treated for high-grade CIN or for cancer should continue to have annual screening for at least 20 years and periodic screening indefinitely. Annual gynecologic examination might still be appropriate even if cytologic screening is not performed.197
Accurate calculation of false-negative rates for the Pap test is difficult; estimates range from <5% to ≥20%. The sensitivity of individual tests may be improved by ensuring adequate sampling of the squamocolumnar junction and the endocervical canal; smears without endocervical or metaplastic cells are inadequate, and in such cases the test must be repeated. The sensitivity of a screening program is increased by repeated testing; studies of the test frequency required to optimize the sensitivity of screening formed the basis of the American College of Obstetrics and Gynecology recommendations.
Most US gynecologists currently prefer newer liquid-based screening methods to conventional Pap tests. A meta-analysis of available data concluded that “liquid-based cervical cytology is neither more sensitive nor more specific for detection of high-grade CIN compared with the conventional Pap,”203 but liquid based tests are more widely used based on the ability to perform HPV typing on fluid remaining after cytologic examination. HPV testing of ASC-US smears followed by colposcopy in patients with HPV-positive lesions has been shown to be a highly accurate and cost-effective means of detecting HSIL in cases of equivocal smears and may also be used to triage postmenopausal women with LSIL.203,204
Patients with abnormal findings on cytologic examination who do not have a gross cervical lesion must be evaluated with colposcopy and directed biopsies. Following application of a 3% acetic-acid solution, the cervix is examined under 10- to 15-fold magnification with a bright, filtered light that enhances the acetowhitening and vascular patterns characteristic of dysplasia or carcinoma. The skilled colposcopist can accurately distinguish between low- and high-grade dysplasia,205 but microinvasive disease cannot consistently be distinguished from intraepithelial lesions on colposcopy.206
In patients with a high-grade Pap smear finding, if no abnormalities are found on colposcopic examination or if the entire squamocolumnar junction cannot be visualized, an additional endocervical sample should be collected. Although some authorities advocate the routine addition of endocervical curettage to colposcopic examination, it is probably reasonable to omit this step in previously untreated women if the entire squamocolumnar junction is visible with a complete ring of unaltered columnar epithelium in the lower canal.207 The rate of detection of endocervical lesions may be higher when specimens are collected using a cytobrush rather than by curettage.208
Cervical cone biopsy is used to diagnose occult endocervical lesions and is an essential step in the diagnosis and management of microinvasive carcinoma of the cervix. Cervical cone biopsy yields an accurate diagnosis and decreases the incidence of inappropriate therapy when (1) the squamocolumnar junction is poorly visualized on colposcopy and a high-grade lesion is suspected, (2) high-grade dysplastic epithelium extends into the endocervical canal, (3) the cytologic findings suggest high-grade dysplasia or carcinoma in situ, (4) a microinvasive carcinoma is found on directed biopsy, (5) the endocervical curettage specimens show high-grade CIN, or (6) the cytologic findings are suggestive of adenocarcinoma in situ.209
Clinical Evaluation of Patients with Invasive Carcinoma
All patients with invasive cervical cancer should be evaluated with a detailed history and physical examination, with particular attention paid to inspection and palpation of the pelvic organs with bimanual and rectovaginal examinations. Standard laboratory studies should include a complete blood cell count and renal function and liver function tests. All patients should have chest radiography to rule out lung metastases. Additional imaging of the abdomen and pelvis should be performed for all patients who have stage IB2 or greater disease and is generally recommended for patients with stage IBI disease. MRI can be used to evaluate the size and extent of the cervical mass as well as suggest invasion into the parametria, bladder, or rectum. Cystoscopy and proctoscopy should be considered in patients with bulky tumors and patients with imaging findings suggestive of organ involvement.
Many clinicians obtain CT or MRI scans to evaluate regional lymph nodes, but these studies have suboptimal accuracy because they fail to detect small metastases and because patients with bulky necrotic tumors often have enlarged reactive lymph nodes that may be free of metastasis.210,211 In a large GOG study that compared the results of radiographic studies with subsequent histologic findings, Heller et al.211 found that the sensitivity of CT in the detection of positive para-aortic nodes was only 34%. PET appears to be a more sensitive, specific, and noninvasive method of evaluating the regional nodes of patients with cervical cancer.169,212
Clinical Staging
The FIGO staging system is the most widely accepted staging system for carcinomas of the cervix.213,214 The latest (2009) update of this system is summarized in Table 72.8.213 (Since the earliest versions of the cervical cancer staging system, there have been numerous changes: the designation of preinvasive disease as a separate category [1950], designation and changes in the definition of microinvasive disease [1962, 1985, and 1994], and subdivisions of the stage I and II categories according to tumor or cervical diameter [1994 and 2009]). Although these changes have gradually improved the discriminatory value of the staging system, the many fluctuations in the definitions of stages IA and IB have complicated efforts to compare the outcomes of patients whose tumors were staged and treated during different periods.215
FIGO stage is based primarily on careful clinical examination. The use of diagnostic imaging techniques to assess tumor size and local extent is encouraged but not mandatory in the 2009 staging system. However, FIGO still does not incorporate evidence of lymph node metastasis gained by surgical staging or advanced imaging studies in the 2009 staging system. Some form of imaging must be performed to evaluate the presence or absence of hydronephrosis, but intravenous pyelography is no longer required. Cystoscopy, sigmoidoscopy, and examination under anesthesia are also optional. However, suspected bladder or rectal involvement must be confirmed by biopsy. Stage should be assigned before any definitive therapy is administered. The clinical stage should never be changed on the basis of subsequent findings. When the stage to which a particular case should be allotted is in doubt, the case should be assigned to the earlier stage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree